Abstract
(1) Background: In recent years, a global epidemiological shift in candidemia has been observed, marked by the emergence of resistant non-albicans Candida species. Candida auris, in particular, has become a significant global concern, causing infections in both pediatric and adult populations within healthcare settings. Despite its widespread impact, there is a limited understanding of the clinical course and transmission dynamics of neonatal systemic Candida auris infections, hindering effective prevention and management. This study focused on the epidemiologic data, the clinical presentation, risk factors, and outcome of C. auris infection in neonatal population. (2) Methods: A systematic review of the literature using PubMed and Scopus databases until December 2023 was conducted. (3) Results: A total of 24 relevant studies were identified, encompassing 476 documented cases of Candida auris infection in neonates. Prematurity emerged as a primary risk factor, alongside total parenteral nutrition, central line insertion, mechanical ventilation, and prior broad-spectrum antibiotic use. The mortality rate reached approximately 42%, with therapeutic details sparingly reported in 12% of cases. Treatment strategies varied, with amphotericin B predominantly used as monotherapy, while combination antifungal agents were used in 44% of cases. Notably, 97.4% of cases exhibited fluconazole resistance, and 67.1% showed resistance to amphotericin B. Limited data were available on resistance to other antifungal agents. (4) Conclusions: Despite the rarity of neonatal Candida auris infections, their global occurrence necessitates comprehensive preparedness in patient care. A deeper understanding of Candida auris pathogenesis is crucial for developing effective strategies to control and prevent neonatal infections caused by this pathogen.
1. Introduction
Candida (C.) auris is a fungal pathogen, considered to be an emerging global health threat due to its rapid spread across the world and the emergence of strains resistant to multiple antifungal agents [1,2]. It is associated with hospital-acquired and endemic nosocomial infections [2,3]. There are two ways in which C. auris can affect the human body. It can either reside on some areas of the body, such as the skin, rectum, or mouth, in a condition known as asymptomatic colonization. In this situation, a person remains asymptomatic but can still transmit the fungi to others. It can also cause serious infections by entering the bloodstream or open wounds. Invasive C. auris infection is the most common clinical manifestation in the Intensive Care Unit (ICU). It could cause sepsis, meningitis, pneumonia, myocarditis, and urinary tract infections with high mortality rates [4]. A recent meta-analysis by Chen et al. [5] revealed that mortality due to fungemia from C. auris was 39–45%, reaching up to 80% in patients with serious underlying health problems [6]. The main risk factors for invasive candidiasis include prolonged hospitalization in the ICU, the use of medical devices such as central venous catheters, medical comorbidities, long-term antibiotic therapy, and individuals of extreme ages, such as elderly adults and newborns [7]. The fact that C. auris has been referred to as “an often resistant and sometimes deadly fungal infection”, “a superbug and extremely drug-resistant infection”, and “C. auris sickens dozens”, makes it clear that there is something perilous about this recently recognized strain. Candida auris is a species of fungus that grows as yeast, isolated for the first time in 2009 in Japan from a patient’s ear, thus the name auris which means ear in Latin [8]. It was also recognized as a pathogen in 15 patients in Korea with chronic otitis media, 3 of whom were children [9]. Its ability to cause severe infections was first described in 2011 when the fungus was isolated from blood samples of patients with fungemia in South Korea [10]. However, according to recent studies, C. auris has likely been present since 1996, but it went unnoticed due to the difficulty of its identification. Cases of fungal infections were reported, but scientists were not aware that they were caused by this specific strain of fungus. The first case to date was retrospectively recognized through DNA sequencing of the isolation from the blood culture of a Korean pediatric patient that had undergone surgery in 1996 [10]. Since then, C. auris strains have been isolated globally in sporadic infections, hospital epidemics, or as colonization among patients, especially in the ICU. These isolations are increasing with geometric progression, at least in the USA.
Currently, the real impact and severity of C. auris infections are yet to be clarified due to their difficulty in being isolated with conventional techniques used in most laboratories [11]. In these clinical situations, it is necessary to perform additional tests such as molecular analyses to confirm the possibility of infection by C. auris. Candida auris possesses three characteristic drawbacks. Firstly, as mentioned previously, it is very difficult to be identified. Secondly, it is progressively becoming resistant to antifungal medication. Thirdly, it can easily spread within healthcare facilities and is very difficult to extinguish from surfaces, rendering infection control policies crucial. Therefore, on March 20th 2023, the CDC published an urgent warning, describing Candida auris as a global health threat, that has already spread to half of the states in the USA. Since 2019, the World Health Organization has considered it to be “an antimicrobial resistance threat”, and it is included in the “Antibiotic Resistance Threats in the United States” list [12,13].
During the coronavirus (SARS-CoV-2) pandemic, several epidemic outbreaks of invasive C. auris infections were reported in hospitalized COVID-19-positive patients in the United States, Brazil, India, Mexico and Pakistan. This demonstrates the rapid spread of C. auris despite strict hygiene protocols applied in the management of SARS-CoV-2. These reports note that a high number of severely ill patients with COVID-19 develop candidemia with high mortality [14,15,16,17,18]. Its ability to disperse from one patient to another in hospital grounds and also through medical devices, including thermometers, is unprecedented in fungi and causes great concern. Therefore, it is mandatory to apply strict strategic measures, in order to avoid the grave combination that enables transmissions in these emerging contagious threats. Recently, cases of C. auris candidemia have been reported in pediatric patients with COVID-19 [16,19]. The high incidence of C. auris candidemia as well as other multi-drug-resistant bacteremia reported during the COVID-19 pandemic could also be attributed to the reckless use of wide-spectrum antibiotics and prolonged hospitalization [20].
Candida infection is the third most common cause of late-onset sepsis in the Neonatal Intensive Care Unit (NICU). Invasive candidiasis is the most common invasive fungal infection in pediatric patients, with high mortality and hospital costs. Candida albicans and Candida parapsilosis are the most frequently isolated types of fungi in neonatal systemic candidiasis [21,22]; however, over the last few years, there has been a rise in cases of systematic C. auris infection reported in neonatal patients in NICUs worldwide. These outbreaks have a poor prognosis, despite antifungal therapy, as C. auris infection is often lethal in neonates [23,24,25]. The incidence of invasive candidiasis cases in NICUs has risen during the last few years due to advances in healthcare. Many factors are involved in this rise, including invasive procedures, such as catheterizations of central veins and arteries, gastrointestinal tract invasive procedures, and the use of broad-spectrum antimicrobial therapies in the context of neonatal sepsis [23]. These infections also complicate the clinical course of term neonates with underlying health problems that are hospitalized in the NICU [26]. There are various international guidelines on the management, therapy, and prevention of invasive candidiasis in neonatal patients, most of which are based on what is known about neonatal C. albicans and C. parapsilosis infections [27,28,29]. However, immune responses, clinical course, and the outcome of invasive candidiasis, in combination with antifungal prescription guidelines vary among Candida species [19,30,31]. Furthermore, there is limited information about the clinical course of systemic C. auris infection and its spread, which undermines the sufficient prevention and management of fungemia in neonatal patients [32,33]. Hence, there is a clinical need to understand the pathogenesis of C. auris infection during the neonatal period as well as to characterize the interactions between neonates and C. auris. This is the reason we conducted a systematic review in order to collect and update our knowledge and practices concerning the risk factors, clinical presentation, and outcomes of Candida auris infection in neonates.
2. Materials and Methods
We followed the methodology of a systematic review to identify, evaluate, and interpret the available research studies that addressed our research objective. A protocol was prepared, following the Preferred Reported Items for Systematic Reviews and Meta-analysis (PRISMA, presented as a Supplementary Material) guidelines [34], which is registered in the PROSPERO database (CRD42023417031) and used in this systematic review.
2.1. Inclusion Criteria
- Randomized controlled trials (RCTs), observational studies, case reports and case series;
- Population: neonates;
- Study design: studies focused on Candida auris infection, defined as positive blood, urine, CSF, or other sterile anatomical site culture in neonates.
2.2. Exclusion Criteria
- Surveys that included children and adults in the same group with neonates and did not offer information that solely involved the neonatal population;
- Review articles, systematic reviews and meta-analyses, and conference proceedings were excluded;
- Studies not published in the English language.
2.3. Research Objective
To obtain information on the clinical presentation, risk factors, and outcomes of Candida auris infection in neonates.
2.4. Main Outcome(s)
- Clinical presentation of Candida auris infection in neonates;
- Risk factors/characteristics of the affected population;
- Outcomes of the infection including mortality.
2.5. Search Strategy–Data Source
This systematic review was performed from May 2023 to December 2023. We used the PubMed and Scopus databases, with the research end date set at 31 December 2023.
The used combination of key words: “candida auris”, “neonate”, “newborn”, “premature”, and “preterm” with logical operators Boolean (AND, OR). Moreover, in order to cover the full expansion of the available literature and reduce the risk of losing studies, we manually searched and reviewed the references of each chosen study, individually, as well as the references of previous systematic reviews on the topic.
2.6. Resolving Conflicts
The data extraction and quality assessment were conducted independently by two researchers (P.K.T., A.E.P.), with conflicts being resolved through discussion and consensus between the researchers or, if needed, with the involvement of a third researcher (R.S.).
2.7. Data Synthesis and Presentation
We recorded the data on a table according to the day of life that the infection occurred, existing comorbidities, risk factors, prematurity, administration of total parenteral nutrition, placement of central catheters, mechanical ventilation and previous use of broad-spectrum antibiotics, number of participants, number of events, and other relevant criteria for the classification of the study population (subpopulations of neonates include preterm infants, very low birth weight infants, those undergoing cardiac or other surgical procedures, infants with congenital malformations, and so on) research study design, date of publication, regardless whether there was chronological ambiguity or not, number of participants, and other relative criteria in order to collect and, if possible, analyze the results of the study. We described potential gaps in the data and offered suggestions for future investigation.
3. Results
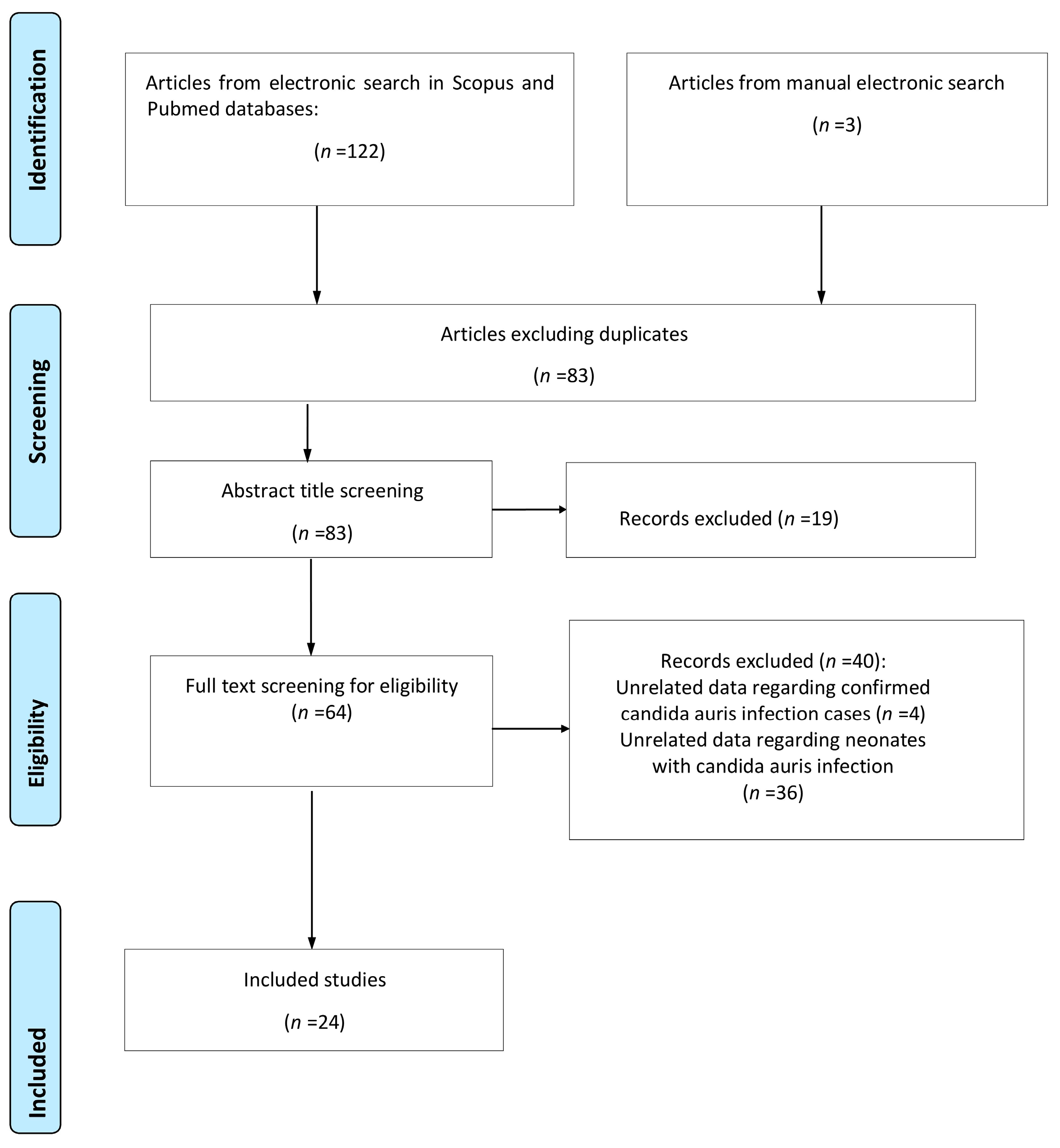
A total of 125 studies were retrieved from the research of the electronic literature databases; 42 were duplicate entries and were removed. The removal of duplicate studies was achieved with the help of a management tool, which is used to remove duplicate studies, installed in the digital literature reference program (EndNote X8). After meticulously studying the titles and abstracts of the remaining studies, 19 of them were excluded either due to the fact that their subject did not serve the purpose of the study or because they met some of the exclusion criteria that was already evident from their title and abstract, while 12 reviews concerned the same field of inquiry but were not focused on the neonatal population. A careful study of the entire text of the remaining 64 studies revealed that only 24 of them met all the inclusion criteria and were therefore included in this systematic review. The flowchart is presented in Figure 1.
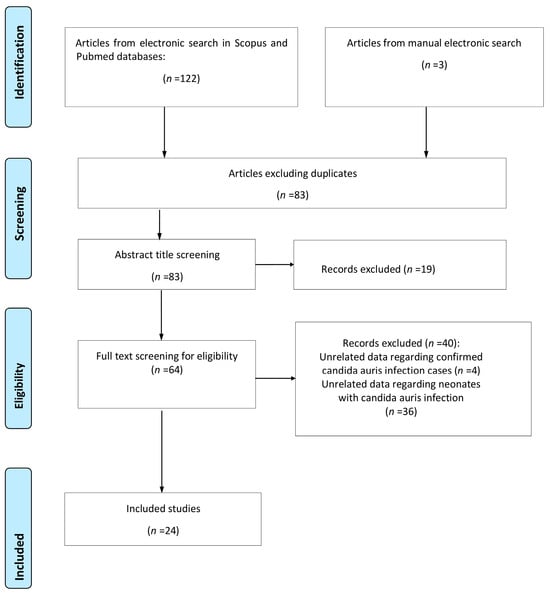
Figure 1.
Flowchart of the systematic review.
Of all the studies included in the present review, three concerned public health surveillance programs, four were laboratory-based surveillance series, five were case series, three were cross-sectional studies, and the remaining nine were cohort studies. The characteristics of each study, concerning cases of neonatal C. auris infection, are extensively presented in Table 1.

Table 1.
Studies concerning cases of neonatal C. auris infection.
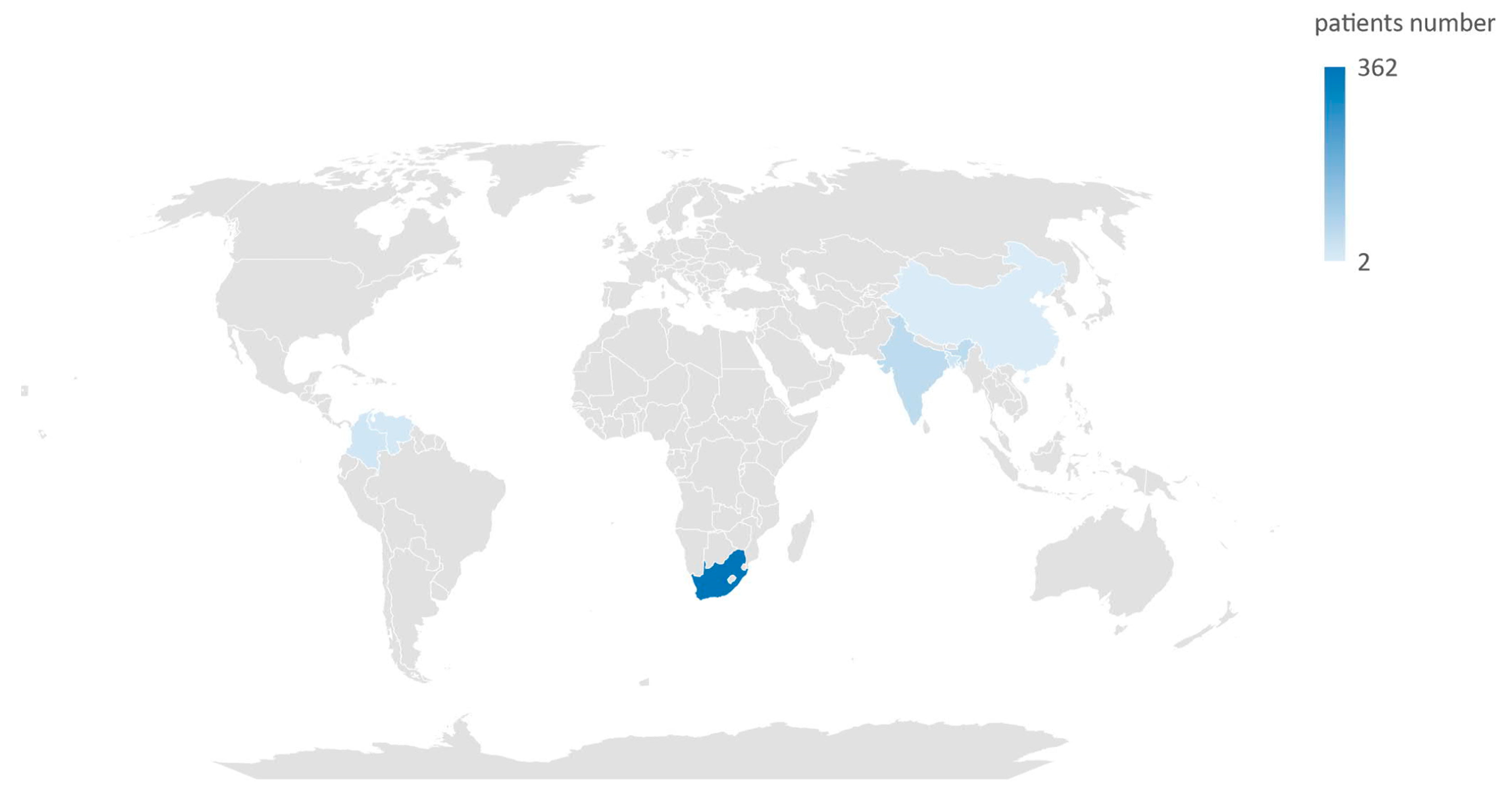
In total, these studies assessed data from 476 neonates. The countries from which the cases of neonatal C. auris infection were documented are Bangladesh, China, Colombia, India, South Africa, and Venezuela (Figure 2).
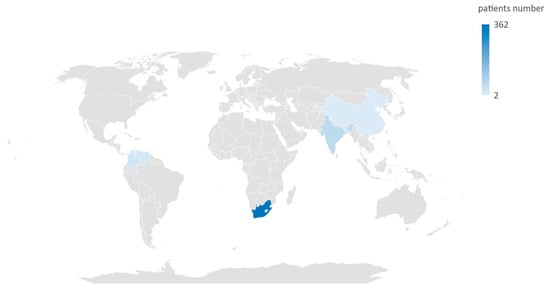
Figure 2.
Map of countries where cases of Candida auris infection were documented in neonatal population.
The demographic data of the study population are presented in Table 2. Unfortunately, among the retrieved studies, there were lots of missing data. For the majority of cases, there were no data concerning the age of gestation. Of the 31 neonates with a documented gestational age, 20 were preterm neonates (64.5%), with a median gestational age of 28 weeks (IQR 26–34). The median patient age (among the 264 cases with reported data) during disease onset was 21 (range: 1–71) days.

Table 2.
Demographic data of the study population.
Apart from prematurity, many other predisposing factors were documented, such as the administration of total parenteral nutrition (TPN), the placement of central line catheters, mechanical ventilation, and previous administration of broad-spectrum antibiotics (with piperacillin tazobactam and meropenem combined with vancomycin being the most used). As far as the underlying conditions are concerned, information was provided only for 42 neonates (8.8% of cases); congenital heart disease (14/42, 33.3%), congenital gastrointestinal disorders (10/42 neonates, 23.8%), and necrotizing enterocolitis (NEC, 4/42, 9.5%) were the most common comorbidities. From the available literature data, 20 out of the aforementioned neonates (48%) had a history of a previous abdominal or cardiac surgery. No neonate had previously received antifungal prophylaxis.
Candida auris was isolated from blood cultures (98.1%), urine (16.5%), peritoneal fluid specimen (2.4%), and cerebrospinal fluid (2.1%) (Table 3).

Table 3.
The isolation site of C. auris.
Information concerning the type of therapy was only provided for 57 cases. The choice of antifungal medication differed significantly between studies, with amphotericin B (AΜΒ) being the most frequently administered agent as monotherapy (Table 4). The mean duration of antifungal therapy (excluding neonates that passed away) was 27.85 days (range: 11–42 days). Information concerning the resistance of the pathogen to antifungal medication was only given for 79 cases. Specifically, resistance to fluconazole was mentioned in 77 out of the 79 cases (97.4%) and to AΜΒ in 53 cases (67.1%).

Table 4.
Treatment strategies and survival rate.
Data concerning the outcome of patients were provided for 93 cases, out of which the infection was successfully treated in 58.1% of patients, whereas 41.9% of neonates passed away. Information regarding survival was not available for 383 cases.
4. Discussion
Very little information is available for neonatal C. auris infection, while the potential severity of the disease requires timely recognition and appropriate management. To our knowledge, this is the first systematic review of the literature focused on the epidemiologic data, the clinical presentation, risk factors and outcome of C. auris infection in the neonatal population.
Invasive fungal infections (IFIs) are the main cause of morbidity and mortality in hospitalized neonates in the NICUs, especially preterm neonates and those with a very low birth weight (VLBW, <1500 g) [55]. These neonates are immunocompromised, exposed to broad-spectrum antibiotics, have an immature epithelial barrier and are subjected to invasive procedures, and consequently, they are at an increased risk for opportunistic fungal infections. The majority of IFIs are caused by Candida species [56]. Invasive Candidiasis (IC) is the third most common cause of late-onset sepsis in VLBW neonates and an important cause of morbidity and mortality in the NICU [56]. The frequency of IC in NICUs ranges between 0.5 and 20% and varies between centers and patient population and is inversely proportional to gestational age and birth weight, with a higher frequency documented in neonates with an extremely low birth weight (ELBW) [26,56,57]. However, cases of neonatal IFI caused by other fungal species are increasingly reported in the international literature. In recent years, we have witnessed a global change in candidemia epidemiology with the appearance of resistant, non-albicans species of Candida, with a particular emphasis on the increase in cases of C. auris infections. Candida auris is spreading globally and causing hospital infections in pediatric and adult populations, particularly in ICUs [58,59,60] and is included in the 2019 Emergency Threats Report of the Centers for Disease Control and Prevention (CDC) of the USA [12]. As is evident from the history of the discovery of this fungus, C. auris can cause severe ear infections. However, its main threat lies on its isolation from the bloodstream. Wound and soft tissue infections, osteomyelitis, central nervous system infections, and generalized infections in immunosuppressed patients have been described in the international literature [60]. According to our systematic review, we located 24 publications [23,24,25,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] from 2013 until today, in which 476 cases of C. auris infections are reported. Unfortunately, there was a lot of information missing among the retrieved studies. For the majority of the recorded cases, data regarding the age of gestation were not provided. However, it is noteworthy that, among the overall number of infants with reported gestational age, 64.5% were premature, suggesting that prematurity, as in other cases of IFI, is one of the main contributing factors to C. auris infection. Apart from prematurity, other contributing factors noted were the administration of total parenteral nutrition, the insertion of umbilical or other central lines, mechanical ventilation and prior administration of broad-spectrum antibiotics, which were also recognized in adult and pediatric patients with C. auris infection [60,61,62]. From the total number of patients with available data concerning underlying health problems, 33.3% had congenital heart disease, 23.8% had congenital gastrointestinal malformations, and 9.5% had necrotizing enterocolitis. These findings suggest that invasive infections caused by C. auris represent an emerging threat, not only for premature neonates but also for term neonates hospitalized in the NICU due to comorbidities, such as complex congenital heart disease or other congenital malformations requiring surgical correction [23,25]. Fungemia is a condition with high mortality, often as high as 50%, while the mortality rate for C. auris is reported to be between 40 and 60% [4,6]. The mortality rate we documented, in the total number of neonates with C. auris infection (93 cases), for which the studies provided information concerning the outcome of the infection, was quite high (approximately 42%), a finding consistent with the high mortality rate reported in studies focused on pediatric and adult patients [6,60].
C. auris has comparable characteristics with common candida species, concerning its force of infection and its ability to adhere to the host’s cell and form a biomembrane. However, in contrast to C. albicans, where the source of infection is usually the patient’s own intestinal microbiota, C. auris infections are mainly caused by the dispersion of the pathogen via the hands, from a colonized patient to another or from the colonized inanimate environment. C. auris causes systematic infections such as septicemia, soft tissue infections, and surgical site infections in seriously affected individuals of all ages, from premature infants to the elderly patients. It is also isolated from respiratory and urine samples, which it often colonizes [63]. A distinctive characteristic of C. auris is that it colonizes the patient’s skin and the hospital environment for extended periods of time and spreads from one patient to another within healthcare facilities, a characteristic not previously observed with other Candida species. These characteristics remind us of those seen in multi-resistant bacteria such as Acinetobacter or Klebsiella [63]. C. auris adheres to the skin due to a unique agent it possesses which does not exist in other Candida species called Surface Colonization Factor (Scf1) [64]. This agent provides the fungus with the ability to effectively adhere to the skin and central line catheters, resulting in infection. This discovery, recently published in the scientific journal Science, is significant because it might potentially lead to a new approach in treating this fungus through the development of new agents that inhibit the Scf1 factor and the fungus’s ability to adhere. Although reports of C. auris infections among neonates and children are rare, its appearance in different countries and continents worldwide represents a new reality that requires proper preparation across all aspects of patient’s healthcare. A better understanding of the mechanisms of pathogenesis will lead to strategies that aim to control and prevent C. auris infections in neonates. Information about experimental models of diffuse C. auris infection in adult mice have been reported. In these models, adult mice were intravenously infected with C. auris, revealing that the tissues with the highest fungal burden and invasion during the infection were the kidneys and the heart [65,66], as opposed to the experimental model of neonatal candidiasis conducted by Flores-Maldonado et al. [19], who realized that the liver and the brain of animal neonates were the tissues with the highest fungal burden and invasion during infection, indicating that the spread of the fungus during infection is different between neonates and adults. Therefore, there is great need to understand the pathogenesis of C. auris infection during the neonatal period and the characteristics of the interaction between C. auris and neonates.
Furthermore, these studies indicate that the pathogenesis and the outcome of systematic neonatal candidiasis varies between different strains of Candida. This is probably because different strains of Candida exhibit different patterns of virulence [67], antifungal sensitivity [68], and species-dependent recognition of the immune system [69]. Specifically, C. auris is a strain of fungus that causes different immune responses in comparison to other strains, such as C. albicans [70]. These differences in immune response are probably due to the composition of the cell wall of C. auris, where mannans and mannoproteins are the main inducers of the immune response, while in C. albicans, the main inducers of the immune response are glucans [71].
According to our systematic review, which includes 476 neonates with C. auris infection, the most common type of invasive infection was bloodstream infection (98.1%, 263/268 patients with available data on the site of infection). In our study, besides primary sepsis, other sites of infection were urinary tract infection (16.5%), meningitis (2.4%), peritonitis (2.4%), and skin abscess (1.2%). These findings are in accordance with information derived from studies on adult patients as well as with a recent systematic analysis conducted by Ashkenazi-Hoffnung and Rosenberg Danziger [60] which provides data on C. auris infection with a focus on children. According to the experimental model of Flores-Maldonado et al. [19], the spleen, kidneys, and lungs were the tissues of the mouse with the lowest colonization, while the liver showed the highest fungal invasion in the early stages of the infection and was associated with the presence of cellular infiltration. In contrast, the brain was the most susceptible tissue to invasion from C. auris, and the invasion persisted until the advanced stages of the infection, findings that were not confirmed by our systematic review. This could be primarily due to the high percentage of missing data (50.4%) in our review regarding the isolation site of C. auris. Additionally, we must take into consideration that in the experimental model, data concerning C. auris dissemination were evaluated via fungal burden and histopathological tissues analysis.
The clinical presentation of C. auris infections, in most cases, is nonspecific, and the providing a distinction from other types of systemic infections is often a challenge [72]. This fact is also reflected in our systematic review where, in the majority of neonates, C. auris infection had a similar clinical presentation with bacterial sepsis. As with other Candida infections, the diagnosis of invasive C. auris infection is made by isolating the yeast in blood cultures or other biological specimens from infected sites, clinically or radiographically ascertained. Identifying C. auris in cultures is often a prolonged procedure in comparison to more common types of Candida. The diagnosis of C. auris is extremely difficult as it is often confused with other rare fungi species (C. haemulonii ή C. duobushaemulonii, C. famata, C. lusitaniae, Rhodotorula glutinis ή Saccharomyces cerevisiae) using conventional fungal identification methods and demands the use of newer methods of identification, such as mass spectrometry MALDI-TOF MS, which are not widely available [60,73]. In order to overcome this problem, new precise and rapid diagnostic methods have been recently developed. Furthermore, morphological distinction detectors can be used for the rapid assessment of resistance mechanisms to echinocandins and azoles. C. auris has been included in the database of the VITEK-2 automated system since 2018. Despite being less available for routine testing, the molecular determination of sequence using polymerase chain reaction (PCR) analyses offers a definite identification of C. auris. Several molecular analyses have been developed, including conventional PCR assays, real-time PCR, magnetic resonance T2 sensing, and loop-mediated isothermal amplification (LAMP) assays [74,75]. As opposed to automated biochemical systems and MALDI-TOF MS, which depend on specimen cultures, DNA can be directly isolated from the patient’s samples, without the need for culture.
One of the main reasons of global concern about C. auris spreading is its sensitivity profile, which limits the choice of therapy. Most C. auris strains are resistant to fluconazole, cross-resistant to other azoles, and have variable increased minimum inhibitory concentrations (MICs) for amphotericin B [76,77]. Echinocandins have the lowest MIC for C. auris in comparison to other categories of systematic antifungal medication. However, resistance has been described even to this medication [78]. Data over time suggest that resistance rates to echinocandins are increasing [79]. Information derived from our systematic review, regarding resistance to antifungal medication, was only available for 79 cases. More specifically, 97.4% of the cases were resistant to fluconazole, and 67.1% of the cases were resistant to AMB. Data concerning the resistance to other categories of antifungal medication were limited. This finding raises concern over the management of neonatal C. auris infections as fluconazole and AMB are the most widely used antifungal medicine in NICUs. Moreover, fluconazole is, up to date, the first choice medication used as a prophylaxis against fungal infections in extremely low birth weight infants [80]. The treatment of C. auris presents with important challenges as it is resistant to various categories of antifungal medication, demanding changes in therapy due to treatment failure. Based on the information given above, the concern on resistance to azoles and AMB has led the CDC and Public Health England to recommend echinocandins as the first-line therapy against C. auris infections [75,81]. However, so far, no correlation has been established between in vitro sensitivity testing and clinical results. Observational data from hospital cases show high mortality rates between patients infected by C. auris, regardless of the choice of antifungal agent, both in adults [35,82] and children [32,44]. Additionally, past exposure to fluconazole and echinocandins has consistently been related to a high risk of C. auris infection [38,83]. In our review, data concerning the type of therapy were provided only for 12% of cases. The choice of antifungal medication differed significantly between studies, with AMB mostly used as monotherapy. The mean duration of antifungal treatment (excluding neonates that passed away) was 27.85 days (range 11–42). In 44% of cases, an invasive infection was treated with a combination of antifungal agents. Conclusions concerning differences in mortality rates according to antifungal treatment could not be drawn due to the small number of patients. In cases of C. auris pediatric infection, treatment recommendations are derived from the Infectious Diseases Society guidelines of 2016 [81] for the therapy of candidiasis, which suggest caspofungin or micafungin as both medications are approved by the FDA for use in children above 2 months of age. For neonates, amphotericin B deoxycholate is the first-line therapy choice. If sensitivity reports show increased (MIC) in all categories of antifungals, there is evidence suggesting that combination therapy with fluconazole and echinocandin, AMB, or azole effectively reduces MIC in vitro [84,85,86].
In settings with high rates of C. auris, some authors recommend antifungal prophylaxis with echinocandins in low birth weight premature neonates as an alternative to the typical prophylaxis with fluconazole [27]. This suggestion comes as a result of the high resistance that C. auris momentarily presents to fluconazole [32,87]. A small, comparative clinical study reported that the use of micafungin, in comparison to fluconazole, for prophylaxis against fungal infections in ELBW neonates was connected with lower rates of C. albicans infection [88]. Furthermore, the safety and pharmacokinetics of micafungin have been evaluated in VLBW [89,90]. As a result, within the context of an endemic–epidemic in a NICU, the prophylactic use of micafungin could be taken into consideration for high risk populations. However, as aforementioned, there is a concern that the echinocandins used could exert selective pressure, leaning in favor of the emergence of C. auris.
C. auris poses a serious challenge to the healthcare system due to its unique characteristics, including the extended transmission among patients, the persistence in hospital environments, misidentification via traditional laboratory methods, antifungal resistance, and the association with high mortality rates. The serious threat posed by C. auris has urged public healthcare services, throughout the world, to detect and report cases to health authorities.
Our study has certain limitations, and its findings should be interpreted with caution. Our main limitation was the high rate of missing data. The gaps and significant heterogeneity in recording basic information, such as the timing of disease manifestation, prenatal risk factors, demographic characteristics of neonates, mode of C. auris transmission, and diagnostic method, among the studies included in our review are evident. This fact served as a primary inhibitor to conducting a meta-analysis.
5. Conclusions
In conclusion, this study portrays that even though reports of neonatal C. auris infections are relatively rare, they have emerged in NICUs and seem to be replacing IFI caused by other fungal species. Their global emergence in many countries and across various continents represents a new reality that demands the effective planning of patient’s healthcare, including laboratory preparedness for adequate detection and high clinical awareness among healthcare providers for any unrecognized or rarely encountered species of Candida, in order to promptly apply infection control measures. Despite effective intervention and dramatic improvement in compliance with health protocols, the transmission of C. auris may persist even to a small extent. In this context, it is emphasized that prevention and early intervention are undoubtedly the preferred means to address this challenging pathogen. A future investigation focused on the risk stratification of patients as well as optimal choices of pre-symptomatic screening will offer important information in order to help healthcare facilities in preemptively identifying these outbreaks within the constraints of resources. The associations of C. auris with hospital outbreaks in the NICUs, invasive infections, high levels of resistance to antifungal medication, and high rates of mortality underline the importance of global collective efforts to raise awareness and limit its spread. Future research should address knowledge gaps concerning appropriate antifungal therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13061586/s1, PRISMA checklist.
Author Contributions
Conceptualization, R.S. and S.B.; methodology, R.S., S.B., and N.I.; data curation, A.E.P., P.K.T., A.K., A.G.T., D.P., K.A.T., E.A.G., Z.I., T.B., and A.E.T.; writing—original draft preparation, R.S., A.E.P., S.B., and N.I.; writing—review and editing, P.K.T., A.K., A.G.T., D.P., K.A.T., E.A.G., Z.I., T.B., and A.E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef]
- Dahiya, S.; Chhillar, A.K.; Sharma, N.; Choudhary, P.; Punia, A.; Balhara, M.; Kaushik, K.; Parmar, V.S. Candida auris and Nosocomial Infection. Curr. Drug Targets 2020, 21, 365–373. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, F.; Jiang, W.; Wang, Y.; Quan, Y.; Zhang, G.; Gu, F.; Yang, Y. Retrospective Analysis of the Clinical Characteristics of Candida auris Infection Worldwide From 2009 to 2020. Front. Microbiol. 2021, 12, 658329. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef]
- Egger, N.B.; Kainz, K.; Schulze, A.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. The rise of Candida auris: From unique traits to co-infection potential. Microb. Cell 2022, 9, 141–144. [Google Scholar] [CrossRef]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Carbone, A.; Oliva, M.; Schinca, E.; Boni, S.; Pontali, E. An Overview on Candida auris in Healthcare Settings. J. Fungi 2023, 9, 913. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Shin, J.H.; Sung, H.; Lee, K.; Kim, E.C.; Ryoo, N.; Lee, J.S.; Jung, S.I.; Park, K.H.; Kee, S.J.; et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: Identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 2009, 48, e57–e61. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.Á.; Pemán, J. What Do We Know about Candida auris? State of the Art, Knowledge Gaps, and Future Directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 29 January 2024).
- Nelson, R. Emergence of resistant Candida auris. Lancet Microbe 2023, 4, e396. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef]
- Moin, S.; Farooqi, J.; Rattani, S.; Nasir, N.; Zaka, S.; Jabeen, K. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med. Mycol. 2021, 59, 1238–1242. [Google Scholar] [CrossRef]
- de Almeida, J.N.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Presta Dias, P.H.; de Miranda Costa, M.M.; de Souza Jordão, R.T.; de Groot, T.; Colombo, A.L. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220. [Google Scholar] [CrossRef]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; Perio, M.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Flores-Maldonado, O.; González, G.M.; Andrade, A.; Montoya, A.; Treviño-Rangel, R.; Silva-Sánchez, A.; Becerril-García, M.A. Dissemination of Candida auris to deep organs in neonatal murine invasive candidiasis. Microb. Pathog. 2021, 161, 105285. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Lankarani, K.; Akbari, M.; Tabrizi, R.; Vali, M.; Sekhavati, E.; Heydari, S.T.; Khodadadi, H.; Ahmadizar, F. Candida auris: Outbreak fungal pathogen in COVID-19 pandemic: A systematic review and meta-analysis. Iran. J. Microbiol. 2022, 14, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Pana, Z.-D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E. Etiology and Outcome of Candidemia in Neonates and Children in Europe: An 11-year Multinational Retrospective Study. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Noni, M.; Stathi, A.; Vaki, I.; Velegraki, A.; Zachariadou, L.; Michos, A. Changing epidemiology of invasive candidiasis in children during a 10-year period. J. Fungi 2019, 5, 19. [Google Scholar] [CrossRef]
- Alvarado-Socarras, J.L.; Vargas-Soler, J.A.; Franco-Paredes, C.; Villegas-Lamus, K.C.; Rojas-Torres, J.P.; Rodriguez-Morales, A.J. A Cluster of Neonatal Infections Caused by Candida auris at a Large Referral Center in Colombia. J. Pediatr. Infect. Dis. Soc. 2021, 10, 549–555. [Google Scholar] [CrossRef]
- Armstrong, P.A.; Rivera, S.M.; Escandon, P.; Caceres, D.H.; Chow, N.; Stuckey, M.J.; Díaz, J.; Gomez, A.; Vélez, N.; Espinosa-Bode, A.; et al. Hospital-associated multicenter outbreak of emerging fungus Candida auris, colombia, 2016. Emerg. Infect. Dis. 2019, 25, 1339–1346. [Google Scholar] [CrossRef]
- Van Schalkwyk, E.; Mpembe, R.S.; Thomas, J.; Shuping, L.; Ismail, H.; Lowman, W.; Karstaedt, A.S.; Chibabhai, V.; Wadula, J.; Avenant, T.; et al. Epidemiologic shift in Candidemia driven by Candida auris, South Africa, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1698–1707. [Google Scholar] [CrossRef]
- Kopanou Taliaka, P.; Tsantes, A.G.; Konstantinidi, A.; Houhoula, D.; Tsante, K.A.; Vaiopoulos, A.G.; Piovani, D.; Nikolopoulos, G.K.; Bonovas, S.; Iacovidou, N.; et al. Risk Factors, Diagnosis, and Treatment of Neonatal Fungal Liver Abscess: A Systematic Review of the Literature. Life 2023, 13, 167. [Google Scholar] [CrossRef]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012, 18, 38–52. [Google Scholar] [CrossRef]
- Mantadakis, E.; Pana, Z.D.; Zaoutis, T. Candidemia in children: Epidemiology, prevention and management. Mycoses 2018, 61, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, E.; Apsemidou, A.; Vyzantiadis, T.A.; Tragiannidis, A. Invasive candidiasis and candidemia in pediatric and neonatal patients: A review of current guidelines. Curr. Med. Mycol. 2018, 4, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.F.; Lai, M.Y.; Lee, C.W.; Chu, S.M.; Wu, I.H.; Huang, H.R.; Lee, I.T.; Chiang, M.C.; Fu, R.H.; Tsai, M.H. Comparison of the incidence, clinical features and outcomes of invasive candidiasis in children and neonates. BMC Infect. Dis. 2018, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Chandramati, J.; Sadanandan, L.; Kumar, A.; Ponthenkandath, S. Neonatal Candida auris infection: Management and prevention strategies—A single centre experience. J. Paediatr. Child Health 2020, 56, 1565–1569. [Google Scholar] [CrossRef]
- Adilia, W. Candida auris, what do paediatricians need to know? Arch. Dis. Child. 2018, 103, 891. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef]
- Calvo, B.; Melo, A.S.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, J.; Han, L.; Qi, L.; Fan, W.; Liu, J.; Wang, Z.; Xia, X.; Chen, J.; Zhang, L. Emergency of fungemia cases caused by fluconazole-resistant Candida auris in Beijing, China. J. Infect. 2018, 77, 561–571. [Google Scholar] [CrossRef]
- Dutta, S.; Rahman; Hossain, K.; Haq, J. Detection of Candida auris and its antifungal susceptibility: First report from Bangladesh. IMC J. Med. Sci. 2019, 13, 18–22. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Jillwin, J.; Iyer, R.; Sharma, A.; Harish, B.N.; Roy, I.; Kindo, A.J.; et al. Characteristics, outcome and risk factors for mortality of paediatric patients with ICU-acquired candidemia in India: A multicentre prospective study. Mycoses 2020, 63, 1149–1163. [Google Scholar] [CrossRef]
- Berrio, I.; Caceres, D.H.; Coronell, R.W.; Salcedo, S.; Mora, L.; Marin, A.; Varón, C.; Lockhart, S.R.; Escandón, P.; Berkow, E.L.; et al. Bloodstream Infections With Candida auris Among Children in Colombia: Clinical Characteristics and Outcomes of 34 Cases. J. Pediatr. Infect. Dis. Soc. 2021, 10, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Withers, A.; Cronin, K.; Mabaso, M.; Brisighelli, G.; Gabler, T.; Harrison, D.; Patel, N.; Westgarth-Taylor, C.; Loveland, J. Neonatal surgical outcomes: A prospective observational study at a Tertiary Academic Hospital in Johannesburg, South Africa. Pediatr. Surg. Int. 2021, 37, 1061–1068. [Google Scholar] [CrossRef]
- Ramya, G.M.; Balakrishnan, U.; Chandrasekaran, A.; Abiramalatha, T.; Amboiram, P.; Sekar, U.; UshaDevi, R. Candida auris, an emerging pathogen—Challenge in the survival of microprimies. Indian J. Med. Microbiol. 2021, 39, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Subramanian, A.; Kindo, A.J. Clinicomycological study of Candida isolates in a tertiary care hospital: A pilot study. J. Med. Soc. 2021, 35, 58. [Google Scholar]
- Shuping, L.; Mpembe, R.; Mhlanga, M.; Naicker, S.D.; Maphanga, T.G.; Tsotetsi, E.; Wadula, J.; Velaphi, S.; Nakwa, F.; Chibabhai, V.; et al. Epidemiology of Culture-confirmed Candidemia Among Hospitalized Children in South Africa, 2012–2017. Pediatr. Infect. Dis. J. 2021, 40, 730–737. [Google Scholar] [CrossRef]
- Chibabhai, V. Incidence of candidemia and prevalence of azole-resistant candidemia at a tertiary South African hospital—A retrospective laboratory analysis 2016–2020. S. Afr. J. Infect. Dis. 2022, 37, 326. [Google Scholar] [CrossRef]
- Mashau, R.C.; Meiring, S.T.; Dramowski, A.; Magobo, R.E.; Quan, V.C.; Perovic, O.; von Gottberg, A.; Cohen, C.; Velaphi, S.; van Schalkwyk, E.; et al. Culture-confirmed neonatal bloodstream infections and meningitis in South Africa, 2014-19: A cross-sectional study. Lancet Glob. Health 2022, 10, e1170–e1178. [Google Scholar] [CrossRef]
- Sathi, F.A.; Paul, S.K.; Ahmed, S.; Alam, M.M.; Nasreen, S.A.; Haque, N.; Islam, A.; Nila, S.S.; Afrin, S.Z.; Aung, M.S.; et al. Prevalence and Antifungal Susceptibility of Clinically Relevant Candida Species, Identification of Candida auris and Kodamaea ohmeri in Bangladesh. Trop. Med. Infect. Dis. 2022, 7, 211. [Google Scholar] [CrossRef]
- Cook, A.; Ferreras-Antolin, L.; Adhisivam, B.; Ballot, D.; Berkley, J.A.; Bernaschi, P.; Carvalheiro, C.G.; Chaikittisuk, N.; Chen, Y.; Chibabhai, V.; et al. Neonatal invasive candidiasis in low- and middle-income countries: Data from the NeoOBS study. Med. Mycol. 2023, 61, myad010. [Google Scholar] [CrossRef]
- Sathi, F.A.; Aung, M.S.; Paul, S.K.; Nasreen, S.A.; Haque, N.; Roy, S.; Ahmed, S.; Alam, M.M.; Khan, S.; Rabbany, M.A.; et al. Clonal Diversity of Candida auris, Candida blankii, and Kodamaea ohmeri Isolated from Septicemia and Otomycosis in Bangladesh as Determined by Multilocus Sequence Typing. J. Fungi 2023, 9, 658. [Google Scholar] [CrossRef]
- Singhal, T.; Shah, S.; Thakkar, P.; Ladi, S. Candida auris as the Predominant Species Causing Invasive Candidiasis in Neonates and Children. Indian J. Pediatr. 2023, 90, 946. [Google Scholar] [CrossRef]
- Shuping, L.; Maphanga, T.G.; Naicker, S.D.; Mpembe, R.; Ngoma, N.; Velaphi, S.; Nakwa, F.; Wadula, J.; Jaglal, P.; Govender, N.P. High Prevalence of Candida auris Colonization during Protracted Neonatal Unit Outbreak, South Africa. Emerg. Infect. Dis. 2023, 29, 1913–1916. [Google Scholar] [CrossRef]
- Ramdin, T.D.; Chibabhai, V.; Saggers, R.T.; Bandini, R.M.; Ballot, D.E. Epidemiology, risk factors and outcomes associated with candidaemia in very low birth weight infants at a tertiary South African Hospital over a 7-year period (2013–2019). Clin. Epidemiol. Glob. Health 2023, 20, 101247. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef]
- Weimer, K.E.D.; Smith, P.B.; Puia-Dumitrescu, M.; Aleem, S. Invasive fungal infections in neonates: A review. Pediatr. Res. 2022, 91, 404–412. [Google Scholar] [CrossRef]
- Kelly, M.S.; Benjamin, D.K., Jr.; Smith, P.B. The epidemiology and diagnosis of invasive candidiasis among premature infants. Clin. Perinatol. 2015, 42, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Berman, J.; Novikov, A.; Bash, E.; Shachor-Meyouhas, Y.; Zakin, S.; Maor, Y.; Tarabia, J.; Schechner, V.; Adler, A. Multidrug-resistant Candida haemulonii and C. auris, tel aviv, Israel. Emerg. Infect. Dis. 2017, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Jain, K.; Chauhan, N. Candida auris Genetics and Emergence. Annu. Rev. Microbiol. 2023, 77, 583–602. [Google Scholar] [CrossRef]
- Ashkenazi-Hoffnung, L.; Rosenberg Danziger, C. Navigating the New Reality: A Review of the Epidemiological, Clinical, and Microbiological Characteristics of Candida auris, with a Focus on Children. J. Fungi 2023, 9, 176. [Google Scholar] [CrossRef]
- Munshi, A.; Almadani, F.; Ossenkopp, J.; Alharbi, M.; Althaqafi, A.; Alsaedi, A.; Al-Amri, A.; Almarhabi, H. Risk factors, antifungal susceptibility, complications, and outcome of Candida auris bloodstream infection in a tertiary care center in the western region of Saudi Arabia. J. Infect. Public Health 2024, 17, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Vinayagamoorthy, K.; Pentapati, K.C.; Prakash, H. Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: A systematic review. Mycoses 2022, 65, 613–624. [Google Scholar] [CrossRef]
- Horton, M.V.; Holt, A.M.; Nett, J.E. Mechanisms of pathogenicity for the emerging fungus Candida auris. PLoS Pathog. 2023, 19, e1011843. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.J.; Anku, J.A.E.; Zhao, G.; Zarnowski, R.; Johnson, C.J.; Hautau, H.; Visser, N.D.; Ibrahim, A.S.; Andes, D.; Nett, J.E.; et al. A Candida auris-specific adhesin, Scf1, governs surface association, colonization, and virulence. Science 2023, 381, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Chowdhary, A.; Nasiry, D.; Faeli, L.; Meis, J.F.; Badali, H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses 2018, 61, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.R.; Pichowicz, A.; Torres-Velez, F.; Song, R.; Singh, N.; Lasek-Nesselquist, E.; De Jesus, M. Impact of Candida auris Infection in a Neutropenic Murine Model. Antimicrob. Agents Chemother. 2020, 64, e01625-19. [Google Scholar] [CrossRef]
- Staniszewska, M. Virulence Factors in Candida species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; García-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, N.E.; Estrada-Mata, E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Bruno, M.; Kersten, S.; Bain, J.M.; Jaeger, M.; Rosati, D.; Kruppa, M.D.; Lowman, D.W.; Rice, P.J.; Graves, B.; Ma, Z.; et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat. Microbiol. 2020, 5, 1516–1531. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef]
- Keighley, C.; Garnham, K.; Harch, S.A.J.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.C. Candida auris: Diagnostic Challenges and Emerging Opportunities for the Clinical Microbiology Laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Alshahni, M.M.; Tamura, T.; Satoh, K.; Iguchi, S.; Kikuchi, K.; Mimaki, M.; Makimura, K. Rapid detection of Candida auris based on loop-mediated isothermal amplification (LAMP). J. Clin. Microbiol. 2018, 56, e00591-18. [Google Scholar] [CrossRef]
- Sexton, D.J.; Bentz, M.L.; Welsh, R.M.; Litvintseva, A.P. Evaluation of a new T2 Magnetic Resonance assay for rapid detection of emergent fungal pathogen Candida auris on clinical skin swab samples. Mycoses 2018, 61, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N. Candida auris candidemia in critically ill, colonized patients: Cumulative incidence and risk factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Samaranayake, L. Emerging and future strategies in the management of recalcitrant Candida auris. Med. Mycol. 2022, 60, myac008. [Google Scholar] [CrossRef] [PubMed]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 6. [Google Scholar] [CrossRef]
- Kilburn, S.; Innes, G.; Quinn, M.; Southwick, K.; Ostrowsky, B.; Greenko, J.A.; Lutterloh, E.; Greeley, R.; Magleby, R.; Chaturvedi, V.; et al. Antifungal Resistance Trends of Candida auris Clinical Isolates in New York and New Jersey from 2016 to 2020. Antimicrob. Agents Chemother. 2022, 66, e0224221. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, D.; He, N.; Wang, X.; Dong, W.; Lei, X. Prophylactic Use of Fluconazole in Very Premature Infants. Front. Pediatr. 2021, 9, 726769. [Google Scholar] [CrossRef]
- CDC. Treatment and Management of Infections and Colonization|Candida auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html (accessed on 30 December 2023).
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and 381 candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- O’Brien, B.; Liang, J.; Chaturvedi, S.; Jacobs, J.L.; Chaturvedi, V. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe 2020, 1, e193–e194. [Google Scholar] [CrossRef] [PubMed]
- Jaggavarapu, S.; Burd, E.M.; Weiss, D.S. Micafungin and amphotericin B synergy against Candida auris. Lancet Microbe 2020, 1, e314–e315. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Chaturvedi, S.; Chaturvedi, V. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob. Agents Chemother. 2020, 64, e02195-19. [Google Scholar] [CrossRef] [PubMed]
- Mesini, A.; Saffioti, C.; Mariani, M.; Florio, A.; Medici, C.; Moscatelli, A.; Castagnola, E. First case of Candida auris colonization in a preterm, extremely low-birth-weight newborn after vaginal delivery. J. Fungi 2021, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Maede, Y.; Ibara, S.; Nagasaki, H.; Inoue, T.; Tokuhisa, T.; Torikai, M.; Ishihara, C.; Matsui, T.; Kodaira, Y. Micafungin versus fluconazole for prophylaxis against fungal infections in premature infants. Pediatr. Int. 2013, 55, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Esposito, S. An overview of micafungin as a treatment option for invasive candidiasis in pediatric patients younger than 4 months old. Expert Opin. Pharmacother. 2022, 23, 1987–1993. [Google Scholar] [CrossRef]
- Ascher, S.; Smith, P.B.; Benjamin, D.K., Jr. Safety of micafungin in infants: Insights into optimal dosing. Expert Opin. Drug Saf. 2011, 10, 281–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).