Primary Cutaneous Melanoma—Management in 2024
Abstract
1. Introduction
2. Methods
3. Results
4. Biopsy Technique
5. Histology Report on Cutaneous Melanoma
- a)
- Breslow thickness/depth (Breslow),
- b)
- The presence or absence of ulceration (Ulceration), including its diameter in mm;
- c)
- Subtype of melanoma (Subtype);
- d)
- Margins measured in mm;
- e)
- Growth phase of melanoma;
- f)
- Presence of perineural invasion/neurotropism;
- g)
- Presence of satellites/microsatellites/in-transit metastases;
- h)
- Presence of lymphovascular invasion and extravascular migratory metastases;
- i)
- Tumor-infiltrating lymphocytes,
- j)
- Presence or absence of regression;
- k)
- Mitotic index, using the hot spot method;
6. The BAUSSS Biomarker
7. Excision Margins
7.1. Melanoma In Situ Margins
7.2. Does the Location Change the Margin?
7.3. Do the Subtype and Site Change the Margin?
8. Biopsy and Definitive Excision in a Single Procedure?
Can One Just Do the Excision Biopsy and Nothing Further?
9. When Is a Sentinel Lymph Node Biopsy (SLNB) Considered?
10. SLNB and Age
10.1. Young Patients
10.2. Older Patients
10.3. Suggested Usage of SLNB in 40- to 60-Year-Old Patients
11. Investigations following Diagnosis
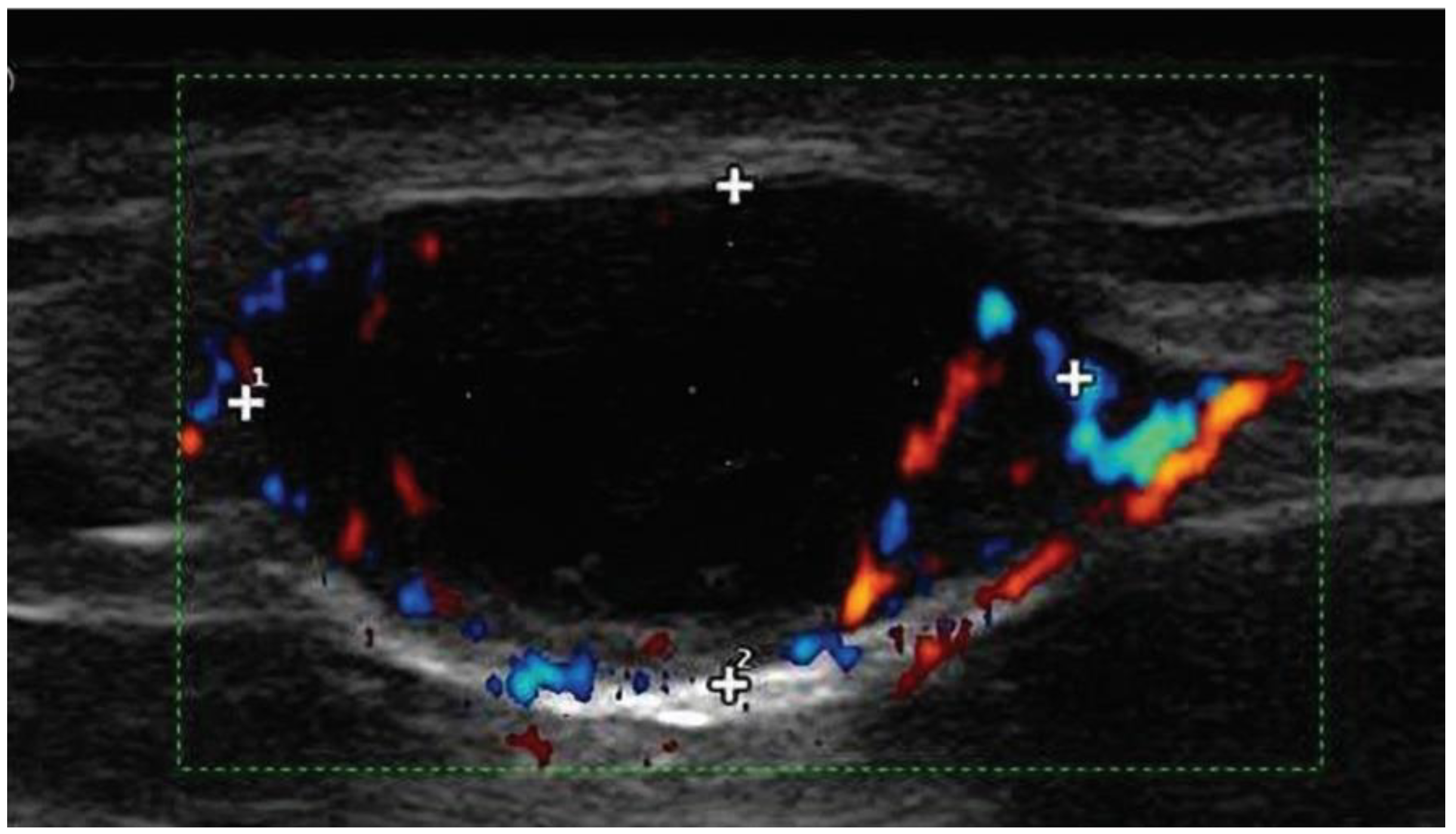
11.1. Ultrasounds of Nodal Basins
11.2. Other Investigations
12. Adjuvant Drug Therapy
12.1. When Are Drug Therapies Indicated?
12.1.1. Nodal Disease
12.1.2. Metastatic Melanoma
12.1.3. High-Risk Primary Cutaneous Melanoma
12.2. BRAF Inhibitors—Including Vemurafenib, Dabrafenib and Encorafenib
12.3. MEK Inhibitors in Combination with BRAF Inhibitors
12.4. PD-1 Drugs: Pembrolizumab, Nivolumab, Atezolizumab and Toripalimab
13. Ipilimumab
| Drug | Indication | Established MSS * or Recurrence Free Advantage | Grade 3 + Adverse Events |
|---|---|---|---|
| Ipilimumab alone | Unresectable or metastatic melanoma | 61% recurrence free at 12 months [106], 34% survival at 3 years [104], 2.9-month median-free survival [105], 43% 2-year survival [101] 65% 5-year recurrence free (Stage III disease) [118] | 46% [106], 43% discontinued [106], 2 deaths [106], 28% [104,105], 54% [118] |
| Ipilimumab and Nivolumab combination | Unresectable or metastatic melanoma | 58% survival at 3 years [104] 11.5 month median progression-free survival [105] 75% recurrence-free survival at 12 months [119] | 59% [104,105,106,107] 71% [119] |
Newer Combination Therapies
14. Total Body Photography (TBP)
15. Follow-Up Appointments
16. Sun Exposure Advice
17. Supplementary Vitamins
18. Psychosocial Support
19. Moving forward—More Trials That Do Not Include SLNB Are Required
20. Limitations
21. Conclusions
22. Dedication
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liszewski, W.; Stewart, J.R.; Vidal, N.Y.; Demer, A.M. Incisional Biopsy Technique Is Associated with Decreased Overall Survival for Cutaneous Melanoma. Dermatol. Surg. 2022, 48, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Kok, Y.; Scott, K.; Pham, A.; Liu, W.; Roberts, H.; Pan, Y.; McLean, C.; Chamberlain, A.; Kelly, J.W.; Mar, V.J. The impact of incomplete clinical information and initial biopsy technique on the histopathological diagnosis of cutaneous melanoma. Australas. J. Dermatol. 2021, 62, e524–e531. [Google Scholar] [CrossRef]
- Restrepo, D.J.; Huayllani, M.T.; Boczar, D.; Sisti, A.; Gabriel, E.; Lemini, R.; Spaulding, A.C.; Bagaria, S.; Manrique, O.J.; Forte, A.J. Biopsy Type Disparities in Patients With Melanoma: Who Receives the Standard of Care? Anticancer Res. 2019, 39, 6359–6363. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Swain, S.; Dowling, J.P.; Wolfe, R.; Simpson, P.; Kelly, J.W. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: Experience of an Australian tertiary referral service. Arch. Dermatol. 2010, 146, 234–239. [Google Scholar] [CrossRef]
- Sharma, K.S.; Lim, P.; Brotherston, M.T. Excision versus incision biopsy in the management of malignant melanoma. J. Dermatol. Treat. 2016, 27, 88–90. [Google Scholar] [CrossRef]
- Jones, S.; Henry, V.; Strong, E.; Sheriff, S.A.; Wanat, K.; Kasprzak, J.; Clark, M.; Shukla, M.; Zenga, J.; Stadler, M.; et al. Clinical Impact and Accuracy of Shave Biopsy for Initial Diagnosis of Cutaneous Melanoma. J. Surg. Res. 2023, 286, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Farberg, A.S.; Rigel, D.S. A comparison of current practice patterns of US dermatologists versus published guidelines for the biopsy, initial management, and follow up of patients with primary cutaneous melanoma. J. Am. Acad. Dermatol. 2016, 75, 1193–1197 e1191. [Google Scholar] [CrossRef]
- Martin, R.C., 2nd; Scoggins, C.R.; Ross, M.I.; Reintgen, D.S.; Noyes, R.D.; Edwards, M.J.; McMasters, K.M. Is incisional biopsy of melanoma harmful? Am. J. Surg. 2005, 190, 913–917. [Google Scholar] [CrossRef]
- Moscarella, E.; Pampena, R.; Palmiotti, G.; Bonamonte, D.; Brancaccio, G.; Piccolo, V.; Longo, C.; Argenziano, G. A meta-analysis on the influence of partial biopsy of primary melanoma on disease recurrence and patient survival. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 279–284. [Google Scholar] [CrossRef]
- Mir, M.; Chan, C.S.; Khan, F.; Krishnan, B.; Orengo, I.; Rosen, T. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J. Am. Acad. Dermatol. 2013, 68, 452–458. [Google Scholar] [CrossRef]
- Saco, M.; Thigpen, J. A retrospective comparison between preoperative and postoperative Breslow depth in primary cutaneous melanoma: How preoperative shave biopsies affect surgical management. J. Drugs Dermatol. 2014, 13, 531–536. [Google Scholar]
- Pflugfelder, A.; Weide, B.; Eigentler, T.K.; Forschner, A.; Leiter, U.; Held, L.; Meier, F.; Garbe, C. Incisional biopsy and melanoma prognosis: Facts and controversies. Clin. Dermatol. 2010, 28, 316–318. [Google Scholar] [CrossRef]
- Monshizadeh, L.; Hanikeri, M.; Beer, T.W.; Heenan, P.J. A critical review of melanoma pathology reports for patients referred to the Western Australian Melanoma Advisory Service. Pathology 2012, 44, 441–447. [Google Scholar] [CrossRef]
- Taylor, L.A.; Eguchi, M.M.; Reisch, L.M.; Radick, A.C.; Shucard, H.; Kerr, K.F.; Piepkorn, M.W.; Knezevich, S.R.; Elder, D.E.; Barnhill, R.L.; et al. Histopathologic synoptic reporting of invasive melanoma: How reliable are the data? Cancer 2021, 127, 3125–3136. [Google Scholar] [CrossRef]
- Kaur, M.R.; Colloby, P.S.; Martin-Clavijo, A.; Marsden, J.R. Melanoma histopathology reporting: Are we complying with the National Minimum Dataset? J. Clin. Pathol. 2007, 60, 1121–1123. [Google Scholar] [CrossRef]
- de Waal, J. Skin tumour specimen shrinkage with excision and formalin fixation-how much and why: A prospective study and discussion of the literature. ANZ J. Surg. 2021, 91, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Kerns, M.J.; Darst, M.A.; Olsen, T.G.; Fenster, M.; Hall, P.; Grevey, S. Shrinkage of cutaneous specimens: Formalin or other factors involved? J. Cutan. Pathol. 2008, 35, 1093–1096. [Google Scholar] [CrossRef]
- El Sharouni, M.A.; Stodell, M.D.; Ahmed, T.; Suijkerbuijk, K.P.M.; Cust, A.E.; Witkamp, A.J.; Sigurdsson, V.; van Diest, P.J.; Scolyer, R.A.; Thompson, J.F.; et al. Sentinel node biopsy in patients with melanoma improves the accuracy of staging when added to clinicopathological features of the primary tumor. Ann. Oncol. 2021, 32, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.A.; Sanders, L.L.; Edwards, L.J.; Seigler, H.F.; Tyler, D.S.; Grichnik, J.M. Identification of higher risk thin melanomas should be based on Breslow depth not Clark level IV. Cancer 2001, 91, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.; Steinman, H.K.; Kyrgidis, A.; Smith, H.; Sladden, M.; Zouboulis, C.C.; Argenziano, G.; Apalla, Z.; Lallas, A.; Longo, C.; et al. Online prediction tools for melanoma survival: A comparison. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Steinman, H.K.; Kyrgidis, A.; Smith, H.; Sladden, M.; Zouboulis, C.C.; Argenziano, G.; Apalla, Z.; Lallas, A.; Longo, C.; et al. Improved methodology in determining melanoma mortality and selecting patients for immunotherapy. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e843–e845. [Google Scholar] [CrossRef]
- Dixon, A.J.; Kyrgidis, A.; Sladden, M.; Nirenberg, A.; Steinman, H.K.; Smith, H.; Zachary, C.B.; Anderson, S.; Leiter-Stöppke, U.; Longo, C.; et al. BAUSSS biomarker further validated as a key risk staging tool for patients with primary melanoma. J. Eur. Acad. Dermatol. Venereol. 2024, 38375764. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.; Skitzki, J.J. Surgical Management of Primary Cutaneous Melanoma. Surg. Clin. N. Am. 2020, 100, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sladden, M.J.; Nieweg, O.E.; Howle, J.; Coventry, B.J.; Thompson, J.F. Updated evidence-based clinical practice guidelines for the diagnosis and management of melanoma: Definitive excision margins for primary cutaneous melanoma. Med. J. Aust. 2018, 208, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Newton-Bishop, J.; A’Hern, R.; Coombes, G.; Timmons, M.; Evans, J.; Cook, M.; Theaker, J.; Fallowfield, M.; O’Neill, T.; et al. Excision margins in high-risk malignant melanoma. N. Engl. J. Med. 2004, 350, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Gillgren, P.; Drzewiecki, K.T.; Niin, M.; Gullestad, H.P.; Hellborg, H.; Mansson-Brahme, E.; Ingvar, C.; Ringborg, U. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: A randomised, multicentre trial. Lancet 2011, 378, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Sladden, M.J. Sufficiency and safety of 2-cm excision margin for stage IIA through stage IIC cutaneous melanoma. Arch. Dermatol. 2012, 148, 1197–1198. [Google Scholar] [CrossRef]
- Kunishige, J.H.; Brodland, D.G.; Zitelli, J.A. Surgical margins for melanoma in situ. J. Am. Acad. Dermatol. 2012, 66, 438–444. [Google Scholar] [CrossRef]
- Felton, S.; Taylor, R.S.; Srivastava, D. Excision Margins for Melanoma In Situ on the Head and Neck. Dermatol. Surg. 2016, 42, 327–334. [Google Scholar] [CrossRef]
- Bigby, M.; Zagarella, S.; Sladden, M.; Popescu, C.M. Time to reconsider the role of sentinel lymph node biopsy in melanoma. J. Am. Acad. Dermatol. 2019, 80, 1168–1171. [Google Scholar] [CrossRef]
- Zitelli, J.A.; Stiegel, E.; Brodland, D.G. The Controversy and Value of Mohs Micrographic Surgery for Melanoma and Melanoma in Situ on the Trunk and Extremities. Dermatol. Surg. 2023, 49, 1061–1065. [Google Scholar] [CrossRef]
- Rubin, A.I. Commentary on Mohs Micrographic Surgery Using MART-1 Immunostaining for Nail Unit Melanoma in Situ. Dermatol. Surg. 2021, 47, 263. [Google Scholar] [CrossRef]
- Phan, K.; Loya, A. Mohs micrographic surgery versus wide local excision for melanoma in situ: Analysis of a nationwide database. Int. J. Dermatol. 2019, 58, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Foulad, D.P.; Doan, L.; Lee, P.K.; Atanaskova Mesinkovska, N. Mohs surgery for the treatment of lentigo maligna and lentigo maligna melanoma—A systematic review. J. Dermatolog Treat. 2021, 32, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Phan, K. Mohs micrographic surgery versus wide local excision for eyelid melanoma: An analysis of a national database. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Puyana, C.; Ham, P.; Tsoukas, M.M. Mohs Micrographic Surgery for the Treatment of External Ear Melanoma: An Outcome Study. Dermatol. Surg. 2020, 46, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Queen, D.; Knackstedt, T.; Polacco, M.A.; Collins, L.K.; Lee, K.; Samie, F.H. Characteristics of non-melanoma skin cancers of the cutaneous perioral and vermilion lip treated by Mohs micrographic surgery. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Woody, M.; Leitenberger, J.; Latour, E.; Bar, A. Invasive Melanoma and Melanoma in Situ Treated with Modified Mohs Micrographic Surgery with En Face Permanent Sectioning: A 10-Year Retrospective Review. Dermatol. Surg. 2020, 46, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Isaq, N.A.; Demer, A.M.; Vidal, N.Y.; Lohman, M.E. Dermatologic surgeons’ approaches to acral lentiginous melanoma: A survey of the American College of Mohs Surgery. Arch. Dermatol. Res. 2023, 316, 17. [Google Scholar] [CrossRef]
- Varey, A.H.R.; Madronio, C.M.; Cust, A.E.; Goumas, C.; Mann, G.J.; Armstrong, B.K.; Scolyer, R.A.; Curtin, A.M.; Thompson, J.F. Poor Adherence to National Clinical Management Guidelines: A Population-Based, Cross-Sectional Study of the Surgical Management of Melanoma in New South Wales, Australia. Ann. Surg. Oncol. 2017, 24, 2080–2088. [Google Scholar] [CrossRef]
- Kelly, J.W.; Henderson, M.A.; Thursfield, V.J.; Slavin, J.; Ainslie, J.; Giles, G.G. The management of primary cutaneous melanoma in Victoria in 1996 and 2000. Med. J. Aust. 2007, 187, 511–514. [Google Scholar] [CrossRef]
- Laeijendecker, A.E.; El Sharouni, M.A.; Sigurdsson, V.; van Diest, P.J. Desmoplastic melanoma: The role of pure and mixed subtype in sentinel lymph node biopsy and survival. Cancer Med. 2020, 9, 671–677. [Google Scholar] [CrossRef]
- Lattanzi, M.; Lee, Y.; Simpson, D.; Moran, U.; Darvishian, F.; Kim, R.H.; Hernando, E.; Polsky, D.; Hanniford, D.; Shapiro, R.; et al. Primary Melanoma Histologic Subtype: Impact on Survival and Response to Therapy. J. Natl. Cancer Inst. 2019, 111, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fong, Z.V.; Tanabe, K.K. Comparison of melanoma guidelines in the U.S.A., Canada, Europe, Australia and New Zealand: A critical appraisal and comprehensive review. Br. J. Dermatol. 2014, 170, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.P.; Thelmo, M.C.; Kim, R.P.; Gokhale, R.; Rhee, J.Y.; Achtem, T.A.; Morita, E.; Allen, R.E., Jr.; Kashani-Sabet, M.; Sagebiel, R.W. Delayed harvesting of sentinel lymph nodes after previous wide local excision of extremity melanoma. Ann. Surg. Oncol. 2003, 10, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Rodgaard, J.C.; Kramer, S.; Stolle, L.B. Sentinel node biopsy (SNB) in malignant melanoma as same day procedure vs delayed procedure: Clinical and economic outcome. J. Plast. Surg. Hand Surg. 2014, 48, 265–269. [Google Scholar] [CrossRef]
- Zijlker, L.P.; Eggermont, A.M.M.; van Akkooi, A.C.J. The end of wide local excision (WLE) margins for melanoma? Eur. J. Cancer 2023, 178, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Rugge, M.; Damiani, G.; De Luca, G.; Zorzi, M.; Fusinato, R.; De Toni, C.; Vecchiato, A.; Del Fiore, P.; Falasco, F.; et al. Impact of Wide Local Excision on Melanoma Patient Survival: A Population-Based Study. Front. Public Health 2022, 10, 806934. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Elashoff, R.; Essner, R.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; et al. Sentinel-node biopsy or nodal observation in melanoma. N. Engl. J. Med. 2006, 355, 1307–1317. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Sladden, M.; Zagarella, S.; Popescu, C.; Bigby, M. When is a sentinel node biopsy indicated for patients with primary melanoma? Comment on the ‘Australian guidelines for the management of cutaneous melanoma’. Australas. J. Dermatol. 2018, 59, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Cochran, A.J.; Thompson, J.F.; Elashoff, R.; Essner, R.; Glass, E.C.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; et al. Sentinel node biopsy for early-stage melanoma: Accuracy and morbidity in MSLT-I, an international multicenter trial. Ann. Surg. 2005, 242, 302–311; discussion 311–313. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Pereiro, C.E.; Zulaica Garate, A.; Garcia-Doval, I. Complications and Sequelae After Sentinel Lymph Node Biopsy in Melanoma: A Retrospective Cohort Study. Actas Dermosifiliogr. 2019, 110, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Moody, J.A.; Ali, R.F.; Carbone, A.C.; Singh, S.; Hardwicke, J.T. Complications of sentinel lymph node biopsy for melanoma—A systematic review of the literature. Eur. J. Surg. Oncol. 2017, 43, 270–277. [Google Scholar] [CrossRef]
- Dixon, A.; Steinman, H.; Anderson, S.; Nirenberg, A.; Dixon, J. Routine usage of sentinel node biopsy in melanoma management must cease. Br. J. Dermatol. 2016, 175, 1340–1341. [Google Scholar] [CrossRef]
- Dixon, A.J.; Kyrgidis, A.; Steinman, H.K.; Dixon, J.B.; Sladden, M.; Garbe, C.; Lallas, A.; Zachary, C.B.; Leiter-Stoppke, U.; Smith, H.; et al. Sentinel lymph node biopsy is unreliable in predicting melanoma mortality for both younger and older patients. J. Eur. Acad. Dermatol. Venereol. 2021. early view. [Google Scholar] [CrossRef]
- Lo, S.N.; Ma, J.; Scolyer, R.A.; Haydu, L.E.; Stretch, J.R.; Saw, R.P.M.; Nieweg, O.E.; Shannon, K.F.; Spillane, A.J.; Ch’ng, S.; et al. Improved Risk Prediction Calculator for Sentinel Node Positivity in Patients With Melanoma: The Melanoma Institute Australia Nomogram. J. Clin. Oncol. 2020, 38, 2719–2727. [Google Scholar] [CrossRef]
- Shannon, A.B.; Sharon, C.E.; Straker, R.J., 3rd; Carr, M.J.; Sinnamon, A.J.; Bogatch, K.; Thaler, A.; Kelly, N.; Vetto, J.T.; Fowler, G.; et al. Sentinel lymph node biopsy in patients with T1a cutaneous malignant melanoma: A multicenter cohort study. J. Am. Acad. Dermatol. 2023, 88, 52–59. [Google Scholar] [CrossRef]
- Zabor, E.C.; Coit, D.; Gershenwald, J.E.; McMasters, K.M.; Michaelson, J.S.; Stromberg, A.J.; Panageas, K.S. Variability in Predictions from Online Tools: A Demonstration Using Internet-Based Melanoma Predictors. Ann. Surg. Oncol. 2018, 25, 2172–2177. [Google Scholar] [CrossRef]
- Ulrich, J.; van Akkooi, A.C.; Eggermont, A.M.; Voit, C.A. Sonographic criteria for diagnosing sentinel node metastases in melanoma patients. Ultraschall Med. 2015, 36, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Oude Ophuis, C.M.C.; Verhoef, C.; Grunhagen, D.J.; Siegel, P.; Schoengen, A.; Rowert-Huber, J.; Eggermont, A.M.M.; Voit, C.A.; van Akkooi, A.C.J. Long-term results of ultrasound guided fine needle aspiration cytology in conjunction with sentinel node biopsy support step-wise approach in melanoma. Eur. J. Surg. Oncol. 2017, 43, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Voit, C.A.; van Akkooi, A.C.J.; Catalano, O.; Eggermont, A.M.M. Pre-SN Ultrasound-FNAC can be Sensitive for Lymph Node Metastases in Melanoma Patients if Performed with the Use of the Berlin Criteria. Ann. Surg. Oncol. 2017, 24, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Voit, C.; Van Akkooi, A.C.; Schafer-Hesterberg, G.; Schoengen, A.; Kowalczyk, K.; Roewert, J.C.; Sterry, W.; Eggermont, A.M. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. J. Clin. Oncol. 2010, 28, 847–852. [Google Scholar] [CrossRef]
- Thompson, J.F.; Haydu, L.E.; Uren, R.F.; Andtbacka, R.H.; Zager, J.S.; Beitsch, P.D.; Agnese, D.M.; Mozzillo, N.; Testori, A.; Bowles, T.L.; et al. Preoperative Ultrasound Assessment of Regional Lymph Nodes in Melanoma Patients Does not Provide Reliable Nodal Staging: Results from a Large Multicenter Trial. Ann. Surg. 2019, 273, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Ipenburg, N.A.; Thompson, J.F.; Uren, R.F.; Chung, D.; Nieweg, O.E. Focused Ultrasound Surveillance of Lymph Nodes Following Lymphoscintigraphy without Sentinel Node Biopsy: A Useful and Safe Strategy in Elderly or Frail Melanoma Patients. Ann. Surg. Oncol. 2019, 26, 2855–2863. [Google Scholar] [CrossRef]
- Froidevaux, S.; Calame-Christe, M.; Schuhmacher, J.; Tanner, H.; Saffrich, R.; Henze, M.; Eberle, A.N. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J. Nucl. Med. 2004, 45, 116–123. [Google Scholar]
- Bleicher, J.; Swords, D.S.; Mali, M.E.; McGuire, L.; Pahlkotter, M.K.; Asare, E.A.; Bowles, T.L.; Hyngstrom, J.R. Recurrence patterns in patients with Stage II melanoma: The evolving role of routine imaging for surveillance. J. Surg. Oncol. 2020, 122, 1770–1777. [Google Scholar] [CrossRef]
- de Oliveira Filho, R.S.; de Oliveira, D.A.; Nisimoto, M.M.; Marti, L.C. A Review of Advanced Cutaneous Melanoma Therapies and Their Mechanisms, from Immunotherapies to Lysine Histone Methyl Transferase Inhibitors. Cancers 2023, 15, 5751. [Google Scholar] [CrossRef]
- Franke, V.; van Akkooi, A.C.J. The extent of surgery for stage III melanoma: How much is appropriate? Lancet Oncol. 2019, 20, e167–e174. [Google Scholar] [CrossRef]
- Kudchadkar, R.R.; Michielin, O.; van Akkooi, A.C.J. Practice-Changing Developments in Stage III Melanoma: Surgery, Adjuvant Targeted Therapy, and Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Bafaloukos, D.; Gogas, H. The treatment of brain metastases in melanoma patients. Cancer Treat. Rev. 2004, 30, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, G.B.; Flaherty, D.C.; Kirchoff, D.D.; Bailey, M.; Vitug, S.; Foshag, L.J.; Faries, M.B.; Bilchik, A.J. Association of Surgical Treatment, Systemic Therapy, and Survival in Patients With Abdominal Visceral Melanoma Metastases, 1965-2014: Relevance of Surgical Cure in the Era of Modern Systemic Therapy. JAMA Surg. 2017, 152, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Hau, H.M.; Tautenhahn, H.M.; Schoenberg, M.B.; Atanasov, G.; Wiltberger, G.; Morgul, M.H.; Uhlmann, D.; Seitz, A.T.; Simon, J.C.; Schmelzle, M.; et al. Liver resection in multimodal concepts improves survival of metastatic melanoma: A single-centre case-matched control study. Anticancer Res. 2014, 34, 6633–6639. [Google Scholar] [PubMed]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur. J. Cancer 2019, 119, 1–10. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Cebon, J.S.; Jameson, M.B.; Fitzharris, B.M.; McNeil, C.M.; Hill, A.G.; Ribas, A.; Atkins, M.B.; Thompson, J.A.; et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): An open-label, phase 1b trial. Lancet Oncol. 2017, 18, 1202–1210. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Long, G.V.; Luke, J.J.; Khattak, M.A.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): Distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 1378–1388. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef] [PubMed]
- McArthur, G.A.; Maio, M.; Arance, A.; Nathan, P.; Blank, C.; Avril, M.F.; Garbe, C.; Hauschild, A.; Schadendorf, D.; Hamid, O.; et al. Vemurafenib in metastatic melanoma patients with brain metastases: An open-label, single-arm, phase 2, multicentre study. Ann. Oncol. 2017, 28, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Long, G.V.; Kurzrock, R.; Kim, K.B.; Arkenau, T.H.; Brown, M.P.; Hamid, O.; Infante, J.R.; Millward, M.; Pavlick, A.C.; et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet 2012, 379, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Long, G.V.; Eroglu, Z.; Infante, J.; Patel, S.; Daud, A.; Johnson, D.B.; Gonzalez, R.; Kefford, R.; Hamid, O.; Schuchter, L.; et al. Long-Term Outcomes in Patients with BRAF V600-Mutant Metastatic Melanoma Who Received Dabrafenib Combined with Trametinib. J. Clin. Oncol. 2018, 36, 667–673. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandala, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dreno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandala, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; Testori, A.; Lorigan, P.C.; et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: Final overall survival results of the randomized BRIM-3 study. Ann. Oncol. 2017, 28, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Ribas, A.; Larkin, J.; Ascierto, P.A.; Hauschild, A.; Thomas, L.; Grob, J.J.; Koralek, D.O.; Rooney, I.; Hsu, J.J.; et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann. Oncol. 2017, 28, 1137–1144. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dreno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandala, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Chiarion-Sileni, V.; et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Flaherty, K.T.; Robert, C.; Arance, A.; de Groot, J.W.B.; Garbe, C.; Gogas, H.J.; Gutzmer, R.; Krajsova, I.; Liszkay, G.; et al. COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib Plus Binimetinib Versus Vemurafenib or Encorafenib in Patients with BRAF V600-Mutant Melanoma. J. Clin. Oncol. 2022, 40, 4178–4188. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Simeone, E.; Ascierto, P.A. Vemurafenib plus cobimetinib in the treatment of mutated metastatic melanoma: The CoBRIM trial. Melanoma Manag. 2015, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Schreuer, M.; Jansen, Y.; Planken, S.; Chevolet, I.; Seremet, T.; Kruse, V.; Neyns, B. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: An open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017, 18, 464–472. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Robert, C.; Carlino, M.S.; McNeil, C.; Ribas, A.; Grob, J.J.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.M.; Blaustein, A.; et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J. Clin. Oncol. 2023, 41, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Yan, X.; Zhou, L.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Mao, L.; Lian, B.; et al. Toripalimab plus axitinib in patients with metastatic mucosal melanoma: 3-year survival update and biomarker analysis. J. Immunother. Cancer 2022, 10, e004036. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhou, Y.; Zhang, G.J.; Wang, Y.W.; Wang, J.G.; Wang, X.H.; Li, Y.F. Successful treatment of metastatic vulvar malignant melanoma with toripalimab: A rare case report and review of the literature. Medicine 2022, 101, e30239. [Google Scholar] [CrossRef]
- Lian, B.; Li, Z.; Wu, N.; Li, M.; Chen, X.; Zheng, H.; Gao, M.; Wang, D.; Sheng, X.; Tian, H.; et al. Phase II clinical trial of neoadjuvant anti-PD-1 (toripalimab) combined with axitinib in resectable mucosal melanoma. Ann. Oncol. 2023, 35, 211–222. [Google Scholar] [CrossRef]
- Lian, B.; Si, L.; Chi, Z.H.; Sheng, X.N.; Kong, Y.; Wang, X.; Tian, H.; Li, K.; Mao, L.L.; Bai, X.; et al. Toripalimab (anti-PD-1) versus high-dose interferon-alpha2b as adjuvant therapy in resected mucosal melanoma: A phase II randomized trial. Ann. Oncol. 2022, 33, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chi, Z.; Guo, J. Toripalimab for the treatment of melanoma. Expert. Opin. Biol. Ther. 2020, 20, 863–869. [Google Scholar] [CrossRef]
- Dummer, R.; Queirolo, P.; Abajo Guijarro, A.M.; Hu, Y.; Wang, D.; de Azevedo, S.J.; Robert, C.; Ascierto, P.A.; Chiarion-Sileni, V.; Pronzato, P.; et al. Atezolizumab, vemurafenib, and cobimetinib in patients with melanoma with CNS metastases (TRICOTEL): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2022, 23, 1145–1155. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAF(V600) mutation-positive advanced melanoma (IMspire150): Second interim analysis of a multicentre, randomised, phase 3 study. Lancet Oncol. 2023, 24, 33–44. [Google Scholar] [CrossRef]
- Patrinely, J.R., Jr.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar] [CrossRef]
- Bajetta, E.; Di Leo, A.; Zampino, M.G.; Sertoli, M.R.; Comella, G.; Barduagni, M.; Giannotti, B.; Queirolo, P.; Tribbia, G.; Bernengo, M.G.; et al. Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. J. Clin. Oncol. 1994, 12, 806–811. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef]
- Arance, A.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Marquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma With Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. 2023, 41, 75–85. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600-Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1428–1438. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Robert, C.; Brase, J.C.; Gusenleitner, D.; Gasal, E.; Garrett, J.; Savchenko, A.; Gorgun, G.; Flaherty, K.T.; Ribas, A.; et al. Spartalizumab or placebo in combination with dabrafenib and trametinib in patients with BRAF V600-mutant melanoma: Exploratory biomarker analyses from a randomized phase 3 trial (COMBI-i). J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Hindie, E. Nivolumab with or without Relatlimab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 1860. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Hodi, F.S.; Long, G.V. Nivolumab with or without Relatlimab in Untreated Advanced Melanoma. Reply. N. Engl. J. Med. 2022, 386, 1860–1861. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Lipson, E.J.; Dummer, R.; Larkin, J.; Long, G.V.; Sanborn, R.E.; Chiarion-Sileni, V.; Dreno, B.; Dalle, S.; Schadendorf, D.; et al. Nivolumab and Relatlimab in Patients with Advanced Melanoma That Had Progressed on Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy: Results From the Phase I/IIa RELATIVITY-020 Trial. J. Clin. Oncol. 2023, 41, 2724–2735. [Google Scholar] [CrossRef]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- Ji-Xu, A.; Dinnes, J.; Matin, R.N. Total body photography for the diagnosis of cutaneous melanoma in adults: A systematic review and meta-analysis. Br. J. Dermatol. 2021, 185, 302–312. [Google Scholar] [CrossRef]
- Hornung, A.; Steeb, T.; Wessely, A.; Brinker, T.J.; Breakell, T.; Erdmann, M.; Berking, C.; Heppt, M.V. The Value of Total Body Photography for the Early Detection of Melanoma: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1726. [Google Scholar] [CrossRef] [PubMed]
- Strunck, J.L.; Smart, T.C.; Boucher, K.M.; Secrest, A.M.; Grossman, D. Improved melanoma outcomes and survival in patients monitored by total body photography: A natural experiment. J. Dermatol. 2020, 47, 342–347. [Google Scholar] [CrossRef]
- Salerni, G.; Carrera, C.; Lovatto, L.; Puig-Butille, J.A.; Badenas, C.; Plana, E.; Puig, S.; Malvehy, J. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J. Am. Acad. Dermatol. 2012, 67, e17–e27. [Google Scholar] [CrossRef]
- Fernandes, N.C.; Marinho Fde, S. Evaluation of outpatient discharge in patients with cutaneous melanoma. Rev. Col. Bras. Cir. 2015, 42, 70–74. [Google Scholar] [CrossRef]
- Jones, M.S.; Torisu-Itakura, H.; Flaherty, D.C.; Schoellhammer, H.F.; Lee, J.; Sim, M.S.; Faries, M.B. Second Primary Melanoma: Risk Factors, Histopathologic Features, Survival, and Implications for Follow-Up. Am. Surg. 2016, 82, 1009–1013. [Google Scholar] [CrossRef]
- Schuurman, M.S.; de Waal, A.C.; Thijs, E.J.M.; van Rossum, M.M.; Kiemeney, L.; Aben, K.K.H. Risk factors for second primary melanoma among Dutch patients with melanoma. Br. J. Dermatol. 2017, 176, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Youlden, D.R.; Brodie, A.M.; DiSipio, T.; Youl, P.; Nair-Shalliker, V.; Baade, P.D. Risk of second primary cancer in survivors of in situ melanoma. J. Investig. Dermatol. 2018, 139, 842–847. [Google Scholar] [CrossRef]
- McCaul, K.A.; Fritschi, L.; Baade, P.; Coory, M. The incidence of second primary invasive melanoma in Queensland, 1982–2003. Cancer Causes Control 2008, 19, 451–458. [Google Scholar] [CrossRef]
- Rueth, N.M.; Cromwell, K.D.; Cormier, J.N. Long-term follow-up for melanoma patients: Is there any evidence of a benefit? Surg. Oncol. Clin. N. Am. 2015, 24, 359–377. [Google Scholar] [CrossRef]
- Diffey, B.L. Time and Place as Modifiers of Personal UV Exposure. Int. J. Environ. Res. Public Health 2018, 15, 1112. [Google Scholar] [CrossRef]
- O’Riordan, D.L.; Steffen, A.D.; Lunde, K.B.; Gies, P. A day at the beach while on tropical vacation: Sun protection practices in a high-risk setting for UV radiation exposure. Arch. Dermatol. 2008, 144, 1449–1455. [Google Scholar] [CrossRef][Green Version]
- Tuchinda, C.; Srivannaboon, S.; Lim, H.W. Photoprotection by window glass, automobile glass, and sunglasses. J. Am. Acad. Dermatol. 2006, 54, 845–854. [Google Scholar] [CrossRef]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef]
- Idorn, L.W.; Philipsen, P.A.; Wulf, H.C. Sun exposure before and after a diagnosis of cutaneous malignant melanoma: Estimated by developments in serum vitamin D, skin pigmentation and interviews. Br. J. Dermatol. 2011, 165, 164–170. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Monazam Esmaeelpour, M.; Naseri, N.; Omari Shekaftik, S. Improving ultraviolet protection properties of cotton textiles using Zinc oxide (ZnO) nanomaterials: An approach for controlling occupational and environmental exposures. Int. J. Environ. Health Res. 2023. [Google Scholar] [CrossRef]
- Kezic, S.; van der Molen, H.F. Occupational skin cancer: Measurements of ultraviolet radiation exposure bring knowledge for prevention. Br. J. Dermatol. 2023, 188, 315–316. [Google Scholar] [CrossRef]
- Modenese, A.; Loney, T.; Rocholl, M.; Symanzik, C.; Gobba, F.; John, S.M.; Straif, K.; Silva Paulo, M. Protocol for a Systematic Review on the Effectiveness of Interventions to Reduce Exposure to Occupational Solar UltraViolet Radiation (UVR) Among Outdoor Workers. Front. Public Health 2021, 9, 756566. [Google Scholar] [CrossRef]
- Beck, N.; Balanay, J.A.G.; Johnson, T. Assessment of occupational exposure to heat stress and solar ultraviolet radiation among groundskeepers in an eastern North Carolina university setting. J. Occup. Environ. Hyg. 2018, 15, 105–116. [Google Scholar] [CrossRef]
- Nakashima, H.; Utsunomiya, A.; Takahashi, J.; Fujii, N.; Okuno, T. Hazard of ultraviolet radiation emitted in gas metal arc welding of mild steel. J. Occup. Health 2016, 58, 452–459. [Google Scholar] [CrossRef]
- Downs, N.J.; Harrison, S.L.; Chavez, D.R.; Parisi, A.V. Solar ultraviolet and the occupational radiant exposure of Queensland school teachers: A comparative study between teaching classifications and behavior patterns. J. Photochem. Photobiol. B 2016, 158, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.K.; Durst, D.A.; Gray, E.; Kwasny, M.; Heo, S.Y.; Banks, A.; Rogers, J.A. Sun exposure reduction by melanoma survivors with wearable sensor providing real-time UV exposure and daily text messages with structured goal setting. Arch. Dermatol. Res. 2021, 313, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Thieden, E.; Philipsen, P.A.; Sandby-Moller, J.; Wulf, H.C. Sunburn related to UV radiation exposure, age, sex, occupation, and sun bed use based on time-stamped personal dosimetry and sun behavior diaries. Arch. Dermatol. 2005, 141, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Thieden, E.; Philipsen, P.A.; Heydenreich, J.; Wulf, H.C. UV radiation exposure related to age, sex, occupation, and sun behavior based on time-stamped personal dosimeter readings. Arch. Dermatol. 2004, 140, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Dixon, B.F. Ultraviolet radiation from welding and possible risk of skin and ocular malignancy. Med. J. Aust. 2004, 181, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Idorn, L.W.; Thieden, E.; Philipsen, P.A.; Wulf, H.C. Influence of having a home garden on personal UVR exposure behavior and risk of cutaneous malignant melanoma in Denmark. Int. J. Cancer 2013, 132, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Bolerazska, B.; Durovcova, E.; Marekova, M. Potential of Using Vitamin D as an Adjuvant Treatment of Malignant Melanoma. Klin. Onkol. 2017, 30, 327–336. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, J.; Van Kelst, S.; Boecxstaens, V.; Stas, M.; Bogaerts, K.; Vanderschueren, D.; Aura, C.; Vandenberghe, K.; Lambrechts, D.; Wolter, P.; et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): A randomized controlled trial. BMC Cancer 2017, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Spadola, G.; Tosti, G.; Mandala, M.; Minisini, A.M.; Queirolo, P.; Aristarco, V.; Baldini, F.; Cocorocchio, E.; Albertazzi, E.; et al. Vitamin D Supplementation and Disease-Free Survival in Stage II Melanoma: A Randomized Placebo Controlled Trial. Nutrients 2021, 13, 1931. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Zegeer, J.; Gerbo, M.; Manrique-Silva, E.; Requena, C.; Traves, V.; Nagore, E. Decreased vitamin D serum levels at melanoma diagnosis are associated with tumor ulceration and high tumor mitotic rate. Melanoma Res. 2019, 29, 664–667. [Google Scholar] [CrossRef]
- Ballotti, R.; Healy, E.; Bertolotto, C. Nicotinamide as a chemopreventive therapy of skin cancers. Too much of good thing? Pigment Cell Melanoma Res. 2019, 32, 601–602. [Google Scholar] [CrossRef]
- Damian, D.L. Nicotinamide for skin cancer chemoprevention. Australas. J. Dermatol. 2017, 58, 174–180. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Dalziell, R.A.; McKenzie, C.A.; Lowe, P.M.; Eris, J.M.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Bielski, V.A.; et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br. J. Dermatol. 2016, 175, 1073–1075. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernandez-Penas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Yelamos, O.; Halpern, A.C.; Weinstock, M.A. Reply to ‘A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients’. Br. J. Dermatol. 2017, 176, 551–552. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Cogorno, L.; Calvi, C.; Marsano, L.A.; Parodi, A. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: A case-control study. Eur. J. Dermatol. 2017, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Scatozza, F.; Moschella, F.; D’Arcangelo, D.; Rossi, S.; Tabolacci, C.; Giampietri, C.; Proietti, E.; Facchiano, F.; Facchiano, A. Nicotinamide inhibits melanoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2020, 39, 211. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Colombo, J.; Trane, L.; Silvestri, F.; Venturi, F.; Zuccaro, B.; Doni, L.; Stanganelli, I.; Covarelli, P. Cutaneous immune-related adverse events and photodamaged skin in patients with metastatic melanoma: Could nicotinamide be useful? Clin. Exp. Dermatol. 2022, 47, 1558–1560. [Google Scholar] [CrossRef]
- Fischbeck, S.; Imruck, B.H.; Blettner, M.; Weyer, V.; Binder, H.; Zeissig, S.R.; Emrich, K.; Friedrich-Mai, P.; Beutel, M.E. Psychosocial Care Needs of Melanoma Survivors: Are They Being Met? PLoS ONE 2015, 10, e0132754. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, S.A.; Shuk, E.; Hay, J.L.; Heneghan, M.; Goulart, J.M.; Panageas, K.; Geller, A.C.; Halpern, A.C. Melanoma survivors: Health behaviors, surveillance, psychosocial factors, and family concerns. Psychooncology 2013, 22, 106–116. [Google Scholar] [CrossRef]
- Rychetnik, L.; McCaffery, K.; Morton, R.; Irwig, L. Psychosocial aspects of post-treatment follow-up for stage I/II melanoma: A systematic review of the literature. Psychooncology 2013, 22, 721–736. [Google Scholar] [CrossRef]
- Tan, J.D.; Butow, P.N.; Boyle, F.M.; Saw, R.P.; O’Reilly, A.J. A qualitative assessment of psychosocial impact, coping and adjustment in high-risk melanoma patients and caregivers. Melanoma Res. 2014, 24, 252–260. [Google Scholar] [CrossRef]
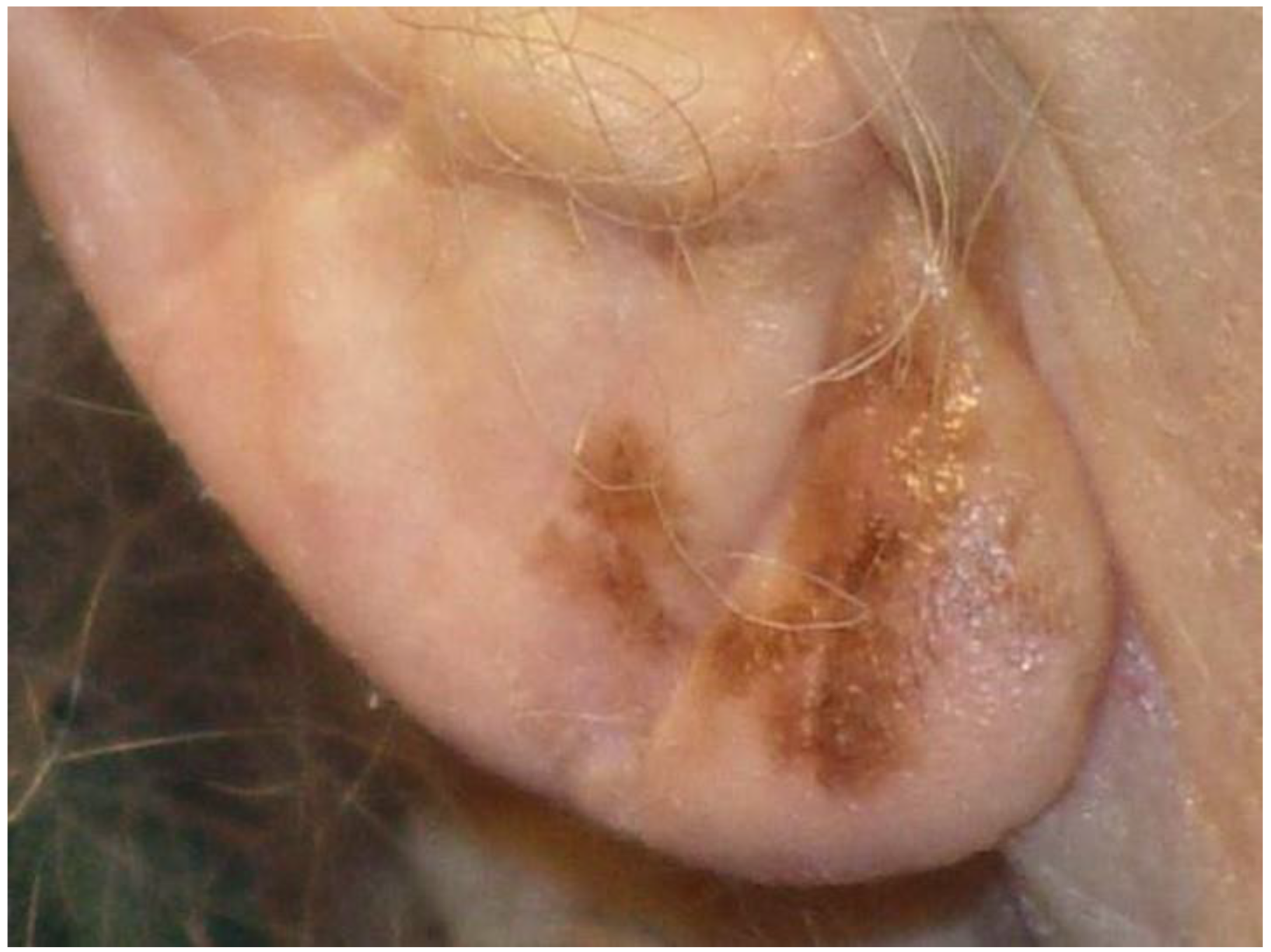

| Breslow Thickness | Radial Margin of Excision | Depth of Excision | |
|---|---|---|---|
| Melanoma in situ (MIS) | Not applicable | 5 to 10 mm * | Subcutaneous tissue |
| Invasive melanoma | Under 1 mm | 10 mm | To but not including deep fascia |
| 1 to 2 mm | 10 to 20 mm ** | ||
| 2 to 4 mm | 20 mm # | ||
| Over 4 mm | 20 mm ## |
| Drug Combination | Indication in Trial | MSS * Established Benefit | Grade 3 + Adverse Events |
|---|---|---|---|
| Dabrafenib and Trametinib | Unresectable stage 3 or 4 melanoma | 72% 12-month survival [85] 44% 3-year survival [86] 28% 5-year survival [87] | 41% [86,88] |
| Dabrafenib and Trametinib | Involvement of lymph nodes following complete resection | 58% 3-year relapse-free survival [88] 86% 3-year overall survival [88] | 41% [88] |
| Dabrafenib alone | Unresectable stage 3 or 4 melanoma | 32% 3-year survival [86] 5.1 month median progression-free survival [80] | 24% [84] |
| Vemurafenib alone | Unresectable stage 3 or 4 melanoma | 65% 12-month survival [85], 17-month median overall survival [89] 15.9-month median overall survival [81] 7.3-month median progression-free survival [90] 17% 4-year survival [91] | 61% [92] 28% [89] 59% [93] |
| Vemurafenib and Cobimetinib | Unresectable or metastatic melanoma | 22.3-month median overall survival [89] 9.9-month median progression-free survival [93] | 75% [92] 37% [89] 65% [93] |
| Encorafenib and Binimetinib | Unresectable or metastatic melanoma | 14.9 months median progression-free survival [90] | 9% increased gammaGT, 6% hypertension, 10% myalgia, 9% arthralgia [90,94] |
| Drug | Indication | MSS */Recurrence Advantage | Grade 3 + Adverse Events |
|---|---|---|---|
| Pembrolizumab | Unresectable or metastatic melanoma | 55% 2-year survival [101] | 32% [101] |
| Pembrolizumab | Lymph node involvement following complete node dissection | 75% 12-month recurrence free [102] | 14.7% [102] |
| Nivolumab | Unresectable or metastatic melanoma | 52% three-year survival [104] 6.9-month median progression-free survival [105] | 21% [104], 22% [105] |
| Nivolumab | Lymph node involvement or metastatic disease following complete resection of lymph node involvement or complete resection of metastatic disease | 70% 12-month recurrence-free survival [106] | 14.4% [106], 10% discontinued [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, A.J.; Sladden, M.; Zouboulis, C.C.; Popescu, C.M.; Nirenberg, A.; Steinman, H.K.; Longo, C.; Dixon, Z.L.; Thomas, J.M. Primary Cutaneous Melanoma—Management in 2024. J. Clin. Med. 2024, 13, 1607. https://doi.org/10.3390/jcm13061607
Dixon AJ, Sladden M, Zouboulis CC, Popescu CM, Nirenberg A, Steinman HK, Longo C, Dixon ZL, Thomas JM. Primary Cutaneous Melanoma—Management in 2024. Journal of Clinical Medicine. 2024; 13(6):1607. https://doi.org/10.3390/jcm13061607
Chicago/Turabian StyleDixon, Anthony Joseph, Michael Sladden, Christos C. Zouboulis, Catalin M. Popescu, Alexander Nirenberg, Howard K. Steinman, Caterina Longo, Zoe Lee Dixon, and Joseph Meirion Thomas. 2024. "Primary Cutaneous Melanoma—Management in 2024" Journal of Clinical Medicine 13, no. 6: 1607. https://doi.org/10.3390/jcm13061607
APA StyleDixon, A. J., Sladden, M., Zouboulis, C. C., Popescu, C. M., Nirenberg, A., Steinman, H. K., Longo, C., Dixon, Z. L., & Thomas, J. M. (2024). Primary Cutaneous Melanoma—Management in 2024. Journal of Clinical Medicine, 13(6), 1607. https://doi.org/10.3390/jcm13061607










