Glioblastoma and Internal Carotid Artery Calcium Score: A Possible Novel Prognostic Partnership?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
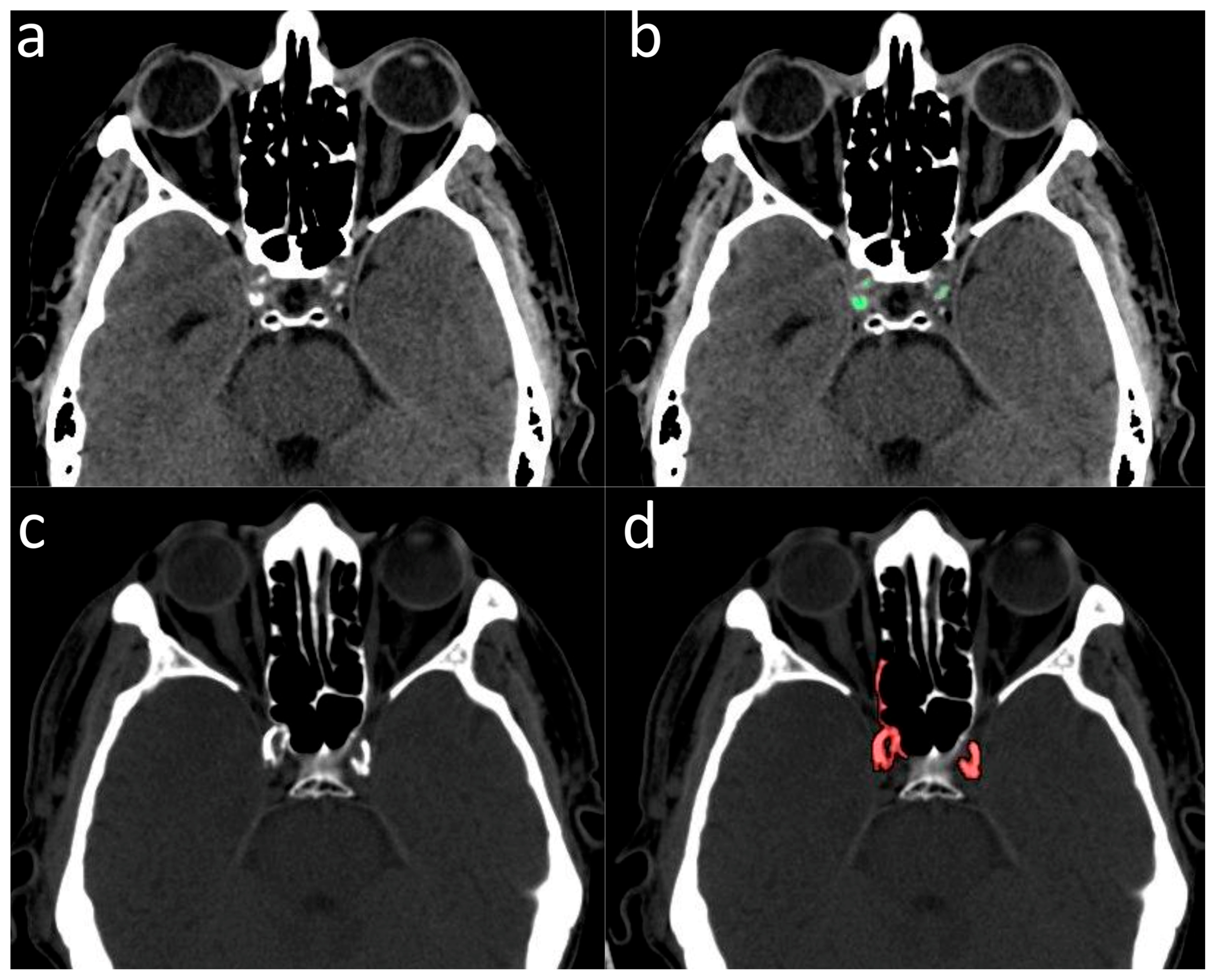
2.2. Validation of ICA Calcium Score as Surrogate Marker of CACs
2.3. Assessment of MR Images and MGMT Methylation of GBM Patients
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme—Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Vanderbeek, A.M.; Rahman, R.; Fell, G.; Ventz, S.; Chen, T.; Redd, R.; Parmigiani, G.; Cloughesy, T.F.; Wen, P.Y.; Trippa, L.; et al. The clinical trials landscape for glioblastoma: Is it adequate to develop new treatments? Neuro. Oncol. 2018, 20, 1034–1043. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Caccese, M.; Simonelli, M.; Villani, V.; Rizzato, S.; Ius, T.; Pasqualetti, F.; Russo, M.; Rudà, R.; Amoroso, R.; Bellu, L.; et al. Definition of the Prognostic Role of MGMT Promoter Methylation Value by Pyrosequencing in Newly Diagnosed IDH Wild-Type Glioblastoma Patients Treated with Radiochemotherapy: A Large Multicenter Study. Cancers 2022, 14, 2425. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.; Ugiliweneza, B.; Woo, S.; Skirboll, S.; Boaky, M. A Surveillance, Epidemiology and End Results-Medicare data analysis of elderly patients with glioblastoma multiforme: Treatment patterns, outcomes and cost. Mol. Clin. Oncol. 2015, 3, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Lombardi, G.; Paolini, S. Glioblastoma in Elderly Patients: Current Management and Future Perspectives. Cancers 2019, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.T.; Hochheimer, C.J.; Rock, A.K.; Dincer, A.; Ravindra, L.; Zhang, F.L.; Opalak, C.F.; Poulos, N.; Sima, A.P.; Broaddus, W.C. Comorbid Medical Conditions as Predictors of Overall Survival in Glioblastoma Patients. Sci. Rep. 2019, 9, 20018. [Google Scholar] [CrossRef] [PubMed]
- Barz, M.; Bette, S.; Janssen, I.; Aftahy, A.K.; Huber, T.; Liesche-Starnecker, F.; Ryang, Y.M.; Wiestler, B.; Combs, S.E.; Meyer, B.; et al. Age-adjusted Charlson comorbidity index in recurrent glioblastoma: A new prognostic factor? BMC Neurol. 2022, 22, 32. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, A.; Khan, A.; Shah, S.U.; Iqbal, H.; Iqbal, S.; Rana, A.I.; Rahman, H.U. Evaluating the use of coronary artery calcium scoring as a tool for coronary artery disease (CAD) risk stratification and its association with coronary stenosis and CAD risk factors: A single-centre, retrospective, cross-sectional study at a tertiary centre in Pakistan. BMJ Open 2022, 12, e057703. [Google Scholar]
- Cuddy, S.; Payne, D.L.; Murphy, D.J.; Dunne, R.M.; Bueno, R.; Blankstein, R.; Di Carli, M.; Mehra, M.R.; Nohria, A.; Groarke, J.D. Incidental Coronary Artery Calcification in Cancer Imaging. JACC Cardio Oncol. 2019, 1, 135–137. [Google Scholar] [CrossRef]
- Chaikriangkrai, K.; Jhun, H.Y.; Shantha, G.P.S.; Abdulhak, A.B.; Sigurdsson, G.; Nabi, F.; Mahmarian, J.J.; Chang, S.M. Coronary artery calcium score as a predictor for incident stroke: Systematic review and meta-analysis. Int. J. Cardiol. 2017, 236, 473–477. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Lei, Y.; Chen, Y.; Cheng, M.; Wei, X.; Liu, J.; Liu, P.; Chen, R.; Yin, X.; et al. Coronary Atherosclerotic Disease and Cancer: Risk Factors and Interrelation. Front. Cardiovasc. Med. 2022, 9, 821267. [Google Scholar] [CrossRef]
- Pasqualetti, F.; Ferrazza, P.; Cocuzza, P.; Fatigante, L.; Pasqualetti, G.; Fabbrini, M.G.; Monzani, F. Radio-chemotherapy with temozolomide in elderly patients with glioblastoma. A mono-institutional experience. Anticancer Res. 2014, 34, 4281–4285. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, F.; Orlandi, P.; Simeon, V.; Cantarella, M.; Giuliani, D.; Di Desidero, T.; Gonnelli, A.; Delishaj, D.; Lombardi, G.; Sechi, A.; et al. Melanocortin Receptor-4 Gene Polymorphisms in Glioblastoma Patients Treated with Concomitant Radio-Chemotherapy. Mol. Neurobiol. 2018, 55, 1396–1404. [Google Scholar] [CrossRef]
- Vaglini, F.; Pardini, C.; Di Desidero, T.; Orlandi, P.; Pasqualetti, F.; Ottani, A.; Pacini, S.; Giuliani, D.; Guarini, S.; Bocci, G. Melanocortin Receptor-4 and Glioblastoma Cells: Effects of the Selective Antagonist ML00253764 Alone and in Combination with Temozolomide In Vitro and In Vivo. Mol. Neurobiol. 2018, 55, 4984–4997. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Gronewold, J.; Lehmann, N.; Moebus, S.; Jöckel, K.H.; Bauer, M.; Erbel, R. Coronary artery calcification is an independent stroke predictor in the general population. Stroke 2013, 44, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Young, R.; Delaney, J.A.; Budoff, M.J.; Polak, J.F.; Blaha, M.J.; Post, W.S.; Michos, E.D.; Kaufman, J.; Stein, J.H. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2017, 6, e005179. [Google Scholar] [CrossRef]
- Shenouda, R.; Vancheri, S.; Maria Bassi, E.; Nicoll, R.; Sobhi, M.; El Sharkawy, E.; Wester, P.; Vancheri, F.; Henein, M.Y. The relationship between carotid and coronary calcification in patients with coronary artery disease. Clin. Physiol. Funct. Imaging 2021, 41, 271–280. [Google Scholar] [CrossRef]
- Pasqualetti, F.; Barberis, A.; Zanotti, S.; Montemurro, N.; De Salvo, G.L.; Soffietti, R.; Mazzanti, C.M.; Ius, T.; Caffo, M.; Paiar, F.; et al. The Impact of Survivorship Bias in Glioblastoma Research. Crit. Rev. Oncol. Hematol. 2023, 188, 104065. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Tanzilli, A.; Telera, S.M.; Terrenato, I.; Vidiri, A.; Fabi, A.; Zucchella, C.; Carapella, C.M.; Marucci, L.; Casini, B.; et al. Comorbidities in elderly patients with glioblastoma: A field-practice study. Future Oncol. 2019, 15, 841–850. [Google Scholar] [CrossRef]
- Ius, T.; Somma, T.; Altieri, R.; Angileri, F.F.; Barbagallo, G.M.; Cappabianca, P.; Certo, F.; Cofano, F.; D’Elia, A.; Della Pepa, G.M.; et al. Is age an additional factor in the treatment of elderly patients with glioblastoma? A new stratification model: An Italian Multicenter Study. Neurosurg. Focus 2020, 49, E13. [Google Scholar] [CrossRef]
- Yang, W.; Pan, X.; Ma, A. The Potential of Exosomal RNAs in Atherosclerosis Diagnosis and Therapy. Front. Neurol. 2020, 11, 572226. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Yu, Q.; Zeng, W.Y.; Zeng, M.; Zhang, X.L.; Zhang, Y.L.; Guo, L.; Jiang, X.J.; Gan, J.L. Killing two birds with one stone: miR-126 involvement in both cancer and atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6145–6168. [Google Scholar]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.; Enrick, M.; Diaz, A.; Yin, L. Is miR-21 A Therapeutic Target in Cardiovascular Disease? Int. J. Drug. Discov. Pharm. 2023, 2, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liang, Q.; Xu, Z.; Cai, Y.; Peng, B.; Li, J.; Zhang, W.; Kang, F.; Hong, Q.; Yan, Y.; et al. Current Understanding of Exosomal MicroRNAs in Glioma Immune Regulation and Therapeutic Responses. Front. Immunol. 2021, 12, 813747. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Florio, T.; Perez-Castro, C. Extracellular Vesicles Loaded miRNAs as Potential Modulators Shared between Glioblastoma, and Parkinson’s and Alzheimer’s Diseases. Front. Cell. Neurosci. 2020, 14, 590034. [Google Scholar] [CrossRef]

| Characteristic | Statistics |
|---|---|
| Gender | |
| M | 56% |
| F | 44% |
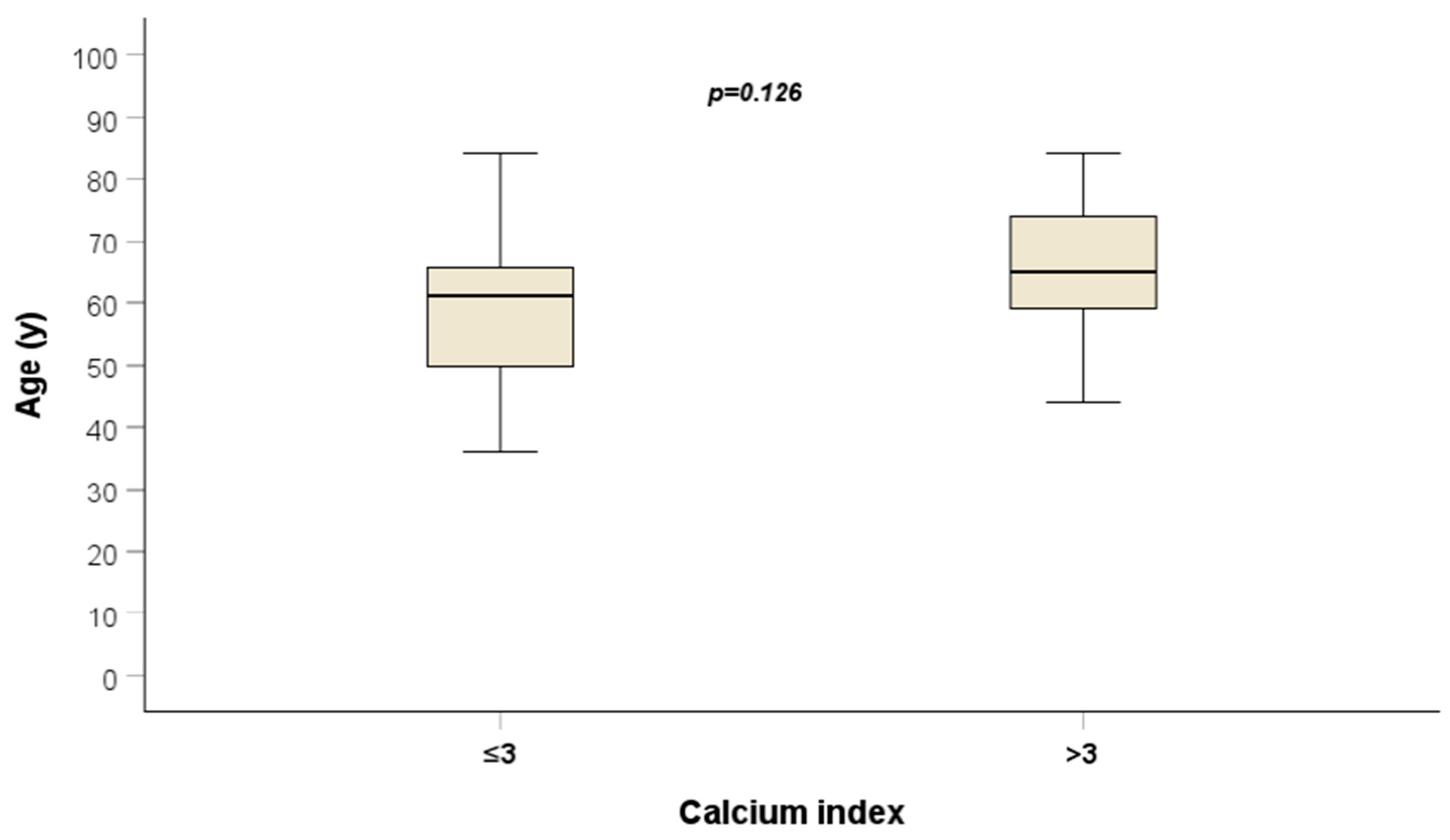
| Median Age (years) | 62 (range: 36–84) |
| MGMT methylation | |
| no | 28.2% |
| yes | 71.8% |
| Extent of surgery | |
| GTR | 36.4% |
| STR | 63.6% |
| ICA calcium score expressed in quartiles | |
| 25th | 3 |
| 50th | 8.5 |
| 75th | 34 |
| Categorical ICA calcium score | |
| ≤3 | 16 (32%) |
| >3 | 34 (68%) |
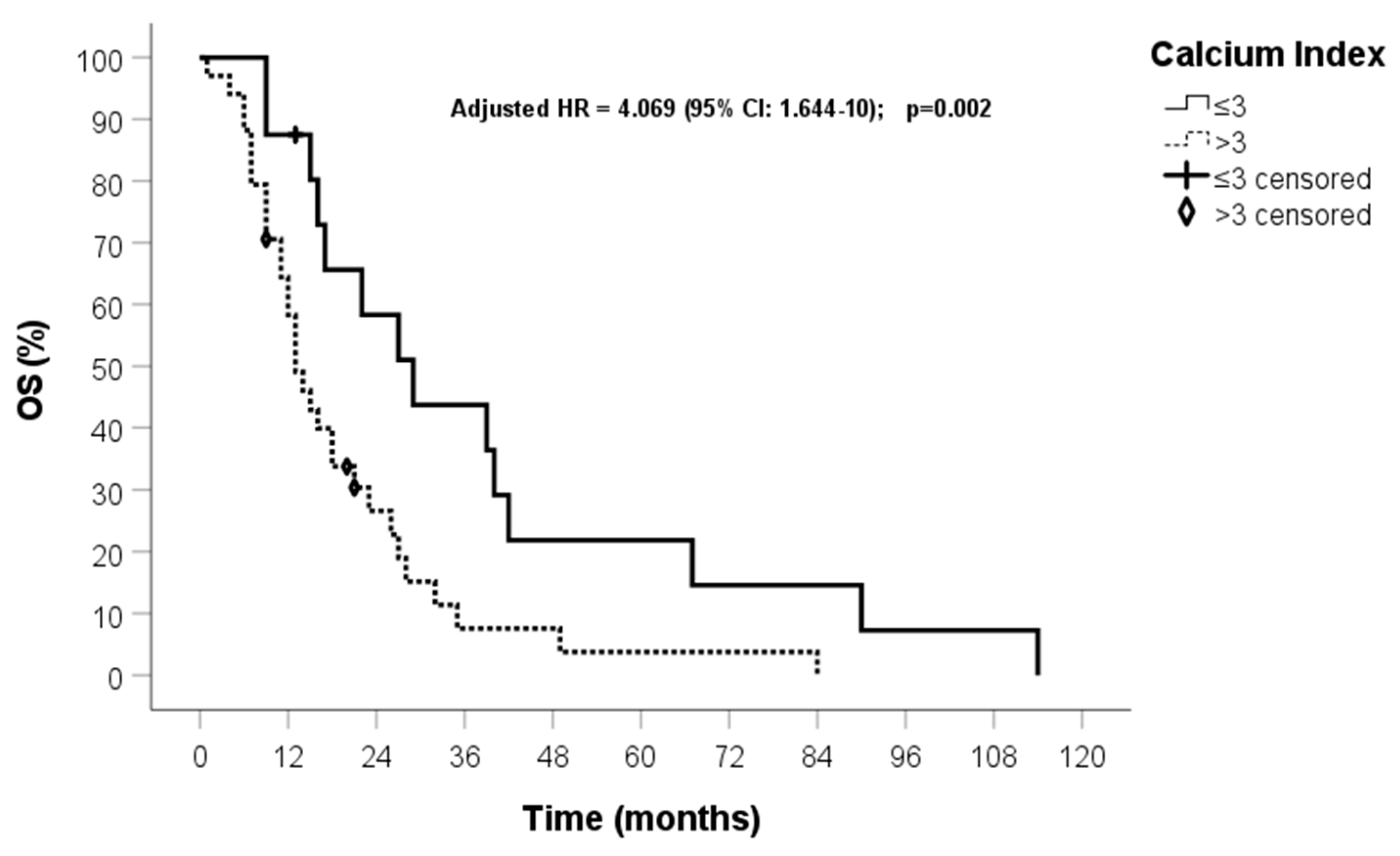
| OS median time (months) | 17 (95% CI: 13.1–20.9) |
| PFS median time (months) | 9 (95% CI: 6.6–11.4) |
| End Point: OS | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Factor | RC | HR (95% CI) | p-Value | RC | HR (95% CI) | p-Value |
| Age | 0.019 | 1.019 (0.991–1.048) | 0.186 | |||
| Gender (0) M, (1) F | −0.282 | 0.754 (0.405–1.404) | 0.373 | |||
| Calcium index (0) ≤3 (1) >3 | 0.893 | 2.443 (1.229–4.855) | 0.011 | 1.912 | 6.767 (2.190–21) | 0.001 |
| MGMT (0) no, (1) yes | −0.859 | 0.423 (0.181–0.992) | 0.048 | −2.712 | 0.066 (0.009–0.472) | 0.007 |
| Extent of surgery (0) GTR, (1) STR | 0.978 | 2.659 (1.188–5.949) | 0.017 | 1.475 | 4.369 (1.520–12.6) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqualetti, F.; Gabelloni, M.; Faggioni, L.; Aquaro, G.D.; De Vietro, F.; Mendola, V.; Spina, N.; Frey, J.; Montemurro, N.; Cantarella, M.; et al. Glioblastoma and Internal Carotid Artery Calcium Score: A Possible Novel Prognostic Partnership? J. Clin. Med. 2024, 13, 1512. https://doi.org/10.3390/jcm13051512
Pasqualetti F, Gabelloni M, Faggioni L, Aquaro GD, De Vietro F, Mendola V, Spina N, Frey J, Montemurro N, Cantarella M, et al. Glioblastoma and Internal Carotid Artery Calcium Score: A Possible Novel Prognostic Partnership? Journal of Clinical Medicine. 2024; 13(5):1512. https://doi.org/10.3390/jcm13051512
Chicago/Turabian StylePasqualetti, Francesco, Michela Gabelloni, Lorenzo Faggioni, Giovanni Donato Aquaro, Fabrizio De Vietro, Vincenzo Mendola, Nicola Spina, Jessica Frey, Nicola Montemurro, Martina Cantarella, and et al. 2024. "Glioblastoma and Internal Carotid Artery Calcium Score: A Possible Novel Prognostic Partnership?" Journal of Clinical Medicine 13, no. 5: 1512. https://doi.org/10.3390/jcm13051512
APA StylePasqualetti, F., Gabelloni, M., Faggioni, L., Aquaro, G. D., De Vietro, F., Mendola, V., Spina, N., Frey, J., Montemurro, N., Cantarella, M., Caccese, M., Gadducci, G., Giannini, N., Valenti, S., Morganti, R., Ius, T., Caffo, M., Vergaro, G., Cosottini, M., ... Paiar, F. (2024). Glioblastoma and Internal Carotid Artery Calcium Score: A Possible Novel Prognostic Partnership? Journal of Clinical Medicine, 13(5), 1512. https://doi.org/10.3390/jcm13051512