The Impact of High BMI on Pregnancy Outcomes and Complications in Women with PCOS Undergoing IVF—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria for Study Selection
2.2. Outcome Measures
2.2.1. Main Outcomes
2.2.2. Secondary Outcome Measures
2.3. Search Strategy
2.4. Screening and Selection of Retrieved Studies
2.5. Risk of Bias Assessment
2.6. Data Extraction and Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
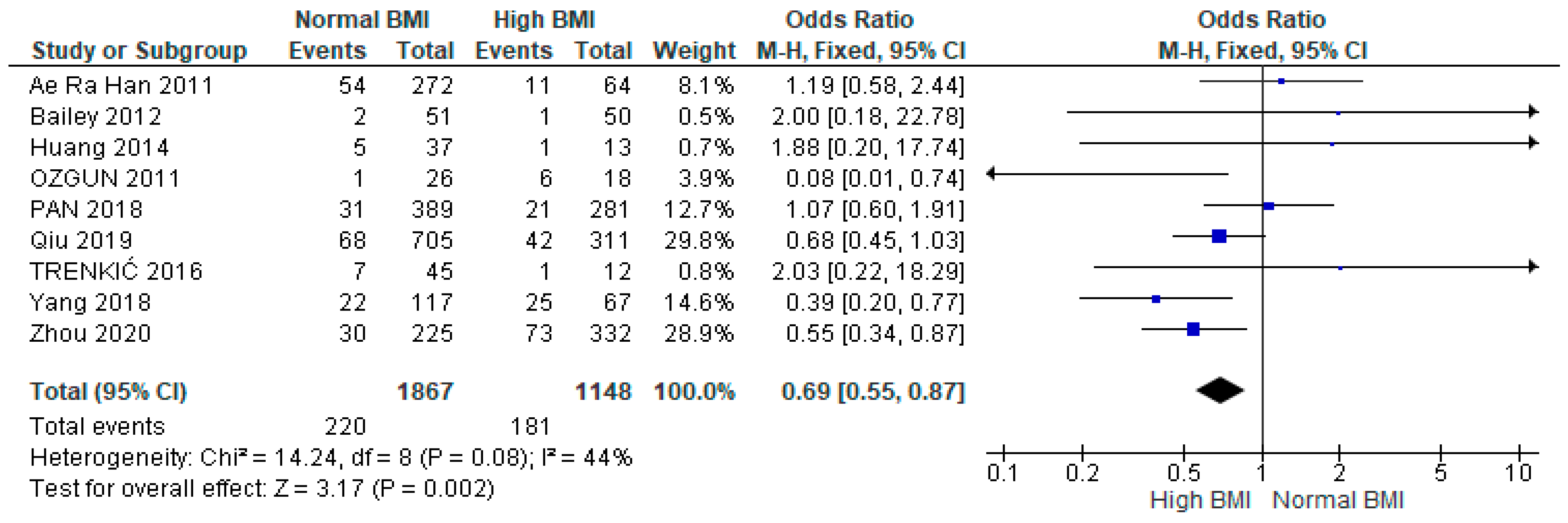
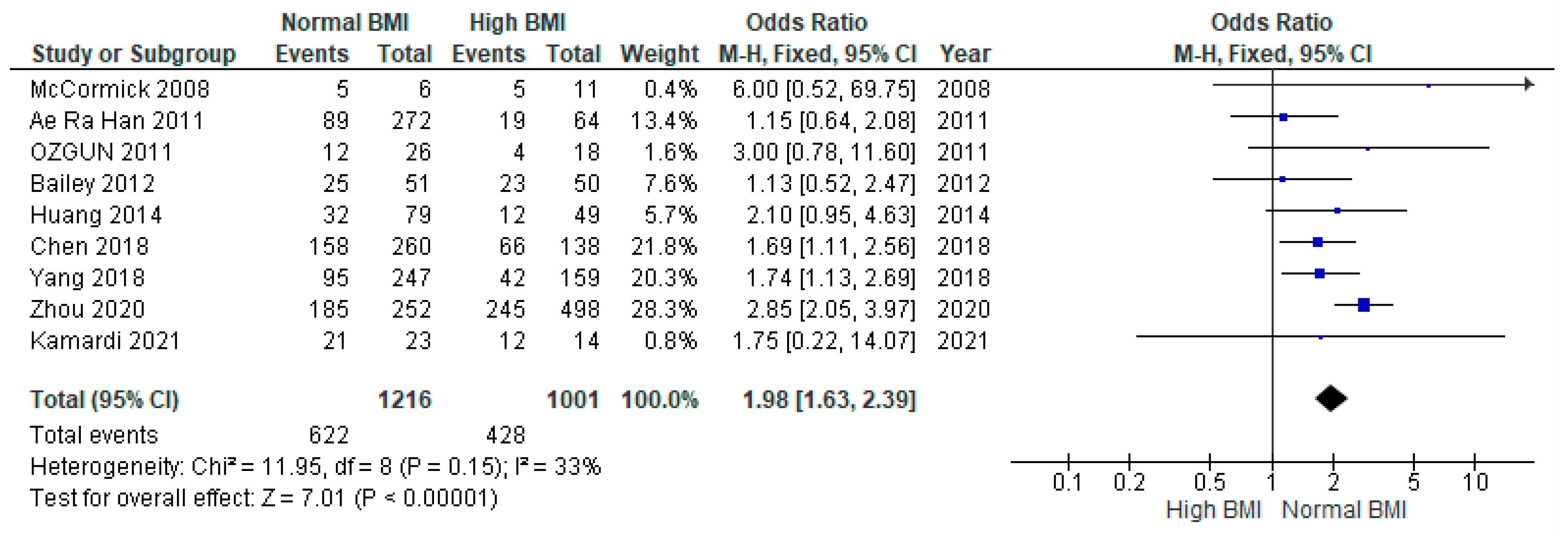
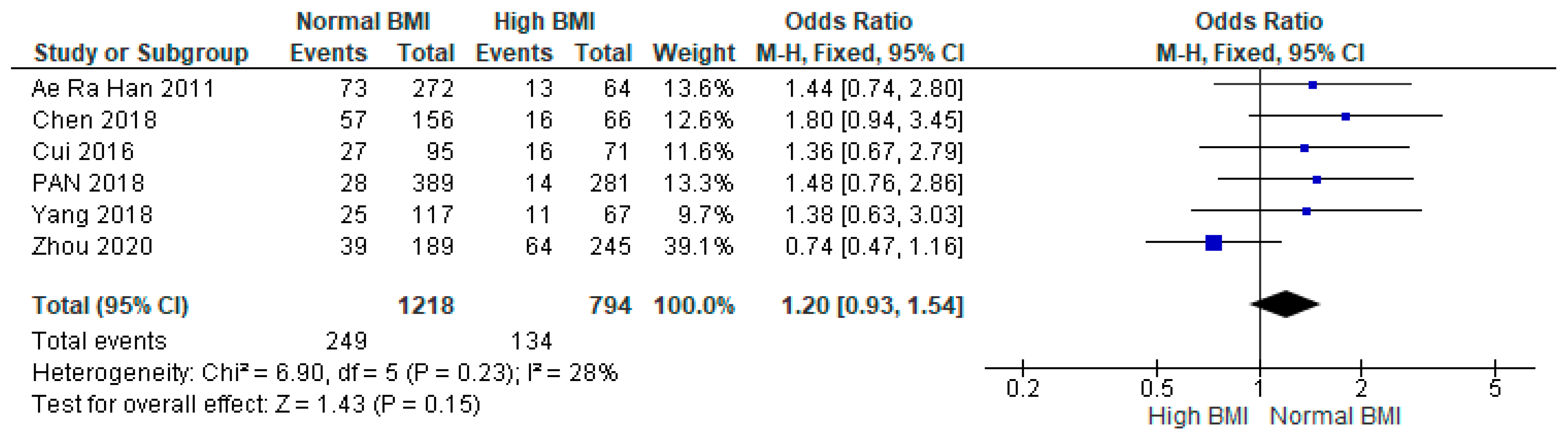
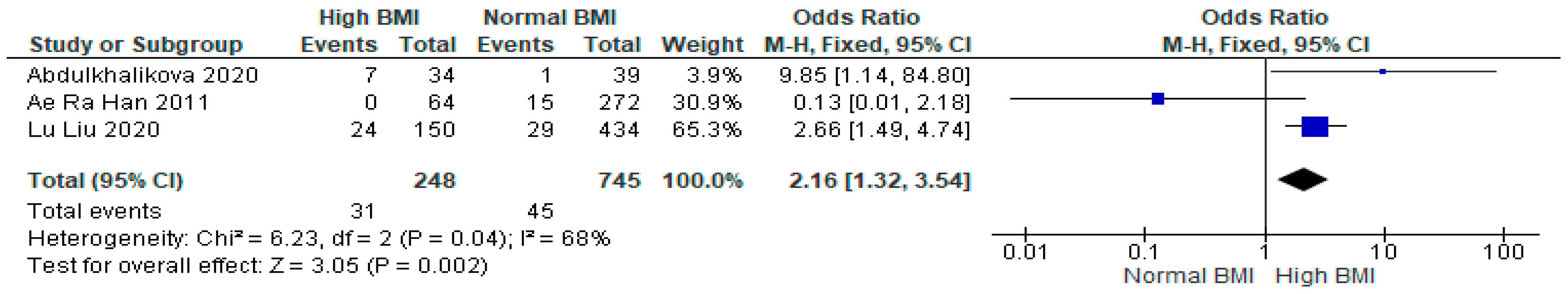
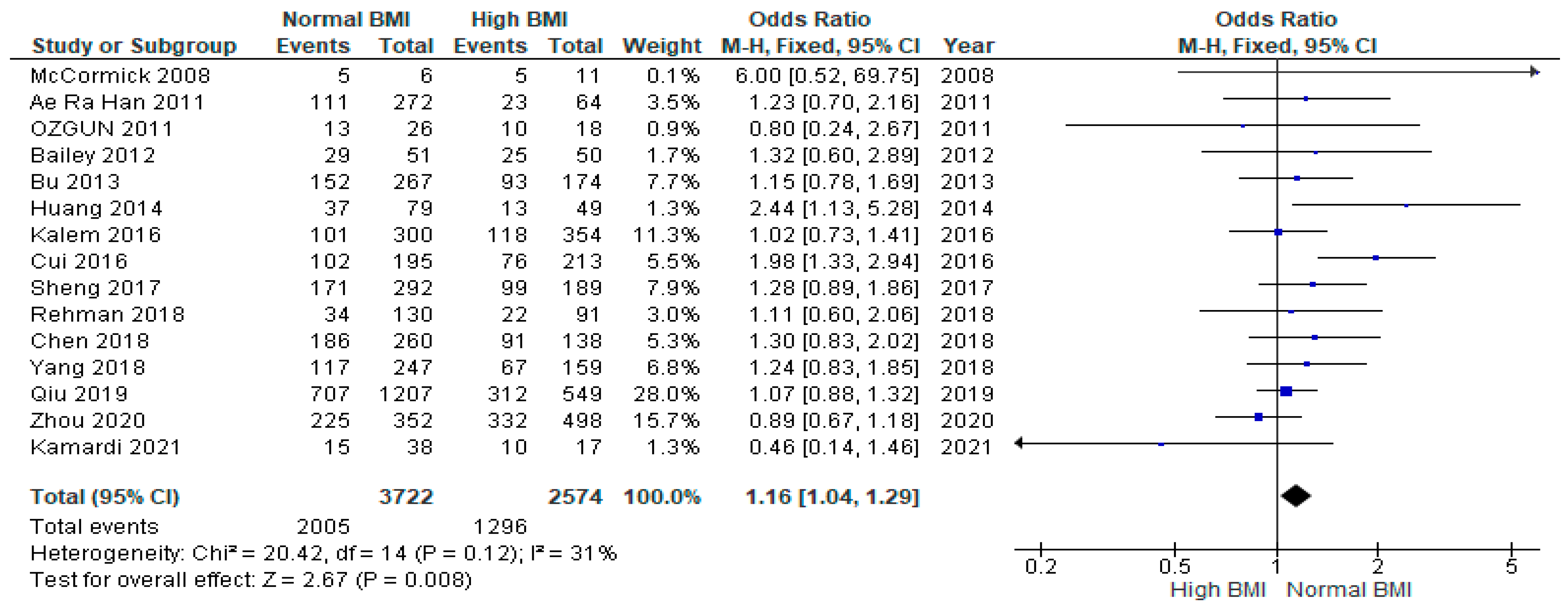
3.3. Clinical Pregnancy
3.4. Miscarriage
3.5. Live Birth
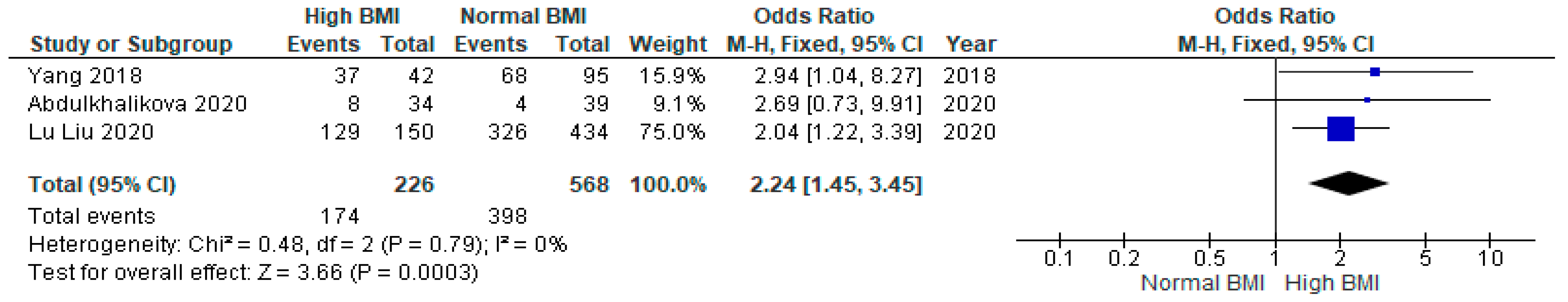
3.6. Preterm Birth
3.7. Gestational Diabetes Mellitus (GDM)
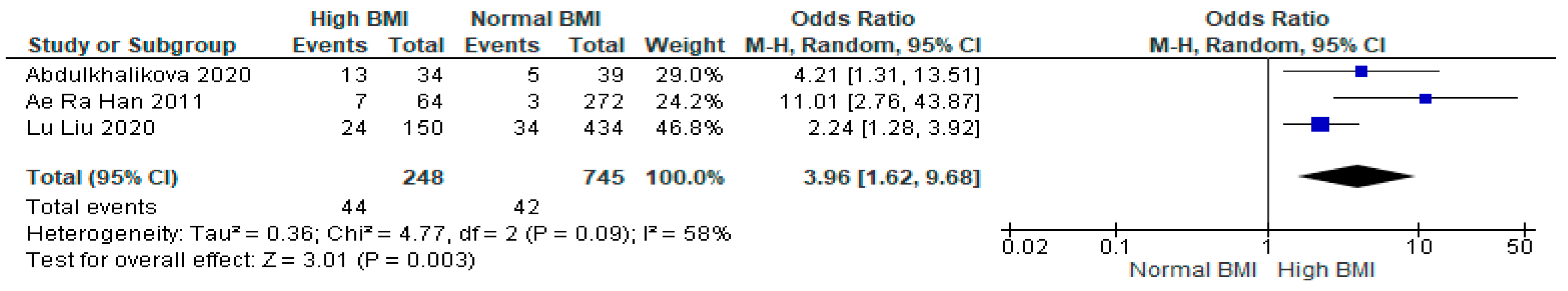
3.8. Gestational Hypertension
3.9. Caesarean Section
4. Discussion
4.1. Comparison with Previous Studies
4.1.1. Pregnancy Outcomes
4.1.2. GDM and Gestational Hypertension
4.1.3. Caesarean Section
4.2. Limitations and Strengths
4.3. Interpretation of the Results
4.4. Clinical Implications
5. Conclusions
BMI and Multiple Pregnancy in PCOS Women Undergoing IVF
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, S.A.; Khan, R.; Amer, S. The Role of NLRP3 Inflammasome in Obesity and PCOS—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 10976. [Google Scholar] [CrossRef]
- Homburg, R.; Crawford, G. The role of AMH in anovulation associated with PCOS: A hypothesis. Hum. Reprod. 2014, 29, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, G.; Mumusoglu, S.; Zengin, D. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Kandarakis, H.; Legro, R.S. The role of genes and environment in the etiology of PCOS. Endocrine 2006, 30, 19–26. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D. Androgen Excess Society. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and polycystic ovary syndrome. Clin. Endocrinol. 2021, 95, 531–541. [Google Scholar] [CrossRef]
- Yang, S.T.; Liu, C.H.; Ma, S.H. Association between Pre-Pregnancy Overweightness/Obesity and Pregnancy Outcomes in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 9094. [Google Scholar] [CrossRef]
- Wallach, E.E.; Buyalos, R.P.; Lee, C.T. Polycystic ovary syndrome: Pathophysiology and outcome with in vitro fertilization. Fertil. Steril. 1996, 65, 1–10. [Google Scholar] [CrossRef]
- Pandey, S.; Shetty, A.; Hamilton, M. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Sha, T.; Wang, X.; Cheng, W. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod. Biomed. Online 2019, 39, 281–293. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Luo, Z. Association between Body Mass Index and Reproductive Outcome in Women with Polycystic Ovary Syndrome Receiving IVF/ICSI-ET. Biomed. Res. Int. 2020, 2020, 6434080. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Tao, Y.; Kuang, Y. Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Fertil. Steril. 2019, 112, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Kim, H.O.; Cha, S.W. Adverse pregnancy outcomes with assisted reproductive technology in non-obese women with polycystic ovary syndrome: A case-control study. Clin. Exp. Reprod. Med. 2011, 38, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liao, X.; Dong, X. Effect of overweight/obesity on IVF-ET outcomes in chinese patients with polycystic ovary syndrome. Int. J. Clin. Exp. Med. 2014, 7, 5872. [Google Scholar] [PubMed]
- McCormick, B.; Thomas, M.; Maxwell, R. Effects of polycystic ovarian syndrome on in vitro fertilization-embryo transfer outcomes are influenced by body mass index. Fertil. Steril. 2008, 90, 2304–2309. [Google Scholar] [CrossRef]
- Kamardi, S.; Surya, I.; Mahendra, I. Impact of body mass index on intracytoplasmic sperm injection in women with polycystic ovary syndrome. Zygote 2021, 29, 229–233. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Ozgun, M.T.; Uludag, S.; Oner, G. The influence of obesity on ICSI outcomes in women with polycystic ovary syndrome. J. Obstet. Gynaecol. 2011, 31, 245–249. [Google Scholar] [CrossRef]
- Bu, Z.; Dai, W.; Guo, Y. Overweight and obesity adversely affect outcomes of assisted reproductive technologies in polycystic ovary syndrome patients. Int. J. Clin. Exp. Med. 2013, 6, 991. [Google Scholar] [PubMed]
- Bailey, A.P.; Hawkins, L.K.; Missmer, S.A. Effect of body mass index on in vitro fertilization outcomes in women with polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2014, 211, 163-e1. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Wang, H.; Wang, W. Impact of Body Mass Index on Outcomes of In Vitro Fertilization/Intracytoplasmic Sperm Injection Among Polycystic Ovarian Syndrome Patients. Cell. Physiol. Biochem. 2016, 39, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Kalem, M.N.; Kalem, Z.; Sarı, T. Effect of body mass index and age on in vitro fertilization in polycystic ovary syndrome. J. Turk. Ger. Gynecol. Assoc. 2016, 17, 83–90. [Google Scholar] [CrossRef]
- Trenkic, M.; Popović, J.; Bjelica, A. Influence of body mass index on in-vitro fertilization outcome in women with polycystic ovary syndrome. Med. Pregl. 2016, 69, 230–236. [Google Scholar] [CrossRef]
- Sheng, Y.; Lu, G.; Liu, J. Effect of body mass index on the outcomes of controlled ovarian hyperstimulation in Chinese women with polycystic ovary syndrome: A multicenter, prospective, observational study. J. Assist. Reprod. Genet. 2016, 34, 61–70. [Google Scholar] [CrossRef]
- Chen, R.; Chen, S.; Liu, M. Pregnancy outcomes of PCOS overweight/obese patients after controlled ovarian stimulation with the GnRH antagonist protocol and frozen embryo transfer. Reprod. Biol. Endocrinol. 2018, 16, 36. [Google Scholar] [CrossRef]
- Pan, X.M.; Lin, Z.; Li, N. Effects of body mass index on the outcomes of in vitro fertilization in Chinese patients with polycystic ovary syndrome: A retrospective cohort study. J. Zhejiang Univ. Sci. B 2018, 19, 490–496. [Google Scholar] [CrossRef]
- Rehman, R.; Mehmood, M.; Ali, R. Influence of body mass index and polycystic ovarian syndrome on ICSI/IVF treatment outcomes: A study conducted in Pakistani women. Int. J. Reprod. Biomed. 2018, 16, 529. [Google Scholar] [CrossRef]
- Yang, W.; Yang, R.; Lin, M. Body mass index and basal androstenedione are independent risk factors for miscarriage in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2018, 16, 119. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Zhang, Y. Effect of pregravid obesity on perinatal outcomes in singleton pregnancies following in vitro fertilization and the weight-loss goals to reduce the risks of poor pregnancy outcomes: A retrospective cohort study. PLoS ONE 2020, 15, e0227766. [Google Scholar] [CrossRef]
- Abdulkhalikova, D.; Korošec, S.; Blickstein, I. Perinatal outcome of in vitro fertilization pregnancies in women with polycystic ovary syndrome by pregravid BMI. J. Perinat. Med. 2021, 49, 514–519. [Google Scholar] [CrossRef]
- Rittenberg, V.; Seshadri, S.; Sunkara, S.K. Effect of body mass index on IVF treatment outcome: An updated systematic review and meta-analysis. Reprod. Biomed. Online 2011, 23, 421–439. [Google Scholar] [CrossRef]
- Wang, J.X.; Davies, M.J.; Norman, R.J. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes. Res. 2002, 10, 551–554. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergstrom, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abe, S.K.; Kanda, M.; Narita, S.; Rahman, M.S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Pang, L.H.; Li, M.J. Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2013, 11, 56. [Google Scholar] [CrossRef]
- Yu, H.F.; Chen, H.S.; Rao, D.P. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4863. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.A.; Ruiter, N.L.; Verhulst, S.M. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil. Steril. 2017, 108, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Badeghiesh, A.; Suarthana, E. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: A population-based study on 9.1 million pregnancies. Hum. Reprod. 2020, 35, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, A.M.; Seifer, D.B. Women with PCOS who undergo IVF: A comprehensive review of therapeutic strategies for successful outcomes. Reprod. Biol. Endocrinol. 2023, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Nolan, J.J.; Baloga, J. Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Diabetes 1995, 44, 1121–1125. [Google Scholar] [CrossRef]
- Sheehan, C.M.; Gotlieb, E.E.; Ayers, S.L. Neighborhood conditions and type 2 diabetes risk among Latino Adolescents with Obesity in Phoenix. Int. J. Environ. Res. Public Health 2022, 19, 7920. [Google Scholar] [CrossRef]
- Wu, T.-H.; Lee, I.T.; Ho, L.T. Combined lipid goal attainment in patients with type 2 diabetes and dyslipidemia: A head-to-head comparative trial of statins. J. Chin. Med. Assoc. 2022, 85, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Feigenbaum, S.L.; Yang, J. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Marlieke, A.; Wilde, D. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Zaki, M.; Basha, W.; El-Bassyouni, H.T.; El-Toukhy, S.; Hussein, T. Evaluation of DNA damage profile in obese women and its association to risk of metabolic syndrome, polycystic ovary syndrome and recurrent preeclampsia. Genes Dis. 2018, 5, 367–373. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S. Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension and gestational diabetes mellitus. J. Clin. Med. 2020, 9, 1980. [Google Scholar] [CrossRef]
- Boomsma, C.; Eijkemans, M.; Hughes, E.G. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 2006, 12, 673–683. [Google Scholar] [CrossRef]
- Bjercke, S.; Dale, P.O.; Tanbo, T. Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol. Obstet. Investig. 2003, 54, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Q.; Xue, X. An update on the progress of endometrial receptivity in women with polycystic ovary syndrome. Reprod. Sci. 2022, 29, 2136–2144. [Google Scholar] [CrossRef]
- Orostica, L.; Astorga, I.; Plaza-Parrochia, F. Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int. J. Obes. 2016, 40, 1715–1722. [Google Scholar] [CrossRef]
- Xue, Z.; Li, J.; Feng, J. Research progress on the mechanism between polycystic ovary syndrome and abnormal endometrium. Front. Physiol. 2021, 12, 788772. [Google Scholar] [CrossRef] [PubMed]
- Snider, A.P.; Wood, J.R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 2019, 158, R79–R90. [Google Scholar] [CrossRef]
- Yang, C.C.; Liao, P.H.; Cheng, Y.H. Diabetes associated with hypertension exacerbated oxidative stress–mediated inflammation, apoptosis and autophagy leading to erectile dysfunction in rats. J. Chin. Med. Assoc. 2022, 85, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Tan, S.L.; MacDougall, J. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum. Reprod. 1993, 8, 959–964. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, R.; Liao, T. A Predictive Model of Live Birth Based on Obesity and Metabolic Parameters in Patients with PCOS Undergoing Frozen-Thawed Embryo Transfer. Front. Endocrinol. 2022, 12, 799871. [Google Scholar] [CrossRef]
- Bednarz, K.; Kowalczyk, K.; Cwynar, M. The role of glp-1 receptor agonists in insulin resistance with concomitant obesity treatment in polycystic ovary syndrome. Int. J. Mol. Sci. 2022, 23, 4334. [Google Scholar] [CrossRef]

| 1st Author, Year | Country | Study Design | Study Population | Measured Outcomes | IVF Stimulation Protocol | NOS Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m) | Sample Size | Age (y) | |||||||||
| Normal | High | N-BMI | H-BMI | N-BMI | H-BMI | ||||||
| McCormick, 2008 [18] | USA | RC | <25 | ≥30 | 6 | 11 | 31.5 ± 3.0 | 31.0 ± 5.0 | CP/LB | Long agonist (98%); Antagonist (2%) | 8 |
| Ozgun, 2011 [21] | Turkey | PC | <30 | ≥30 | 26 | 18 | 26.8 ± 4.5 | 26.7 ± 2.9 | CP/BP/M/LB | Long Agonist | 8 |
| Huang, 2014 [17] | China | RC | <24 | ≥24 | 79 | 49 | 30.5 ± 4.1 | 29.4 ± 3.4 | CP/M/LB | ND | 7 |
| Bailey, 2014 [23] | USA | RC | <25 | ≥25 | 51 | 50 | 32 ± 3.5 | 32.4 ± 3.2 | CP/BP/M/LB | ND | 8 |
| Cui, 2016 [24] | China | RC | <24 | ≥24 | 195 | 213 | 26.99 ± 3.3 | 27.5 ± 3.4 | CP/BP/PTB | Long Agonist | 6 |
| Sheng, 2017 [27] | China | PC | <24 | ≥24 | 292 | 189 | 28.5 ± 2.7 | 26.3 ± 3.1 | CP | Long Agonist | 7 |
| Pan, 2018 [29] | China | RC | <24 | ≥24 | 389 | 281 | 27.7 ± 3.17 | 29.4 ± 3.53 | M/PTB | Long Agonist | 6 |
| Yang, 2018 [31] | China | RC | <25 | ≥25 | 247 | 159 | 29.1 | 29.5 | CB/LB/PTB/CS | Antagonist | 7 |
| Chen, 2018 [28] | China | RC | <24 | ≥24 | 260 | 138 | 28.8 ± 2.7 | 28.9 ± 3.0 | CP/LB/PTB | Antagonist | 7 |
| Qiu, 2019 [15] | China | RC | <25 | ≥25 | 1207 | 549 | 28.91 ± 3.2 | 30.0 ± 3.6 | CP/M | Antagonist | 6 |
| Zhou, 2020 [14] | China | RC | <25 | ≥25 | 352 | 498 | 27.7 ± 2.5 | 27.9 ± 3.1 | CP/MP/M/LB/PTB | Ultra-Long Agonist | 7 |
| Rehman, 2018 [30] | Pakistan | CS | <25 | ≥25 | 130 | 91 | 32.4 ± 4.4 | 32.0 ± 4.8 | CP | Long protocol | 8 |
| Kalem, 2016 [25] | Turkey | RC | <25 | ≥25 | 300 | 354 | ND | * | CP/MP | Long agonist/Antagonist | 7 |
| Bu, 2013 [22] | China | RC | <24 | ≥24 | 267 | 174 | 28.3 ± 4.0 | 29.1 ± 4.1 | CP | Long Agonist | 7 |
| Ae Ra Han, 2011 [16] | Korea | RC | <25 | ≥25 | 272 | 64 | 31.2 ± 2.7 | 31.6 ± 3.1 | CP/MP/M/LB/PTB/GDM/GH | Long Agonist | 8 |
| Kamardi, 2021 [19] | Indonesia | RC | <25 | ≥25 | 38 | 17 | 30.1 ± 4.0 | 30.4 ± 4.3 | CP/BP | Long Agonist | 8 |
| Abdulkhalikova, 2020 [33] | Slovenia | RC | <25 | ≥25 | 39 | 34 | 33.4 ± 4.2 | 33.5 ± 4.0 | GDM/GH/CS | ND | 6 |
| Liu, 2020 [32] | China | RC | <24 | ≥24 | 434 | 150 | 29 (27–32) | 31 (27–34) | GDM/GH/CS | ND | 7 |
| Trenkić, 2016 [26] | Serbia | RC | ≤25 | >25 | 45 | 12 | 31.6 ± 3.99 | 31.5 ± 4.3 | M/GDM/GH | Long agonist (62%); Flexible agonist (38%) | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, S.A.; Khan, R.; Amer, S. The Impact of High BMI on Pregnancy Outcomes and Complications in Women with PCOS Undergoing IVF—A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1578. https://doi.org/10.3390/jcm13061578
Alenezi SA, Khan R, Amer S. The Impact of High BMI on Pregnancy Outcomes and Complications in Women with PCOS Undergoing IVF—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(6):1578. https://doi.org/10.3390/jcm13061578
Chicago/Turabian StyleAlenezi, Salih Atalah, Raheela Khan, and Saad Amer. 2024. "The Impact of High BMI on Pregnancy Outcomes and Complications in Women with PCOS Undergoing IVF—A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 6: 1578. https://doi.org/10.3390/jcm13061578
APA StyleAlenezi, S. A., Khan, R., & Amer, S. (2024). The Impact of High BMI on Pregnancy Outcomes and Complications in Women with PCOS Undergoing IVF—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(6), 1578. https://doi.org/10.3390/jcm13061578