Atypical Manifestations of Syphilis: A 10-Year Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
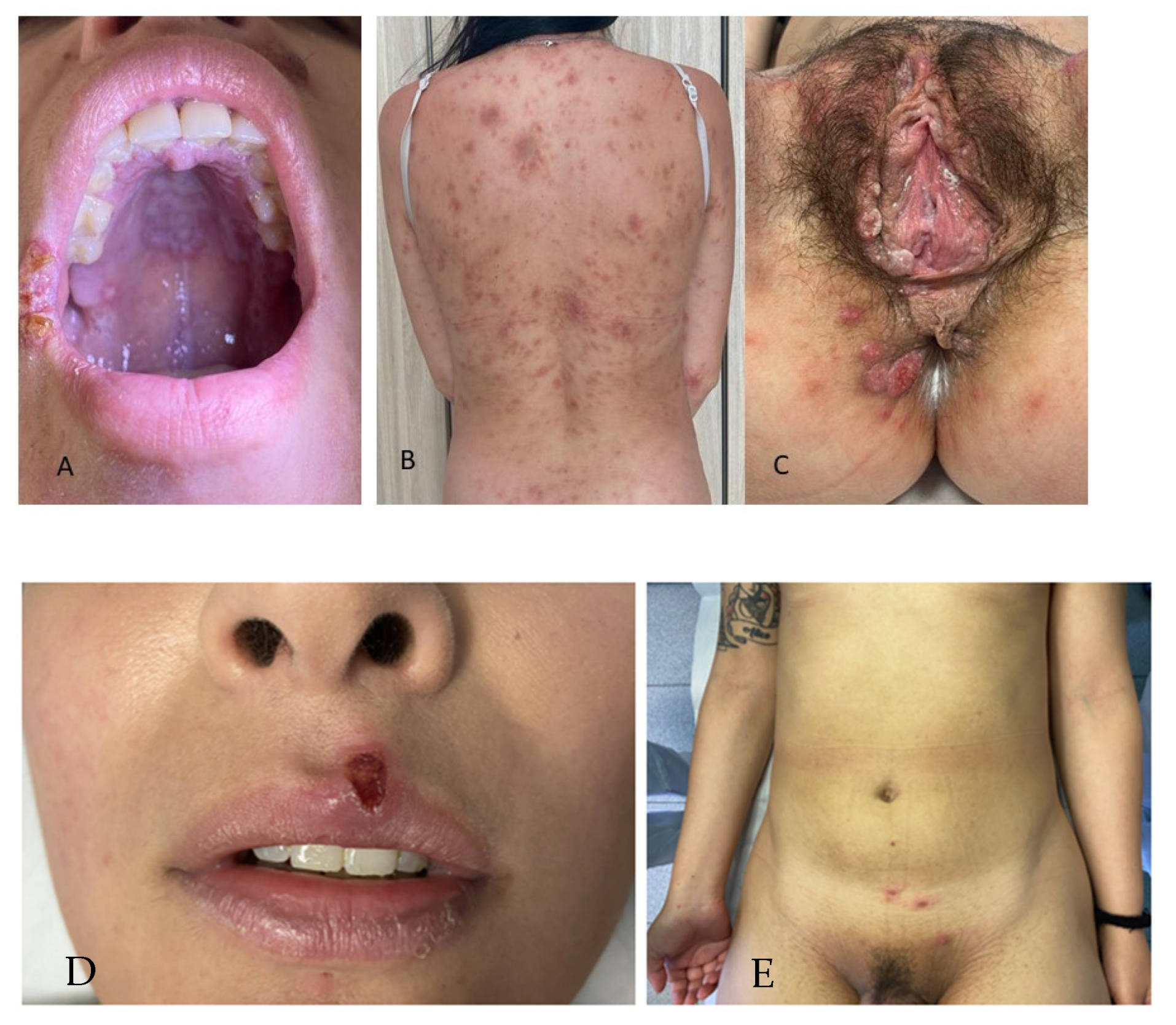
Atypical Syphilis Manifestations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peeling, R.W.; Mabey, D.; Chen, X.S.; Garcia, P.J. Syphilis. Lancet 2023, 402, 336–346. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Merlo, G.; Sartoris, G.; Parodi, A. Is the Standard Treatment for Early Syphilis Sufficient to Prevent Cardiovascular and Neurologic Syphilis? Am. J. Cardiol. 2016, 117, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Ciccarese, G.; Rebora, A. Treatment of late-stage syphilis. JAMA 2015, 313, 969. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Syphilis Epidemiological Report. 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/syphilis-aer-2018.pdf (accessed on 15 February 2024).
- Mitjà, O.; Padovese, V.; Folch, C.; Rossoni, I.; Marks, M.; Rodríguez, I.; Arias, M.A.; Telenti, A.; Ciuffi, A.; Blondeel, K.; et al. Epidemiology and determinants of reemerging bacterial sexually transmitted infections (STIs) and emerging STIs in Europe. Lancet Reg. Health Eur. 2023, 34, 100742. [Google Scholar] [CrossRef]
- Fu, L.; Tian, T.; Wang, B.; Lu, Z.; Bian, J.; Zhang, W.; Wu, X.; Li, X.; Siow, R.C.; Fang, E.F.; et al. Global, regional, and national burden of HIV and other sexually transmitted infections in older adults aged 60–89 years from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2024, 5, e17–e30. [Google Scholar] [CrossRef]
- Ghanem, K.G.; Ram, S.; Rice, P.A. The Modern Epidemic of Syphilis. N. Engl. J. Med. 2020, 382, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Traeger, M.W.; Cornelisse, V.J.; Asselin, J.; Price, B.; Roth, N.J.; Willcox, J.; Tee, B.K.; Fairley, C.K.; Chang, C.C.; Armishaw, J.; et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019, 321, 1380–1390. [Google Scholar] [CrossRef]
- Azarnoosh, M.; Johansen, I.S.; Martin-Iguacel, R. Incidence of sexually transmitted infections after initiating HIV pre-exposure prophylaxis among MSM in southern Denmark. Am. J. Men. Health 2021, 15, 15579883211018917. [Google Scholar] [CrossRef]
- Ciccarese, G.; Drago, F.; Copello, F.; Bodini, G.; Rebora, A.; Parodi, A. Study on the impact of sexually transmitted infections on Quality of Life, mood and sexual function. Ital. J. Dermatol. Venerol. 2021, 156, 686–691. [Google Scholar] [CrossRef]
- Lleó, M.I.; Escribano, P.C.; Prieto, B.M. Manifestaciones cutáneas atipícas en la sífilis. Actas Dermosifiliogr. 2016, 107, 275–283. [Google Scholar] [CrossRef]
- Ciccarese, G.; Herzum, A.; Rebora, A.; Drago, F. Prevalence of genital, oral, and anal HPV infection among STI patients in Italy. J. Med. Virol. 2017, 89, 1121–1124. [Google Scholar] [CrossRef]
- Drago, F.; Herzum, A.; Ciccarese, G.; Dezzana, M.; Casazza, S.; Pastorino, A.; Bandelloni, R.; Parodi, A. Ureaplasma parvum as a possible enhancer agent of HPV-induced cervical intraepithelial neoplasia: Preliminary results. J. Med. Virol. 2016, 88, 2023–2024. [Google Scholar] [CrossRef]
- Chapel, T.A. The variability of syphilitic chancres. Sex. Transm. Dis. 1978, 5, 68–70. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Cogorno, L.; Tomasini, C.F.; Cozzani, E.C.; Riva, S.F.; Parodi, A. Primary syphilis of the oropharynx: An unusual location of a chancre. Int. J. STD AIDS 2015, 26, 679–681. [Google Scholar] [CrossRef]
- Drago, F.; Agnoletti, A.F.; Ciccarese, G.; Cozzani, E.; Parodi, A. Two cases of oligosymptomatic neurosyphilis in immunocompetent patients: Atypical neurosyphilis presentation. Int. J. STD AIDS 2016, 27, 155–156. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Tomasini, C.F.; Calamaro, P.; Boggio, M.; Rebora, A.; Parodi, A. First report of tertiary syphilis presenting as lipoatrophic panniculitis in an immunocompetent patient. Int. J. STD AIDS 2017, 28, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Gasparini, G.; Ciccarese, G.; Drago, F.; Trave, I.; Vellone, V.; Biatta, C.M.; Cabiddu, F.; Ribizzi, G.; Parodi, A. Concurrent benign tertiary syphilis and asymptomatic neurosyphilis in an immunocompetent patient. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e151–e152. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.; Ciccarese, G.; Drago, F.; Cozzani, E.; Buligan, C.; Turina, M.; Parodi, A. Annular and arciform lesions of the palms as unique manifestations of secondary syphilis. Int. J. STD AIDS 2020, 31, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Ciccarese, G.; Parodi, A. Follmann balanitis and anetoderma in secondary syphilis. Dermatol. Rep. 2021, 14, 9271. [Google Scholar] [CrossRef]
- Battistella, M.; Le Cleach, L.; Lacert, A.; Perrin, P. Extensive nodular secondary syphilis with prozone phenomenon. Arch. Dermatol. 2008, 144, 1078–1079. [Google Scholar] [CrossRef]
- Pournaras, C.C.; Masouye, I.; Piletta, P.; Piguet, V.; Saurat, J.H.; French, L.E. Extensive annular verrucous late secondary syphilis. Br. J. Dermatol. 2005, 152, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Balagula, Y.; Mattei, P.L.; Wisco, O.J.; Erdag, G.; Chien, A.L. The great imitator revisited: The spectrum of atypical cutaneous manifestations of secondary syphilis. Int. J. Dermatol. 2014, 53, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Ivars Lleó, M.; Clavo Escribano, P.; Menéndez Prieto, B. Atypical Cutaneous Manifestations in Syphilis. Actas Dermosifiliogr. 2016, 107, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, S. Cutaneous manifestations of syphilis: Recognition and management. Am. J. Clin. Dermatol. 2006, 7, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.J.; Greenberg, H.L.; Khachemoune, A. Unusual primary syphilis: Presentation of a likely case with a review of the stages of acquired syphilis, its differential diagnoses, management, and current recommendations. Int. J. Dermatol. 2016, 55, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Merlo, G.; Ciccarese, G.; Agnoletti, A.F.; Cozzani, E.; Rebora, A.; Parodi, A. Changes in neurosyphilis presentation: A survey on 286 patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Dourmishev, L.A.; Dourmishev, A.L. Syphilis: Uncommon presentations in adults. Clin. Dermatol. 2005, 23, 555–564. [Google Scholar] [CrossRef]
- Kanayama, S.; Nagata, S.; Akiyama, Y.; Miyazato, Y.; Ishikane, M.; Inoue, M.; Ohmagari, N.; Hara, T. Cerebral syphilitic Gumma in the modern era: A report of an unusual case and brief review of recent published reports. Br. J. Neurosurg. 2022, 23, 1–6. [Google Scholar] [CrossRef]
- Tucker, J.D.; Shah, S.; Jarell, A.D.; Tsai, K.Y.; Zembowicz, A.; Kroshinsky, D. Lues maligna in early HIV infection case report and review of the literature. Sex. Transm. Dis. 2009, 36, 512–514. [Google Scholar] [CrossRef]
- Skalnaya, A.; Fominykh, V.; Ivashchenko, R.; Averchenkov, D.; Grazhdantseva, L.; Frigo, N.; Negasheva, E.; Dolya, O.; Brylev, L.; Guekht, A. Neurosyphilis in the modern era: Literature review and case series. J. Clin. Neurosci. 2019, 69, 67–73. [Google Scholar] [CrossRef]
- Ramoni, S.; Genovese, G.; Pastena, A.; Casazza, G.; Lunghi, G.; Marzano, A.V.; Cusini, M. Clinical and laboratory features of 244 men with primary syphilis: A 5-year single-centre retrospective study. Sex. Transm. Infect. 2021, 97, 479–484. [Google Scholar] [CrossRef]
- Larsen, C.N.; Larsen, H.K. Herpetiform Manifestation of Primary Syphilis: A Case Series. Acta Derm. Venereol. 2020, 100, adv00072. [Google Scholar] [CrossRef]
- Towns, J.M.; Leslie, D.E.; Denham, I.; Azzato, F.; Fairley, C.K.; Chen, M. Painful and multiple anogenital lesions are common in men with Treponema pallidum PCR-positive primary syphilis without herpes simplex virus coinfection: A cross-sectional clinic-based study. Sex. Transm. Infect. 2016, 92, 110–115. [Google Scholar] [CrossRef]
- Gong, H.Z.; Wu, M.Y.; Li, J.; Zheng, H.Y. The Great Imitator: Atypical Cutaneous Manifestations of Primary Syphilitic Chancre. Chin. Med. Sci. J. 2021, 36, 279–283. [Google Scholar]
- Arando, M.; Fernandez-Naval, C.; Mota-Foix, M.; Martinez, D.; Armengol, P.; Barberá, M.J.; Esperalba, J.; Vall-Mayans, M. Early syphilis: Risk factors and clinical manifestations focusing on HIV-positive patients. BMC Infect. Dis. 2019, 19, 727. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, J.C.; de Lima, L.L.; Heibel, M.; Schettini, A.; Talhari, S.; Talhari, C. Atypical manifestations of recent syphilis: Study of 19 cases. An. Bras. Dermatol. 2020, 95, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Murata, K.; Ogawa, Y. Primary syphilis of the oropharynx mimicking a neoplasm. Int. J. STD AIDS 2023, 34, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, C.; Zama, M.; Hyodo, T.; Shiraishi, R.; Kamimura, R.; Yagisawa, S.; Hasegawa, T.; Komiyama, Y.; Izumi, S.; Wakui, T.; et al. Primary syphilis with a tongue ulcer mimicking tongue cancer: A case report. J. Int. Med. Res. 2023, 51, 3000605231161223. [Google Scholar] [CrossRef]
- Magnaterra, E.; Grandi, V.; Pisano, L. The Great Imitator: Primary Syphilis Clinically Mimicking Oral Squamous Cell Carcinoma. Am. J. Med. 2022, 135, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, K.; Fahimi, S.; Huebers, C.; Géraud, C. The great impostor: Secondary syphilis imitating palmoplantar psoriasis. J. Dtsch. Dermatol. Ges. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Rahmatika, A.S.; Murtiastutik, D.; Hidayati, A.N.; Astindari; Sari, M.; Widyantari, S.; Agusni, R.I.; Astari, L. Unusual presentation of secondary syphilis mimicking erythema multiforme in HIV positive patient: A case report. Pan Afr. Med. J. 2023, 46, 55. [Google Scholar]
- Zhang, J.; Li, X.; Shi, D. A case of secondary syphilis misdiagnosed as psoriasis vulgaris: Discover the real cause beyond the surface with reflection confocal microscopy and leave the great imitator nowhere to hide. Skin Res. Technol. 2023, 29, e13374. [Google Scholar] [CrossRef]
- Yousefian, F.; Wang, H.; Yadlapati, S.; Cohen, J.A.; Browning, J.C. Granulomatous Secondary Syphilis: An Uncommon Presentation of the Great Imitator. Am. J. Med. 2023, 136, e25–e26. [Google Scholar] [CrossRef]
- Jin, Y.; Zhong, W.; Wang, X.; Huang, C.; Lu, Y. The Great Imitator’s Trick on Bones: Syphilitic Osteomyelitis. Acta Derm. Venereol. 2024, 104, adv18626. [Google Scholar] [CrossRef]
- Aal Hamad, A.; Al Hadhrami, Z.; Al Lawati, A.; Al Busaidi, I.; Mahmood, S. Syphilis-Related Nephropathy: A Rare Manifestation of a Re-emerging Disease. Cureus 2023, 15, e50105. [Google Scholar] [CrossRef]
- Kaur, B.; Khanna, D. A Narrative Review of the Many Psychiatric Manifestations of Neurosyphilis: The Great Imitator. Cureus 2023, 15, e44866. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt Mde, J.; Brito, A.C.; Nascimento, B.A.; Carvalho, A.H.; Nascimento, M.D. A case of secondary syphilis mimicking palmoplantar psoriasis in HIV infected patient. An. Bras. Dermatol. 2015, 90, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lambert, P.J.; Maghari, A.; Lambert, W.C. Arcuate, annular, and polycyclic inflammatory and infectious lesions. Clin. Dermatol. 2011, 29, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bastos, T.C.; Maia, D.C.; Gomes, N.M.; Menezes, C.K.; Francesconi, V.; Francesconi, F. Syphilis associated with paretic neurosyphilis mimicking Reiter’s syndrome in HIV-infected patients. An. Bras. Dermatol. 2015, 90, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Oh, S.T.; Lee, J.Y.; Cho, B.K. Secondary syphilis presenting as scrotal eczema. J. Am. Acad. Dermatol. 2007, 57, 1099–1101. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, D.; Park, J.H.; Kang, M.S.; Cho, S.H.; Park, S.W. Scrotal eczema-like lesion of secondary syphilis in an HIV-positive patient. Acta Derm. Venereol. 2005, 85, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; du Vivier, A. Anetoderma secondary to syphilis. J. R. Soc. Med. 1983, 76, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.V.; Lellis, R.F.; Porto, R.L.; Mattei, G.M. Anetoderma due to secondary syphilis: Report of two cases and discussion of the histopathological findings. Int. J. STD AIDS 2017, 28, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Rompalo, A.M.; Lawlor, J.; Seaman, P.; Quinn, T.C.; Zenilman, J.M.; Hook, E.W., 3rd. Modification of the syphilitic genital ulcer manifestations by coexistent HIV infection. Sex. Transm. Dis. 2001, 28, 448z. [Google Scholar] [CrossRef] [PubMed]
- Zellan, J.; Augenbraun, M. Syphilis in the HIV-infected patient: An update on epidemiology, diagnosis, and management. Curr. HIV/AIDS Rep. 2004, 1, 142–147. [Google Scholar] [CrossRef]
- Sidana, R.; Mangala, H.C.; Murugesh, S.B.; Ravindra, K. Prozone phenomenon in secondary syphilis. Indian J. Sex. Transm. Dis. 2011, 32, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Aroza-Espinar, M.; Merlán-Hermida, A.; Suárez-Hormiga, L.; Pérez-Arellano, J.L. Malignant syphilis in HIV negative patient treated with ibrutinib. Rev. Esp. Quimioter. 2023, 36, 632–634. [Google Scholar] [CrossRef]
- Karanfilian, K.M.; Almohssen, A.A.; Kapila, R.; Schwartz, R.A. Malignant syphilis: A new and revised definition. Int. J. Dermatol. 2023, 62, 369–375. [Google Scholar] [CrossRef]
- Addetia, A.; Lin, M.J.; Phung, Q.; Xie, H.; Huang, M.L.; Ciccarese, G.; Dal Conte, I.; Cusini, M.; Drago, F.; Giacani, L.; et al. Estimation of Full-Length TprK Diversity in Treponema pallidum subsp. pallidum. mBio 2020, 11, e02726-20. [Google Scholar]
- Landry, T.; Smyczek, P.; Cooper, R.; Gratrix, J.; Bertholet, L.; Read, R.; Romanowski, B.; Singh, A.E. Retrospective review of tertiary and neurosyphilis cases in Alberta, 1973–2017. BMJ Open 2019, 9, e025995. [Google Scholar] [CrossRef]
- Hutchinson, C.M.; Hook, E.W., 3rd. Syphilis in adults. Med. Clin. N. Am. 1990, 74, 1389–1416. [Google Scholar] [CrossRef]
- Dunlop, E.M. Survival of treponemes after treatment: Comments, clinical conclusions, and recommendations. Genitourin. Med. 1985, 61, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Tramont, E.C. Persistence of Treponema pallidum following penicillin G therapy: Report of two cases. JAMA 1976, 236, 2206–2207. [Google Scholar] [CrossRef]
- Zhou, P.; Gu, X.; Lu, H.; Guan, Z.; Qian, Y. Re-evaluation of serological criteria for early syphilis treatment efficacy: Progression to neurosyphilis despite therapy. Sex. Transm. Infect. 2012, 88, 342–345. [Google Scholar] [CrossRef]
- Conde-Sendín, M.A.; Amela-Peris, R.; Aladro-Benito, Y.; Maroto, A.A. Current clinical spectrum of neurosyphilis in immunocompetent patients. Eur. Neurol. 2004, 52, 29–35. [Google Scholar] [CrossRef]
- Spiegel, D.R.; Qureshi, N. The successful treatment of disinhibition due to a possible case of non-human immunodeficiency virus neurosyphilis: A proposed pathophysiological explanation of the symptoms and treatment. Gen. Hosp. Psychiatry 2010, 32, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Ciccarese, G.; Broccolo, F.; Sartoris, G.; Stura, P.; Esposito, S.; Rebora, A.; Parodi, A. A new enhanced antibiotic treatment for early and late syphilis. J. Glob. Antimicrob. Resist. 2016, 5, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fan, Y.; Chen, J.; Yang, J.; Gao, L.; Wu, X.; Xu, X.; Zhang, Y.; Yue, P.; Cao, W.; et al. Efficacy and Safety of Treatments for Different Stages of Syphilis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials and Observational Studies. Microbiol. Spectr. 2022, 10, e0297722. [Google Scholar] [CrossRef]
- Reschke, R.; Kunz, M.; Ziemer, M. Gummatous Cutaneous Syphilis. Dtsch. Arztebl. Int. 2022, 119, 457. [Google Scholar] [CrossRef]
- Angerer, M.; Lübbersmeyer, F.; Gübitz, R.; Wülfing, C.; Dieckmann, K.P. Tertiary Syphilitic Gumma Mimicking Testicular Neoplasms. Cureus 2023, 15, e37392. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Hayashi, S.; Kaminaga, T.; Hamasaki, Y.; Igawa, K. Cutaneous tertiary syphilitic gumma on the scalp. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e350–e352. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, S.W.; Saeed, L.; Thurlow, C.M.; Vilfort, K.; Pillay, A.; Rojek, N.W.; Doan, L.T.; Lee, B.A. Round Bodies Detected by Treponema pallidum Immunohistochemical Stain in Two Cases of Cutaneous Syphilitic Gummata. Am. J. Dermatopathol. 2023, 46, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bjekić, M.; Marković, M.; Sipetić, S. Clinical manifestations of primary syphilis in homosexual men. Braz. J. Infect. Dis. 2012, 16, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Irmawati, Y.E.; Pudjiati, S.R. Malignant syphilis: An early feature of underlying HIV infection in an MSM patient. Germs 2023, 13, 168–171. [Google Scholar] [CrossRef]
- Lafferty, W.E.; Hughes, J.P.; Handsfield, H.H. Sexually transmitted diseases in men who have sex with men. Acquisition of gonorrhea and nongonococcal urethritis by fellatio and implications for STD/HIV prevention. Sex. Transm. Dis. 1997, 24, 272–278. [Google Scholar] [CrossRef]
- Kenyon, C.; Tsoumanis, A.; Osbak, K.; Van Esbroeck, M.; Florence, E.; Crucitti, T.; Kestens, L. Repeat syphilis has a different immune response compared with initial syphilis: An analysis of biomarker kinetics in two cohorts. Sex. Transm. Infect. 2018, 94, 180–186. [Google Scholar] [CrossRef]
- Marra, C.M.; Maxwell, C.L.; Sahi, S.K.; Tantalo, L.C.; Dunaway, S.B.; Lukehart, S.A. Previous Syphilis Alters the Course of Subsequent Episodes of Syphilis. Clin. Infect. Dis. 2022, 74, e1–e5. [Google Scholar] [CrossRef]



| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Odds Ratio (CI 95%) | p-Value | Odds Ratio (CI 95%) | p-Value |
| Sex (reference, female) | 0.40 (0.15–1.09) | 0.07 | ||
| Race (reference, Latin) | 4.15 (0.61–1.86) | 0.89 | ||
| Age | 1.62 (0.87–3.93) | 0.07 | ||
| Sexual orientation (reference, homosexual/bisexual) | 0.34 (0.12–0.96) | 0.04 | 0.45 (0.24–0.91) | 0.03 |
| Diagnosis (reference, PS) | SS 1.9 (0.54–6.58) | 0.31 | ||
| TS-NS 4.75 (0.82–27.49) | 0.08 | |||
| HIV status (reference, negative) | 1.11 (0.12–9.69) | 0.92 | ||
| Reinfection (reference, no) | 16.76 (1.83–153.48) | 0.01 | 18 (1.33–172.11) | 0.02 |
| TPHA (reference, ≥5120) | 0.87 (0.35–2.15) | 0.01 | ||
| VDRL (reference, ≤32) | 2.18 (1.08–5.38) | 0.03 | 2.11 (1.13–5.15) | 0.02 |
| T. pallidum IgM antibodies (reference, negative) | 1.33 (0.52–6.78) | 0.97 | ||
| Coinfections by other sexually transmitted bacteria and by HPV (reference, negative) | 1.17 (0.38–3.59) | 0.77 | ||
| HBV/HCV coinfections (reference, negative) | 0.50 (0.02–10.87) | 0.66 |
| Syphilis with Clinical Signs: Primary, Secondary, and Tertiary Syphilis and Neurosyphilis | ||
|---|---|---|
| Total n° = 139 | Atypical Syphilis n° = 36 | |
| Gender | ||
| Male | 111 (80%) | 25 (69%) |
| Female | 28 (20%) | 11 (31%) |
| Mean age at diagnosis, year (range) | 38 (17–75) | 42 (23–67) |
| Ethnicity | ||
| Caucasian | 129 (93%) | 36 (100%) |
| Latin | 10 (7%) | 0 |
| Sexual orientation | ||
| Heterosexual | 62 (45%) | 23 (64%) |
| Homosexual | 72 (51%) | 12 (33%) |
| Bisexual | 5 (4%) | 1 (3%) |
| Stage of the disease | ||
| Primary | 42 (30%) | 9 (25% of the atypical syphilis, 21% of the primary syphilis) |
| Secondary | 84 (60%) | 22 (61% of the atypical syphilis, 26% of the secondary syphilis) |
| Tertiary | 3 (2%) | 2 (5% of the atypical syphilis, 66% of the tertiary syphilis) |
| Neurosyphilis | 10 (7%) | 3 (8% of the atypical syphilis, 30% of the neurosyphilis) |
| HIV status | ||
| Positive | 7 (5%) | 2 (5%) |
| Negative | 132 (95%) | 34 (95%) |
| Serology at diagnosis | ||
| TPHA mean titers (range) | 5120 (80–10,240) | 5120 (640–10,240) |
| VDRL mean titers (range) | 32 (neg-64) | 32 (neg-64) |
| Positive IgM anti-T. pallidum (tested on 98 patients) | 100 (72%) | 26 |
| Bacterial sexually transmitted coinfections (tested on 110 patients) | ||
| Patients positive for at least one pathogen | 48 (44.4%) | 10 (28%) |
| Genital/anal C. trachomatis infections | 4 (3.6%) | 2 (5%) |
| Genital/anal N. gonorrhoeae infections | 2 (1.8%) | 0 |
| Genital/anal infections by Micoplasmataceae | 40 (37.2%) | 10 (28%) |
| Genital T. vaginalis infections | 2 (1.8%) | 0 |
| Other concurrent STIs | ||
| Genital warts | 2 (1.5%) | 0 |
| Genital high-risk HPV infection (tested on 110 patients) | 47 (34%) | 0 |
| Anal high-risk HPV infection (tested on 110 patients) | 15 (11%) | 2 (5%) |
| Oral high-risk HPV infection (tested on 110 patients) | 25 (18%) | 3 (8%) |
| Chronic HBV infection | 1 (0.7%) | 0 |
| Herpes genitalis | 1 (0.7%) | 1 (3%) |
| Monkeypox | 1 (0.7%) | 0 |
| Syphilis reinfection | 9 (6.9%) | 5 (14%) |
| Atypical manifestations | 36 (25.8%) | |
| Atypical Syphilis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient n° | Sex | Age at Diagnosis | Sexual Orientation | Stage of Disease | Clinical Features | HIV Status | Reinfection | VDRL Titer | TPHA Titers |
| 1 | M | 32 | homosexual | PS | oropharyngeal chancre | neg | no | 64 | 10,240 |
| 2 | M | 44 | homosexual | PS | multiple chancres | neg | no | 64 | 2560 |
| 3 | M | 47 | heterosexual | PS | phagedenic chancre | neg | no | neg | 640 |
| 4 | M | 53 | heterosexual | PS | phagedenic chancre + multiple chancres | neg | no | 2 | 2560 |
| 5 | M | 39 | heterosexual | PS | multiple chancres | neg | no | 32 | 1280 |
| 6 | M | 47 | homosexual | PS | oropharyngeal chancre | neg | no | 16 | 2560 |
| 7 | M | 31 | homosexual | PS | multiple chancres | neg | no | 64 | 1280 |
| 8 | M | 53 | heterosexual | PS | Follman balanitis: glans lesions with rosette-like appearance | neg | no | 32 | 10,240 |
| 9 | M | 23 | heterosexual | PS | Follman balanitis | neg | no | 16 | 5120 |
| 10 | M | 31 | homosexual | SS | exclusive inguinal and cervical lymphadenopathy | neg | yes | neg | 10,240 |
| 11 | F | 43 | heterosexual | SS | erythematous–scaly plaques on the lower legs (psoriasiform syphilis) | neg | no | 16 | 10,240 |
| 12 | M | 28 | homosexual | SS | annular lesions of the trunk and arms | neg | yes | 16 | 1280 |
| 13 | M | 54 | heterosexual | SS | annular lesions of the palms | neg | yes | 32 | 10,240 |
| 14 | M | 28 | homosexual | SS | annular lesions of the trunk | pos | no | 16 | 5120 |
| 15 | M | 47 | heterosexual | SS | erythematous macules and papules of the palms and soles and scrotal eczematous plaques | neg | yes | 32 | 10,240 |
| 16 | M | 26 | homosexual | SS | overlapping stages: Follman balanitis and anetoderma | neg | no | 2 | 1280 |
| 17 | F | 47 | heterosexual | SS | few diffuse necrotic ulcerative lesions (lues maligna) | neg | no | 16 | 5120 |
| 18 | F | 34 | bisexual | SS | overlapping stages: chancre of the upper lip, papules of the arms, multiple necrotic ulcerative lesions (lues maligna) of the genitalia | neg | no | 32 | 5120 |
| 19 | F | 27 | heterosexual | SS | exclusive inguinal and cervical lymphadenopathy | neg | no | 64 | 10,240 |
| 20 | F | 31 | heterosexual | SS | itchy erythematous scaly papules and plaques on the trunk (psoriasiform syphilis) | neg | no | 32 | 10,240 |
| 21 | M | 41 | homosexual | SS | erythematous–scaly plaques on the trunk and legs (psoriasiform syphilis) | neg | no | 8 | 2560 |
| 22 | M | 40 | homosexual | SS | diffuse erythematous–scaly plaques (psoriasiform syphilis) | neg | no | 16 | 2560 |
| 23 | F | 38 | heterosexual | SS | diffuse erythematous–scaly plaques (psoriasiform syphilis) | neg | no | 32 | 1280 |
| 24 | M | 30 | homosexual | SS | annular lesions of the trunk | neg | yes | 16 | 5120 |
| 25 | M | 35 | homosexual | SS | annular lesions of the trunk | pos | no | neg | 10,240 |
| 26 | F | 37 | heterosexual | SS | overlapping stages: chancre of the labia majora, papules of the trunk and arms | neg | no | 16 | 10,240 |
| 27 | M | 29 | heterosexual | SS | overlapping stages: Follman balanitis and maculo-papular exanthem | neg | no | 16 | 5120 |
| 28 | M | 50 | heterosexual | SS | erythematous papules of the palms and soles and scrotal eczematous plaques | neg | no | 8 | 10,240 |
| 29 | F | 35 | heterosexual | SS | few diffuse necrotic ulcerative lesions (lues maligna) | neg | no | 8 | 2560 |
| 30 | F | 30 | heterosexual | SS | overlapping stages: chancre of the upper lip, macules and papules of the trunk and arms | neg | no | 32 | 10,240 |
| 31 | M | 40 | heterosexual | SS | itchy erythematous–scaly papules and plaques on the trunk (psoriasiform syphilis) | neg | no | 16 | 1280 |
| 32 | F | 67 | heterosexual | TS | panniculitis | neg | no | 16 | 1280 |
| 33 | F | 59 | heterosexual | TS and NS | erythematous violaceous arciform plaque with peripheral ulceration | neg | no | 64 | 10,240 |
| 34 | M | 59 | heterosexual | NS | vertigo, headache, gait instability, generalized tonic–clonic seizures | neg | no | 64 | 1280 |
| 35 | M | 64 | heterosexual | NS | dizziness, tinnitus, fainting episodes presented with headache, gait instability, and leg numbness | neg | no | 64 | 640 |
| 36 | M | 61 | heterosexual | NS | vertigo, headache, gait instability | neg | no | 32 | 640 |
| Case Series of Atypical Syphilis Manifestations Published in the Past 20 Years | ||||
|---|---|---|---|---|
| First Author | Year | Stage of Disease Studied | N° of Studied Patients | Rate of Atypical Syphilis |
| Towns JM. et al. [35] | 2016 | primary | 183 | 49% |
| Arando M. et al. [37] | 2019 | primary, secondary, and early latent | 274 | 31% |
| Skalnaya A. et al. [32] | 2019 | neurosyphilis | 6 | 50% |
| Sardinha JC. et al. [38] | 2020 | primary and secondary | 19 | 100% |
| Larsen C. et al. [34] | 2020 | primary | 12 | 100% |
| Ramoni S. et al. [33] | 2021 | primary | 244 | 50.4% |
| Gong HZ. et al. [36] | 2021 | primary | 27 | 100% |
| Present study | 2023 | all | 139 | 26% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccarese, G.; Facciorusso, A.; Mastrolonardo, M.; Herzum, A.; Parodi, A.; Drago, F. Atypical Manifestations of Syphilis: A 10-Year Retrospective Study. J. Clin. Med. 2024, 13, 1603. https://doi.org/10.3390/jcm13061603
Ciccarese G, Facciorusso A, Mastrolonardo M, Herzum A, Parodi A, Drago F. Atypical Manifestations of Syphilis: A 10-Year Retrospective Study. Journal of Clinical Medicine. 2024; 13(6):1603. https://doi.org/10.3390/jcm13061603
Chicago/Turabian StyleCiccarese, Giulia, Antonio Facciorusso, Mario Mastrolonardo, Astrid Herzum, Aurora Parodi, and Francesco Drago. 2024. "Atypical Manifestations of Syphilis: A 10-Year Retrospective Study" Journal of Clinical Medicine 13, no. 6: 1603. https://doi.org/10.3390/jcm13061603
APA StyleCiccarese, G., Facciorusso, A., Mastrolonardo, M., Herzum, A., Parodi, A., & Drago, F. (2024). Atypical Manifestations of Syphilis: A 10-Year Retrospective Study. Journal of Clinical Medicine, 13(6), 1603. https://doi.org/10.3390/jcm13061603








