Non-Invasive Recording of Ocular-Following Responses in Children: A Promising Tool for Stereo Deficiency Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. OFR Recording Apparatus
2.3. Behavioral Paradigm
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fielder, A.R.; Moseley, M.J. Does Stereopsis Matter in Humans? Eye 1996, 10, 233–238. [Google Scholar] [CrossRef]
- Kumar Morya, A.; Solanki, K.; Bhandari, S.; Naidu, A. Binocular Functions. In Eye Motility; IntechOpen: London, UK, 2019; pp. 19–44. [Google Scholar]
- Westheimer, G. Clinical Evaluation of Stereopsis. Vis. Res. 2013, 90, 38–42. [Google Scholar] [CrossRef]
- De Valois, R.L.; Smith, C.J.; Kitai, S.T.; Karoly, A.J. Response of Single Cells in Monkey Lateral Geniculate Nucleus to Monochromatic Light. Science 1958, 127, 238–239. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Receptive Fields and Functional Architecture of Monkey Striate Cortex. J. Physiol. 1968, 195, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Barlow, H.B.; Blakemore, C.; Pettigrew, J.D. The Neural Mechanism of Binocular Depth Discrimination. J. Physiol. 1967, 193, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Poggio, G.F.; Fischer, B. Binocular Interaction and Depth Sensitivity in Striate and Prestriate Cortex of Behaving Rhesus Monkey. J. Neurophysiol. 1977, 40, 1392–1405. [Google Scholar] [CrossRef]
- DeAngelis, G.C.; Ohzawa, I.; Freeman, R.D. Depth Is Encoded in the Visual Cortex by a Specialized Receptive Field Structure. Nature 1991, 352, 156–159. [Google Scholar] [CrossRef]
- Prince, S.J.D.; Pointon, A.D.; Cumming, B.G.; Parker, A.J. Quantitative Analysis of the Responses of V1 Neurons to Horizontal Disparity in Dynamic Random-Dot Stereograms. J. Neurophysiol. 2002, 87, 191–208. [Google Scholar] [CrossRef]
- Bishop, P.O.; Pettigrew, J.D. Neural Mechanisms of Binocular Vision. Vis. Res. 1986, 26, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Cumming, B.G.; DeAngelis, G.C. The Physiology of Stereopsis. Annu. Rev. Neurosci. 2001, 24, 203–238. [Google Scholar] [CrossRef]
- Katz, L.C.; Crowley, J.C. Development of Cortical Circuits: Lessons from Ocular Dominance Columns. Nat. Rev. Neurosci. 2002, 3, 34–42. [Google Scholar] [CrossRef]
- Huberman, A.D.; Feller, M.B.; Chapman, B. Mechanisms Underlying Development of Visual Maps and Receptive Fields. Annu. Rev. Neurosci. 2008, 31, 479–509. [Google Scholar] [CrossRef]
- Birch, E.E.; Stager, D.R. Monocular Acuity and Stereopsis in Infantile Esotropia. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1624–1630. [Google Scholar]
- Birch, E.E.; Stager, D.R.; Berry, P.; Everett, M.E. Prospective Assessment of Acuity and Stereopsis in Amblyoptic Infantile Estotropes Following Early Surgery. Investig. Ophthalmol. Vis. Sci. 1990, 31, 758–765. [Google Scholar]
- Robaei, D.; Huynh, S.C.; Kifley, A.; Gole, G.A.; Mitchell, P. Stereoacuity and Ocular Associations at Age 12 Years: Findings from a Population-Based Study. J. AAPOS 2007, 11, 356–361. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Binocular Interaction in Striate Cortex of Kittens Reared with Artificial Squint. J. Neurophysiol. 1965, 28, 1041–1059. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, C. The Conditions Required for the Maintenance of Binocularity in the Kitten’s Visual Cortex. J. Physiol. 1976, 261, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Chino, Y.M.; Smith, E.L.; Yoshida, K.; Cheng, H.; Hamamoto, J. Binocular Interactions in Striate Cortical Neurons of Cats Reared with Discordant Visual Inputs. J. Neurosci. 1994, 14, 5050–5067. [Google Scholar] [CrossRef]
- Scholl, B.; Tan, A.Y.Y.; Priebe, N.J. Strabismus Disrupts Binocular Synaptic Integration in Primary Visual Cortex. J. Neurosci. 2013, 33, 17108–17122. [Google Scholar] [CrossRef]
- Crawford, M.L.J.; Von Noorden, G.K. The Effects of Short-Term Experimental Strabismus on the Visual System in Macaca Mulatta. Investig. Ophthalmol. Vis. Sci. 1979, 18, 496–505. [Google Scholar]
- Smith, E.L.; Chino, Y.M.; Ni, J.; Cheng, H.; Crawford, M.L.J.; Harwerth, R.S. Residual Binocular Interactions in the Striate Cortex of Monkeys Reared with Abnormal Binocular Vision. J. Neurophysiol. 1997, 78, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Kumagami, T.; Zhang, B.; Smith, E.L.; Chino, Y.M. Effect of Onset Age of Strabismus on the Binocular Responses of Neurons in the Monkey Visual Cortex. Investig. Ophthalmol. Vis. Sci. 2000, 41, 948–954. [Google Scholar]
- Mori, T.; Matsuura, K.; Zhang, B.; Smith, E.L.; Chino, Y.M. Effects of the Duration of Early Strabismus on the Binocular Responses of Neurons in the Monkey Visual Cortex (V1). Investig. Ophthalmol. Vis. Sci. 2002, 43, 1262–1269. [Google Scholar]
- Economides, J.R.; Adams, D.L.; Horton, J.C. Interocular Suppression in Primary Visual Cortex in Strabismus. J. Neurosci. 2021, 41, 5522–5533. [Google Scholar] [CrossRef]
- Rowan Candy, T. The Importance of the Interaction between Ocular Motor Function and Vision during Human Infancy. Annu. Rev. Vis. Sci. 2019, 5, 201–221. [Google Scholar] [CrossRef]
- Levi, D.M.; Knill, D.C.; Bavelier, D. Stereopsis and Amblyopia: A Mini-Review. Vis. Res. 2015, 114, 17–30. [Google Scholar] [CrossRef]
- Seemiller, E.S.; Cumming, B.G.; Candy, T.R. Human Infants Can Generate Vergence Responses to Retinal Disparity by 5 to 10 Weeks of Age. J. Vis. 2018, 18, 17. [Google Scholar] [CrossRef]
- Von Noorden, G.K.; Campos, E.C. Binocular Vision and Ocular Motility: Theory and Management of Strabismus, 6th ed.; Mosby: St. Louis, MO, USA, 2002; p. 653. [Google Scholar]
- Fawcett, S.L.; Wang, Y.-Z.Z.; Birch, E.E. The Critical Period for Susceptibility of Human Stereopsis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 521. [Google Scholar] [CrossRef]
- Bruce, A.; Santorelli, G. Prevalence and Risk Factors of Strabismus in a UK Multi-Ethnic Birth Cohort. Strabismus 2016, 24, 153–160. [Google Scholar] [CrossRef]
- Friedman, D.S.; Repka, M.X.; Katz, J.; Giordano, L.; Ibironke, J.; Hawse, P.; Tielsch, J.M. Prevalence of Amblyopia and Strabismus in White and African American Children Aged 6 through 71 Months. The Baltimore Pediatric Eye Disease Study. Ophthalmology 2009, 116, 2128–2134.e2. [Google Scholar] [CrossRef]
- Simons, K. Preschool Vision Screening: Rationale, Methodology and Outcome. Surv. Ophthalmol. 1996, 41, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Giaschi, D.; Narasimhan, S.; Solski, A.; Harrison, E.; Wilcox, L.M. On the Typical Development of Stereopsis: Fine and Coarse Processing. Vis. Res. 2013, 89, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Giaschi, D.; Lo, R.; Narasimhan, S.; Lyons, C.; Wilcox, L.M. Sparing of Coarse Stereopsis in Stereodeficient Children with a History of Amblyopia. J. Vis. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.S.; Choi, D.G. Stereopsis and Fusion in Anisometropia According to the Presence of Amblyopia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Read, J.C.A. Stereo Vision and Strabismus. Eye 2015, 29, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Daw, N.W. Critical Periods and Amblyopia. Arch. Ophthalmol. 1998, 116, 502. [Google Scholar] [CrossRef]
- Chopin, A.; Bavelier, D.; Levi, D.M. The Prevalence and Diagnosis of ‘Stereoblindness’ in Adults Less than 60 Years of Age: A Best Evidence Synthesis. Ophthalmic Physiol. Opt. 2019, 39, 66–85. [Google Scholar] [CrossRef]
- Birch, E.E. Amblyopia and Binocular Vision. Prog. Retin. Eye Res. 2013, 33, 67–84. [Google Scholar] [CrossRef]
- Birch, E.E.; Fawcett, S.; Stager, D.R. Why Does Early Surgical Alignment Improve Stereoacuity Outcomes in Infantile Esotropia? J. AAPOS 2000, 4, 10–14. [Google Scholar] [CrossRef]
- Andalib, D.; Nabie, R.; Poormohammad, B. Factors Affecting Improvement of Stereopsis Following Successful Surgical Correction of Childhood Strabismus in Adults. Strabismus 2015, 23, 80–84. [Google Scholar] [CrossRef]
- Yagasaki, T.; Yokoyama, Y.; Tsukui, M. Relationship between Stereopsis Outcome and Timing of Surgical Alignment in Infantile Esotropia. J. AAPOS 2020, 24, e1–e78. [Google Scholar] [CrossRef]
- Paulette, S.; Maureen, M.; Velma, D.; Graham, Q.; Elise, C.; Lynn, C.; Marjean, T.K.; Bruce, M.; Deborah, O.-B.; Maryann, R.; et al. Comparison of Preschool Vision Screening Tests as Administered by Licensed Eye Care Professionals in the Vision in Preschoolers Study. Ophthalmology 2004, 111, 637–650. [Google Scholar] [CrossRef]
- Ciner, E.B.; Ying, G.S.; Kulp, M.T.; Maguire, M.G.; Quinn, G.E.; Orel-Bixler, D.; Cyert, L.A.; Moore, B.; Huang, J. Stereoacuity of Preschool Children with and without Vision Disorders. Optom. Vis. Sci. 2014, 91, 351–358. [Google Scholar] [CrossRef]
- Jonas, D.E.; Amick, H.R.; Wallace, I.F.; Feltner, C.; Van Der Schaaf, E.B.; Brown, C.L.; Baker, C. Vision Screening in Children Aged 6 Months to 5 Years: Evidence Report and Systematic Review for the US Preventive Services Task Force. J. Am. Med. Assoc. 2017, 318, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.E.; Leske, D.A.; Hatt, S.R.; Holmes, J.M. Defining Real Change in Measures of Stereoacuity. Ophthalmology 2009, 116, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.A.; Kawano, K.; Optican, L.M. Short-Latency Ocular Following Responses of Monkey. I. Dependence on Temporospatial Properties of Visual Input. J. Neurophysiol. 1986, 56, 1321–1354. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.A. Visual Stabilization of the Eyes in Primates. Curr. Opin. Neurobiol. 1997, 7, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.A. The Neural Processing of 3-D Visual Information: Evidence from Eye Movements. Eur. J. Neurosci. 1998, 10, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Kawano, K. Ocular Tracking: Behavior and Neurophysiology. Curr. Opin. Neurobiol. 1999, 9, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Masson, G.S. From 1D to 2D via 3D: Dynamics of Surface Motion Segmentation for Ocular Tracking in Primates. J. Physiol. 2004, 98, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.A.; Busettini, C.; Masson, G.S.; Yang, D.S. Short-Latency Eye Movements: Evidence for Rapid, Parallel Processing of Optic Flow. Opt. Flow Beyond 2004, 79–107. [Google Scholar]
- Takemura, A.; Murata, Y.; Kawano, K.; Miles, F.A. Deficits in Short-Latency Tracking Eye Movements after Chemical Lesions in Monkey Cortical Areas MT and MST. J. Neurosci. 2007, 27, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Quaia, C.; Optican, L.M.; Cumming, B.G. Binocular Summation for Reflexive Eye Movements. J. Vis. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Quaia, C.; Fitzgibbon, E.J.; Optican, L.M.; Cumming, B.G. Binocular Summation for Reflexive Eye Movements: A Potential Diagnostic Tool for Stereodeficiencies. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, A.; Quaia, C.; Ajčević, M.; Diplotti, L.; Cumming, B.G.; Pensiero, S.; Accardo, A. Ocular-Following Responses in School-Age Children. PLoS ONE 2022, 17, e0277443. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Li, X.Q.; Sun, B.; Tian, G.F. Development of Stereopsis among Children. Chin. J. Ophthalmol. 2009, 45, 323–327. [Google Scholar] [CrossRef]
- Chopin, A.; Silver, M.A.; Sheynin, Y.; Ding, J.; Levi, D.M. Transfer of Perceptual Learning From Local Stereopsis to Global Stereopsis in Adults with Amblyopia: A Preliminary Study. Front. Neurosci. 2021, 15, 1244. [Google Scholar] [CrossRef]
- Sheliga, B.M.; Quaia, C.; Cumming, B.G.; FitzGibbon, E.J. Spatial Summation Properties of the Human Ocular Following Response (OFR): Dependence upon the Spatial Frequency of the Stimulus. Vis. Res. 2012, 68, 1–13. [Google Scholar] [CrossRef]
- Quaia, C.; Sheliga, B.M.; FitzGibbon, E.J.; Optican, L.M. Ocular Following in Humans: Spatial Properties. J. Vis. 2012, 12, 13. [Google Scholar] [CrossRef]
- Miladinović, A.; Quaia, C.; Ajčević, M.; Diplotti, L.; Kresevic, S.; Pensiero, S.; Accardo, A. Characteristics of Ocular Following Responses (OFRs) in Children with Stereodeficiencies. In Proceedings of the Mediterranean Conference on Medical and Biological Engineering and Computing, Sarajevo, Bosnia and Herzegovina, 14–16 September 2023; pp. 438–446. [Google Scholar]
- Cooper, J.; Jamal, N. Convergence Insufficiency—A Major Review. Optometry 2012, 83, 137–158. [Google Scholar]
- Lyon, D.W.; Goss, D.A.; Horner, D.; Downey, J.P.; Rainey, B. Normative Data for Modified Thorington Phorias and Prism Bar Vergences from the Benton-IU Study. Optometry 2005, 76, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.; Srinivasan, K.; Thomas, J. Normative Data for near Point of Convergence, Accommodation, and Phoria. Oman J. Ophthalmol. 2015, 8, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, J.; Villarreal, G.; Abrahamsson, M.; Cavazos, H.; Sjöström, A.; Sjöstrand, J. Screening Merits of the Lang II, Frisby, Randot, Titmus, and TNO Stereo Tests. J. AAPOS 2001, 5, 316–322. [Google Scholar] [CrossRef] [PubMed]


| Subj | Age | Sex | Cycloplegic Refraction | LogMAR | Strabismus Angle | ||
|---|---|---|---|---|---|---|---|
| Right Eye (RE) | Left Eye (LE) | RE | LE | (PD) | |||
| HC1 | 7 | M | - | - | ≤0.0 | ≤0.0 | - |
| HC2 | 8 | F | - | - | ≤0.0 | ≤0.0 | - |
| HC3 | 9 | F | - | - | ≤0.0 | ≤0.0 | - |
| HC4 | 9 | M | - | - | ≤0.0 | ≤0.0 | - |
| HC5 | 11 | M | - | - | ≤0.0 | ≤0.0 | - |
| HC6 | 12 | F | - | - | ≤0.0 | ≤0.0 | - |
| P1 | 7 | M | +1.25 + 0.50/80 | +1.50 | 0.2 | 0.0 | 6 XT |
| P2 | 8 | F | +2.00 | +2.25 | 0.0 | 0.4 | 10 ET |
| P3 | 9 | F | +2.25 + 1.00/80 | +3.50 + 2.50/80 | 0.0 | 0.4 | - |
| P4 | 9 | M | +0.50 | +0.50 | 0.2 | 0.0 | RE HYTR 4 |
| P5 | 11 | M | +3.00 + 0.50/95 | +3.25 + 1.50/105 | 0.0 | 0.3 | 8 ET |
| P6 | 12 | F | +2.25 + 0.50/95 | +2.25 + 0.50/75 | 0.0 | 0.2 | 10 ET |
| Subj | Δyc ± SD (N) UP | Δyc ± SD (N) DW | p | p_np | OFRc ± SD |
|---|---|---|---|---|---|
| HC1 | 0.227 ± 0.234 (12) | −0.133 ± 0.096 (13) | <0.001 | <0.001 | 0.360 ± 0.253 |
| HC2 | 0.220 ± 0.088 (6) | −0.174 ± 0.105 (13) | <0.001 | <0.001 | 0.394 ± 0.137 |
| HC3 | 0.424 ± 0.157 (10) | −0.277 ± 0.183 (11) | <0.001 | <0.001 | 0.700 ± 0.241 |
| HC4 | 0.293 ± 0.101 (13) | −0.242 ± 0.186 (15) | <0.001 | <0.001 | 0.534 ± 0.212 |
| HC5 | 0.216 ± 0.131 (12) | −0.204 ± 0.132 (11) | <0.001 | <0.001 | 0.420 ± 0.186 |
| HC6 | 0.157 ± 0.125 (16) | −0.123 ± 0.152 (11) | <0.001 | <0.001 | 0.280 ± 0.197 |
| P1 | 0.192 ± 0.119 (18) | −0.116 ± 0.104 (18) | <0.001 | <0.001 | 0.309 ± 0.158 |
| P2 | 0.124 ± 0.177 (14) | −0.423 ± 0.276 (14) | <0.001 | <0.001 | 0.547 ± 0.328 |
| P3 | 0.187 ± 0.104 (12) | −0.173 ± 0.117 (9) | <0.001 | <0.001 | 0.360 ± 0.157 |
| P4 | 0.090 ± 0.184 (12) | −0.117 ± 0.141 (16) | 0.003 | 0.003 | 0.207 ± 0.232 |
| P5 | 0.278 ± 0.136 (11) | −0.157 ± 0.216 (10) | <0.001 | 0.001 | 0.435 ± 0.255 |
| P6 | 0.126 ± 0.101 (14) | −0.236 ± 0.176 (17) | <0.001 | <0.001 | 0.362 ± 0.202 |
| Subj | Δyac ± SD (N) UP | Δyac ± SD (N) DW | p | p_np | OFRac ± SD |
|---|---|---|---|---|---|
| HC1 | 0.102 ± 0.111 (9) | −0.127 ± 0.041 (12) | <0.001 | <0.001 | 0.230 ± 0.118 |
| HC2 | 0.168 ± 0.175 (11) | 0.001 ± 0.149 (12) | 0.028 | 0.006 | 0.167 ± 0.230 |
| HC3 | 0.158 ± 0.120 (08) | −0.291 ± 0.171 (12) | <0.001 | <0.001 | 0.450 ± 0.209 |
| HC4 | 0.232 ± 0.154 (10) | −0.124 ± 0.049 (6) | <0.001 | <0.001 | 0.356 ± 0.161 |
| HC5 | 0.100 ± 0.165 (15) | −0.045 ± 0.188 (14) | 0.042 | 0.017 | 0.145 ± 0.250 |
| HC6 | 0.158 ± 0.086 (16) | 0.047 ± 0.289 (12) | 0.172 | 0.024 | 0.111 ± 0.301 |
| P1 | 0.163 ± 0.115 (17) | −0.255 ± 0.141 (22) | <0.001 | <0.001 | 0.418 ± 0.182 |
| P2 | 0.155 ± 0.256 (12) | −0.353 ± 0.172 (13) | <0.001 | <0.001 | 0.508 ± 0.308 |
| P3 | 0.143 ± 0.132 (9) | −0.211 ± 0.127 (10) | <0.001 | 0.001 | 0.354 ± 0.183 |
| P4 | 0.131 ± 0.132 (13) | −0.127 ± 0.091 (15) | <0.001 | <0.001 | 0.258 ± 0.160 |
| P5 | 0.288 ± 0.138 (6) | −0.278 ± 0.094 (7) | <0.001 | 0.001 | 0.566 ± 0.167 |
| P6 | 0.187 ± 0.104 (17) | −0.206 ± 0.117 (14) | <0.001 | <0.001 | 0.393 ± 0.157 |
| Subj | OFRc ± SD | OFRc SEM | OFRac ± SD | OFRac SEM | p |
|---|---|---|---|---|---|
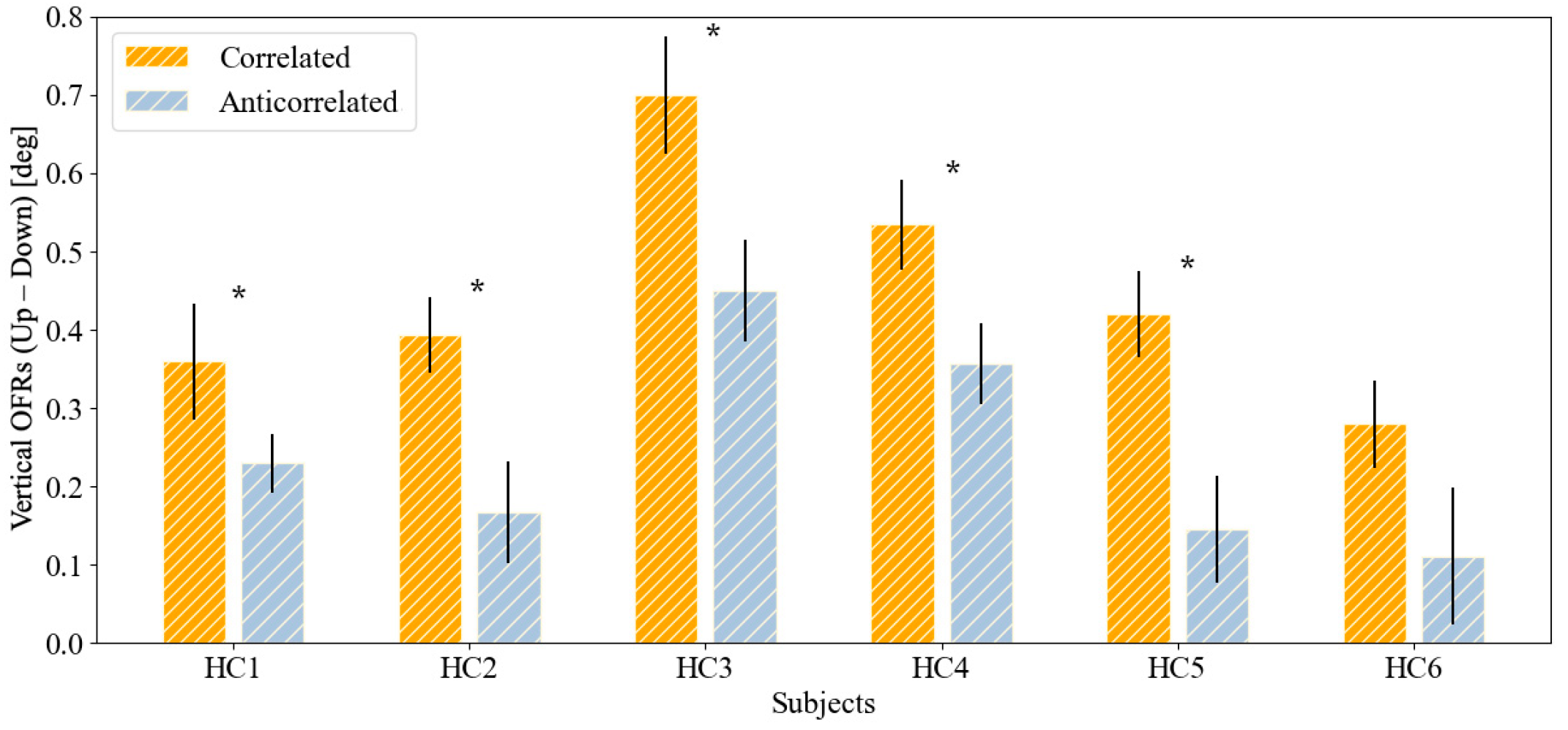
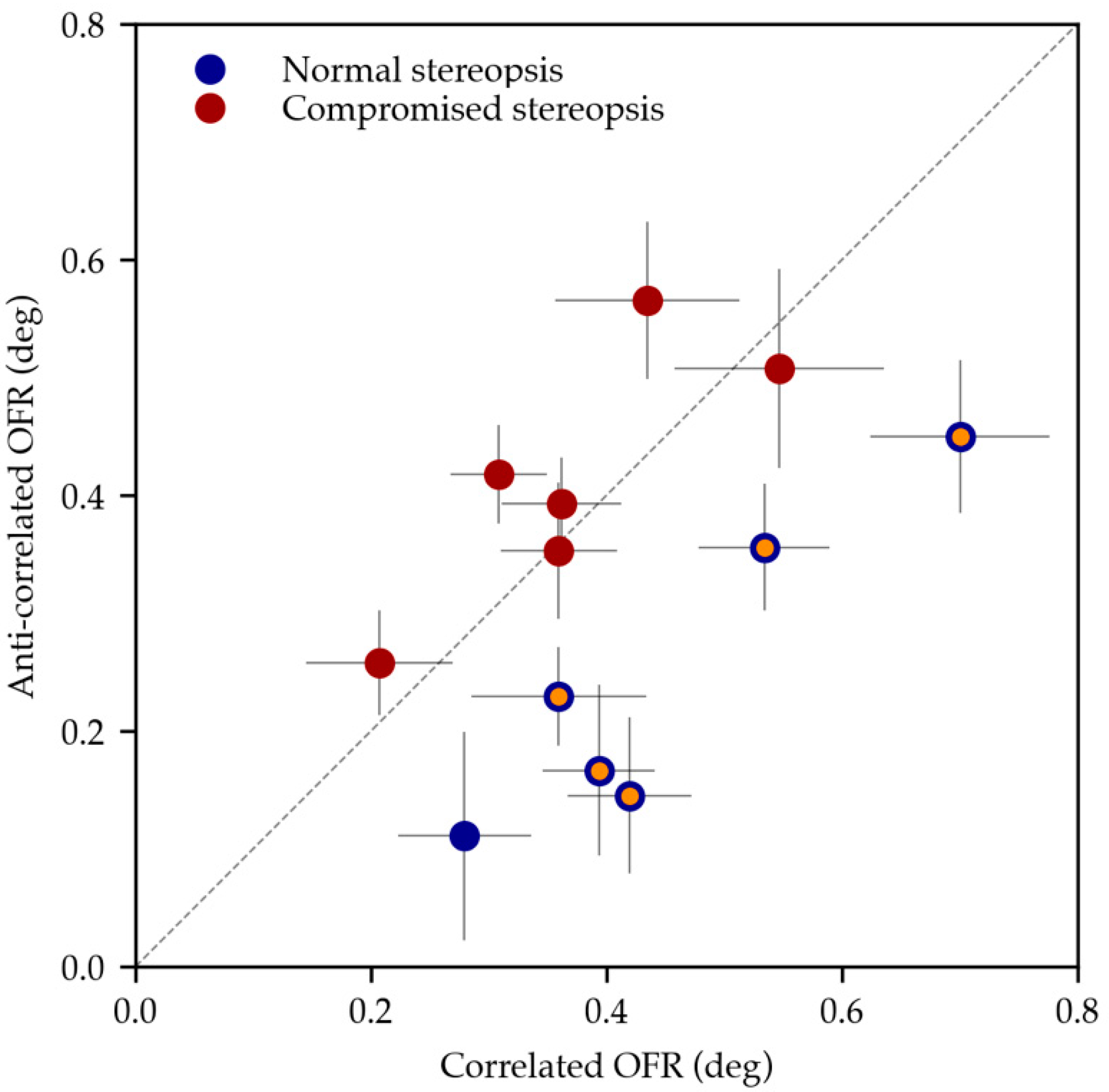
| HC1 | 0.360 ± 0.253 | 0.073 | 0.230 ± 0.118 | 0.038 | 0.048 * |
| HC2 | 0.394 ± 0.137 | 0.045 | 0.167 ± 0.230 | 0.064 | 0.010 * |
| HC3 | 0.700 ± 0.241 | 0.073 | 0.450 ± 0.209 | 0.065 | 0.016 * |
| HC4 | 0.534 ± 0.212 | 0.057 | 0.356 ± 0.161 | 0.054 | 0.014 * |
| HC5 | 0.420 ± 0.186 | 0.056 | 0.145 ± 0.250 | 0.065 | <0.001 * |
| HC6 | 0.280 ± 0.197 | 0.054 | 0.111 ± 0.301 | 0.084 | 0.088 |
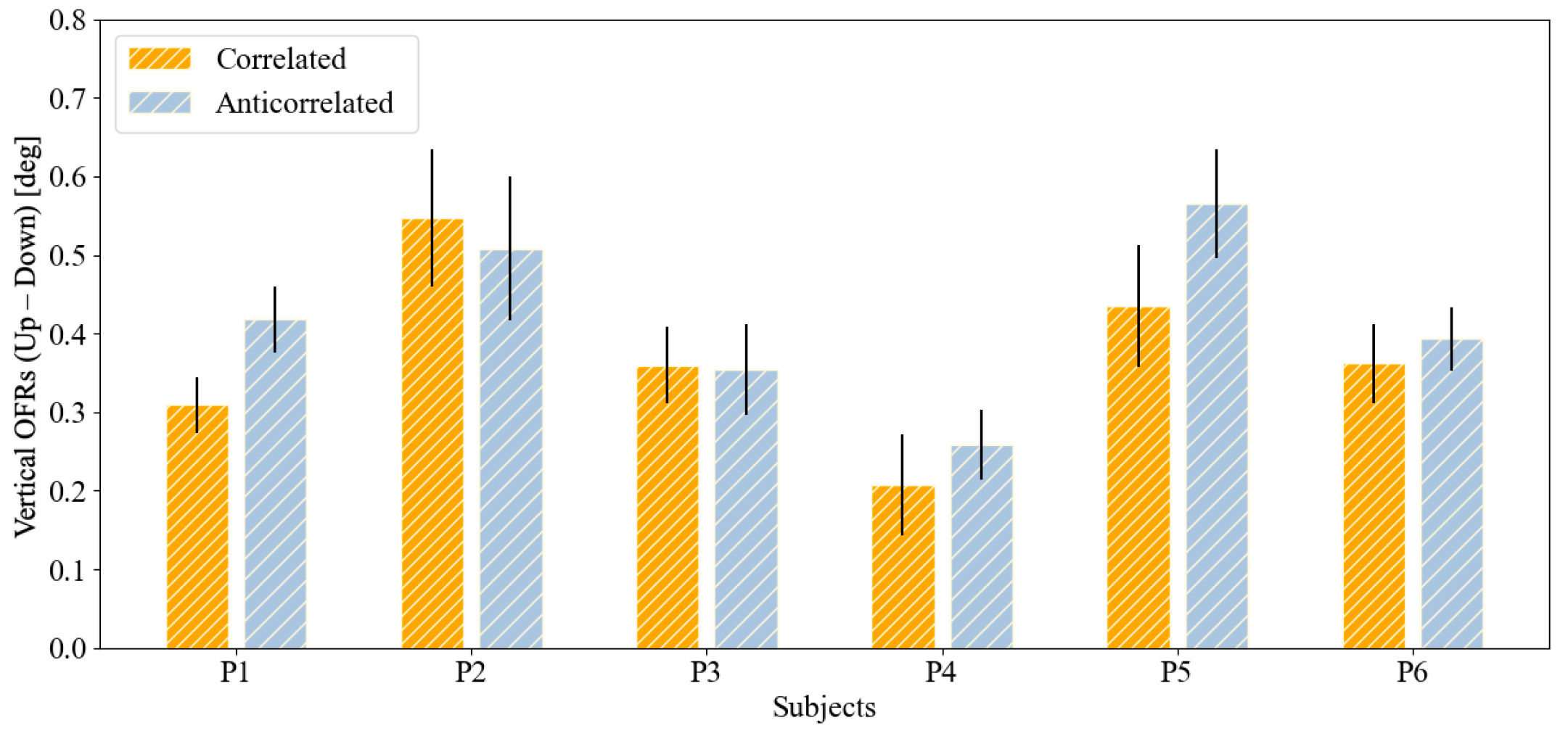
| P1 | 0.309 ± 0.158 | 0.038 | 0.418 ± 0.182 | 0.040 | 0.092 |
| P2 | 0.547 ± 0.328 | 0.088 | 0.508 ± 0.308 | 0.089 | 0.738 |
| P3 | 0.360 ± 0.157 | 0.049 | 0.354 ± 0.183 | 0.059 | 0.936 |
| P4 | 0.207 ± 0.232 | 0.065 | 0.258 ± 0.160 | 0.043 | 0.554 |
| P5 | 0.435 ± 0.255 | 0.079 | 0.566 ± 0.167 | 0.069 | 0.184 |
| P6 | 0.362 ± 0.202 | 0.049 | 0.393 ± 0.157 | 0.042 | 0.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miladinović, A.; Quaia, C.; Ajčević, M.; Diplotti, L.; Michieletto, P.; Accardo, A.; Pensiero, S. Non-Invasive Recording of Ocular-Following Responses in Children: A Promising Tool for Stereo Deficiency Evaluation. J. Clin. Med. 2024, 13, 1596. https://doi.org/10.3390/jcm13061596
Miladinović A, Quaia C, Ajčević M, Diplotti L, Michieletto P, Accardo A, Pensiero S. Non-Invasive Recording of Ocular-Following Responses in Children: A Promising Tool for Stereo Deficiency Evaluation. Journal of Clinical Medicine. 2024; 13(6):1596. https://doi.org/10.3390/jcm13061596
Chicago/Turabian StyleMiladinović, Aleksandar, Christian Quaia, Miloš Ajčević, Laura Diplotti, Paola Michieletto, Agostino Accardo, and Stefano Pensiero. 2024. "Non-Invasive Recording of Ocular-Following Responses in Children: A Promising Tool for Stereo Deficiency Evaluation" Journal of Clinical Medicine 13, no. 6: 1596. https://doi.org/10.3390/jcm13061596






