The Reasons for the Low Uptake of New Antidiabetic Drugs with Cardiovascular Effects—A Family Doctor Perspective
Abstract
:1. Introduction
2. Hesitancy in Delivering a New Antidiabetic Drug Treatment Strategy Related to Postulates of Clinical Inertia
3. Trends in the ADA/EASD Guidelines on the T2D Patient Management Strategy and the Effect on New Antidiabetic Drug Uptake
4. T2D Patient Complexity and Endeavor towards Precision Medicine
5. Discussion
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2D | type 2 diabetes |
| CV | cardiovascular |
| GLP-1ra | glucagon-like peptide 1 receptor agonists |
| SGLT-2in | sodium-glucose cotransporter-2 inhibitors |
| CVD | cardiovascular disease |
| ADA | American Diabetes Association |
| EASD | European Association for the Study of Diabetes |
| HbA1c | hemoglobin A1c |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| CAD | coronary artery disease |
| ASCVD | atherosclerotic cardiovascular disease |
| CVOT’s | cardiovascular outcome trials |
| CHF | chronic heart failure |
| CKD | chronic kidney disease |
| AI | Artificial Intelligence |
| DKA | diabetic ketoacidosis |
| PS | propensity score |
References
- Phillips, L.S.; Branch, W.T.; Cook, C.B.; Doyle, J.P.; El-Kebbi, I.M.; Gallina, D.L.; Miller, C.D.; Ziemer, D.C.; Barnes, C.S. Clinical Inertia. Ann. Intern. Med. 2001, 135, 825–834. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Sperl-Hillen, J.M.; Johnson, P.E.; Rush, W.A.; Biltz, G. Clinical Inertia and Outpatient Medical Errors. In Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology); Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2005. [Google Scholar]
- Byrnes, P.D. Why Haven’t I Changed That? Therapeutic Inertia in General Practice. Aust. Fam. Physician 2011, 40, 24–28. [Google Scholar]
- Lavoie, K.L.; Rash, J.A.; Campbell, T.S. Changing Provider Behavior in the Context of Chronic Disease Management: Focus on Clinical Inertia. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Gomes, M.B.; Pocock, S.; Shestakova, M.V.; Pintat, S.; Fenici, P.; Hammar, N.; Medina, J. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes. Metab. 2018, 20, 427–437. [Google Scholar] [CrossRef]
- Allen, J.D.; Curtiss, F.R.; Fairman, K.A. Nonadherence, Clinical Inertia, or Therapeutic Inertia? J. Manag. Care Pharm. 2009, 15, 690–695. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234, Correction in Lancet 2023, 402, 1132. [Google Scholar] [CrossRef] [PubMed]
- de Belvis, A.G.; Pelone, F.; Biasco, A.; Ricciardi, W.; Volpe, M. Can Primary Care Professionals’ Adherence to Evidence Based Medicine Tools Improve Quality of Care in Type 2 Diabetes Mellitus? A Systematic Review. Diabetes Res. Clin. Pract. 2009, 85, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.A.; Charpentier, G.; Doggen, K.; Kuss, O.; Lindblad, U.; Kellner, C.; Nolan, J.; Pazderska, A.; Rutten, G.; Trento, M.; et al. Quality of care of people with type 2 diabetes in eight European countries: Findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care 2013, 36, 2628–2638. [Google Scholar] [CrossRef]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef]
- Jing, X.; Chen, J.; Dong, Y.; Han, D.; Zhao, H.; Wang, X.; Gao, F.; Li, C.; Cui, Z.; Liu, Y.; et al. Related factors of quality of life of type 2 diabetes patients: A systematic review and meta-analysis. Health Qual. Life Outcomes 2018, 16, 189. [Google Scholar] [CrossRef]
- Rutten, G.E.H.M.; Alzaid, A. Person-centred type 2 diabetes care: Time for a paradigm shift. Lancet Diabetes Endocrinol. 2018, 6, 264–266. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Mitchell, S.; Malanda, B.; Damasceno, A.; Eckel, R.H.; Gaita, D.; Kotseva, K.; Januzzi, J.L.; Mensah, G.; Plutzky, J.; Prystupiuk, M.; et al. Roadmap on the Prevention of Cardiovascular Disease among People Living with Diabetes. Glob. Heart 2019, 14, 215–240. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, S.; Aggarwal, R.; Chauhan, M.K. Diabetes and cardiovascular disease: Inter-relation of risk factors and treatment. Future J. Pharm. Sci. 2020, 6, 130. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Schernthaner, G.; Shehadeh, N.; Ametov, A.S.; Bazarova, A.V.; Ebrahimi, F.; Fasching, P.; Janež, A.; Kempler, P.; Konrāde, I.; Lalić, N.M.; et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 185. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Bejan-Angoulvant, T.; Saadatian-Elahi, M.; Lafont, S.; Bergeonneau, C.; Kassaï, B.; Erpeldinger, S.; Wright, J.M.; Gueyffier, F.; Cornu, C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. BMJ 2011, 343, d4169. [Google Scholar] [CrossRef]
- Khunti, K.; Davies, M.J. Clinical Inertia Versus Overtreatment in Glycaemic Management. Lancet Diabetes Endocrinol. 2018, 6, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Mata-Cases, M.; Mauricio, D.; Real, J.; Vlacho, B.; Romera-Liebana, L.; Molist-Brunet, N.; Cedenilla, M.; Franch-Nadal, J. Potential Risk of Overtreatment in Patients with Type 2 Diabetes Aged 75 Years or Older: Data from a Population Database in Catalonia, Spain. J. Clin. Med. 2022, 11, 5134. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, J.A.; Cánovas, G.; Durán, A. Factors Associated with Adherence to Clinical Practice Guidelines for Patients with Type 2 Diabetes Mellitus: Results of a Spanish Delphi Consensus. J. Diabetes Res. 2021, 2021, 9970859. [Google Scholar] [CrossRef]
- Pantalone, K.M.; Wells, B.J.; Chagin, K.M.; Ejzykowicz, F.; Yu, C.; Milinovich, A.; Bauman, J.M.; Kattan, M.W.; Rajpathak, S.; Zimmerman, R.S. Intensification of Diabetes Therapy and Time Until A1C Goal Attainment Among Patients with Newly Diagnosed Type 2 Diabetes Who Fail Metformin Monotherapy within a Large Integrated Health System. Diabetes Care 2016, 39, 1527–1534. [Google Scholar] [CrossRef]
- Khunti, K.; Wolden, M.L.; Thorsted, B.L.; Andersen, M.; Davies, M.J. Clinical Inertia in People with Type 2 Diabetes: A Retrospective Cohort Study of More Than 80,000 People. Diabetes Care 2013, 36, 3411–3417. [Google Scholar] [CrossRef] [PubMed]
- Suraci, C.; Mulas, F.; Rossi, M.C.; Gentile, S.; Giorda, C.B. Management of Newly Diagnosed Patients with Type 2 Diabetes: What Are the Attitudes of Physicians? A SUBITO!AMD Survey on the Early Diabetes Treatment in Italy. Acta Diabetol. 2012, 49, 429–433. [Google Scholar] [CrossRef]
- Pawaskar, M.; Bilir, S.P.; Kowal, S.; Li, Q.; Weiss, T.; Davies, G. Cost-effectiveness of intensification with SGLT2 inhibitors for type 2 diabetes. Am. J. Manag. Care 2021, 27, e269–e277. [Google Scholar] [CrossRef]
- Farcher, R.; Graber, S.M.; Thüring, N.; Blozik, E.; Huber, C.A. Does the Implementation of an Incentive Scheme Increase Adherence to Diabetes Guidelines? A Retrospective Cohort Study of Managed Care Enrollees. BMC Health Serv. Res. 2023, 3, 707. [Google Scholar] [CrossRef]
- Höglinger, M.; Wirth, B.; Carlander, M.; Caviglia, C.; Frei, C.; Rhomberg, B.; Rohrbasser, A.; Trottmann, M.; Eichler, K. Impact of a diabetes disease management program on guideline-adherent care, hospitalization risk and health care costs: A propensity score matching study using real-world data. Eur. J. Health Econ. 2023, 24, 469–478. [Google Scholar] [CrossRef]
- Bosnić, Z.; Babič, F.; Anderková, V.; Štefanić, M.; Wittlinger, T.; Majnarić, L.T. A Critical Appraisal of the Diagnostic and Prognostic Utility of the Anti-Inflammatory Marker IL-37 in a Clinical Setting: A Case Study of Patients with Diabetes Type 2. Int. J. Environ. Res. Public Health 2023, 20, 3695. [Google Scholar] [CrossRef]
- Bosnić, Z.; Babič, F.; Wittlinger, T.; Anderková, V.; Šahinović, I.; Majnarić, L.T. Influence of Age, Gender, Frailty, and Body Mass Index on Serum IL-17a Levels in Mature Type 2 Diabetic Patients. Med. Sci. Monit. 2023, 29, e940128. [Google Scholar] [CrossRef]
- Qumseya, B.; Goddard, A.; Qumseya, A.; Estores, D.; Draganov, P.V.; Forsmark, C. Barriers to Clinical Practice Guideline Implementation among Physicians: A Physician Survey. Int. J. Gen. Med. 2021, 14, 7591–7598. [Google Scholar] [CrossRef]
- Lugtenberg, M.; Burgers, J.S.; Westert, G.P. Effects of Evidence-Based Clinical Practice Guidelines on Quality of Care: A Systematic Review. Qual. Saf. Health Care 2009, 18, 385–392. [Google Scholar] [CrossRef]
- Wang, W.; Choi, D.; Yu, C.H. Effective Web-Based Clinical Practice Guidelines Resources: Recommendations from a Mixed Methods Usability Study. BMC Prim. Care 2023, 24, 29. [Google Scholar] [CrossRef]
- De Angelis, G.; Davies, B.; King, J.; McEwan, J.; Cavallo, S.; Loew, L.; Wells, G.A.; Brosseau, L. Information and Communication Technologies for the Dissemination of Clinical Practice Guidelines to Health Professionals: A Systematic Review. JMIR Med. Educ. 2016, 2, e16. [Google Scholar] [CrossRef]
- Lichtner, G.; Spies, C.; Jurth, C.; Bienert, T.; Mueller, A.; Kumpf, O.; Piechotta, V.; Skoetz, N.; Nothacker, M.; Boeker, M.; et al. Automated Monitoring of Adherence to Evidenced-Based Clinical Guideline Recommendations: Design and Implementation Study. J. Med. Internet Res. 2023, 25, e41177. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Mitchell, G.; Rich, J.; Best, M. Definition of Whole Person Care in General Practice in the English Language Literature: A Systematic Review. BMJ Open 2018, 8, e023758. [Google Scholar] [CrossRef] [PubMed]
- Vlacho, B.; Simarro, F.L.; Mata-Cases, M.; Miravet, S.; Escribano-Serrano, J.; Asensio, D.; Cortes, X.; Franch-Nadal, J. Adherence to antidiabetic treatment among patients managed in primary care centres in Spain: The INTENSE study. Prim. Care Diabetes 2022, 16, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Dankers, M.; van den Berk-Bulsink, M.J.E.; van Dalfsen-Slingerland, M.; Nelissen-Vrancken, H.J.M.G.; Mantel-Teeuwisse, A.K.; van Dijk, L. Non-Adherence to Guideline Recommendations for Insulins: A Qualitative Study Amongst Primary Care Practitioners. BMC Prim. Care 2022, 23, 150. [Google Scholar] [CrossRef]
- Holmes-Truscott, E.; Blackberry, I.; O’Neal, D.N.; Furler, J.S.; Speight, J. Willingness to Initiate Insulin among Adults with Type 2 Diabetes in Australian Primary Care: Results from the Stepping up Study. Diabetes Res. Clin. Pract. 2016, 114, 126–135. [Google Scholar] [CrossRef]
- Albarracin, D.; Shavitt, S. Attitudes and Attitude Change. Annu. Rev. Psychol. 2018, 69, 299–327. [Google Scholar] [CrossRef]
- Wangler, J.; Jansky, M. What Is the Significance of Guidelines in the Primary Care Setting?: Results of an Exploratory Online Survey of General Practitioners in Germany. Wien. Med. Wochenschr. 2021, 171, 321–329. [Google Scholar] [CrossRef]
- Wang, T.; Tan, J.B.; Liu, X.L.; Zhao, I. Barriers and Enablers to Implementing Clinical Practice Guidelines in Primary Care: An Overview of Systematic Reviews. BMJ Open 2023, 13, e062158. [Google Scholar] [CrossRef]
- Rushforth, B.; McCrorie, C.; Glidewell, L.; Midgley, E.; Foy, R. Barriers to Effective Management of Type 2 Diabetes in Primary Care: Qualitative Systematic Review. Br. J. Gen. Pract. 2016, 66, e114–e127. [Google Scholar] [CrossRef]
- Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Marx, N.; Johansen, O.E.; Inzucchi, S.E.; Rosenstock, J.; George, J.T. FDA Guidance on Antihyperglyacemic Therapies for Type 2 Diabetes: One Decade Later. Diabetes Obes. Metab. 2019, 1, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.R.; Janus, C.; Jensen, S.B.K.; Juhl, C.R.; Olsen, L.M.; Christensen, R.M.; Svane, M.S.; Bandholm, T.; Bojsen-Møller, K.N.; Blond, M.B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Jabbour, S.; Cos, X.; Mudaliar, S.; Mende, C.; Bonaca, M.; Fioretto, P. Sodium-Glucose Co-Transporter-2 Inhibitors in Patients with Type 2 Diabetes: Barriers and Solutions for Improving Uptake in Routine Clinical Practice. Diabetes Obes. Metab. 2022, 24, 1187–1196. [Google Scholar] [CrossRef]
- Young, K.G.; McInnes, E.H.; Massey, R.J.; Kahkoska, A.R.; Pilla, S.J.; Raghavan, S.; Stanislawski, M.A.; Tobias, D.K.; McGovern, A.P.; Dawed, A.Y.; et al. Treatment Effect Heterogeneity Following Type 2 Diabetes Treatment with GLP1-Receptor Agonists and SGLT2-inibitors: A Systematic Review. Commun. Med. 2023, 3, 131. [Google Scholar] [CrossRef]
- Melo, M.; Gavina, C.; Silva-Nunes, J.; Andrade, L.; Carvalho, D. Heterogeneity Amongst GLP-1 RA Cardiovascular Outcome Trials Results: Can Definition of Established Cardiovascular Disease Be the Missing Link? Diabetol. Metab. Syndr. 2021, 13, 81. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Bilić Ćurčić, I.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors-Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef] [PubMed]
- Tilinca, M.C.; Tiuca, R.A.; Tilea, I.; Varga, A. The SGLT-2 Inhibitors in Personalized Therapy of Diabetes Mellitus Patients. J. Pers. Med. 2021, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Adams, A.S.; Trinacty, C.M.; Zhang, F.; Kleinman, K.; Soumerai, S.B.; Meigs, J.B.; Ross-Degnan, D. Relationship between Patient Medication Adherence and Subsequent Clinical Inertia in Type 2 Diabetes Glycemic Management. Diabetes Care 2007, 30, 807–812. [Google Scholar] [CrossRef]
- Khunti, K.; Aroda, V.R.; Bhatt, D.L.; Bozkurt, B.; Buse, J.B.; Heerspink, H.L.; Inzucchi, S.E.; Lam, C.S.P.; Marx, N.; McMurray, J.J.V.; et al. Re-examining the widespread policy of stopping sodium-glucose cotransporter-2 inhibitors during acute illness: A perspective based on the updated evidence. Diabetes Obes. Metab. 2022, 24, 2071–2080. [Google Scholar] [CrossRef]
- Faillie, J.L. Pharmacological aspects of the safety of gliflozins. Pharmacol. Res. 2017, 118, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.E.; Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. SGLT2-inibition increases total, LDL, and HDL cholesterol and lowers triglycerides: Meta-analyses of 60 randomized trials, overall and by dose, ethnicity, and drug type. Atherosclerosis 2023, 9, 117236. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Procino, F.; Sardone, R.; Trimboli, P.; Giannelli, G. Generalizability of Sodium-Glucose Co-Transporter-2 Inhibitors Cardiovascular Outcome Trials to the Type 2 Diabetes Population: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2020, 19, 87. [Google Scholar] [CrossRef]
- Schnell, O.; Battelino, T.; Bergenstal, R.; Birkenfeld, A.L.; Ceriello, A.; Cheng, A.; Davies, M.; Edelman, S.; Forst, T.; Giorgino, F.; et al. CVOT Summit 2022 Report: New cardiovascular, kidney, and glycemic outcomes. Cardiovasc. Diabetol. 2023, 22, 59. [Google Scholar] [CrossRef]
- Goldman, A.; Fishman, B.; Twig, G.; Raschi, E.; Cukierman-Yaffe, T.; Moshkovits, Y.; Pomerantz, A.; Ben-Zvi, I.; Dankner, R.; Maor, E. The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥75 years): A retrospective, pharmacovigilance study. Cardiovasc. Diabetol. 2023, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, M.; Zegers-van Schaick, J.M.; Westert, G.P.; Burgers, J.S. Why Don’t Physicians Adhere to Guideline Recommendations in Practice? An Analysis of Barriers among Dutch General Practitioners. Implement. Sci. 2009, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Woo, J. Management of comorbidities in older persons with type 2 diabetes. J. Am. Med. Dir. Assoc. 2017, 18, 639–645. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Harris, K.; Woodward, M.; Chalmers, J.; Cooper, M.; Hamet, P.; Harrap, S.; Heller, S.; MacMahon, S.; Mancia, G.; et al. The Impact of Frailty on the Effectiveness and Safety of Intensive Glucose Control and Blood Pressure-Lowering Therapy for People with Type 2 Diabetes: Results From the ADVANCE Trial. Diabetes Care 2021, 44, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Down, S.; Brown, P.; Puttanna, A.; Sinclair, A. Diabetes and Frailty: An Expert Consensus Statement on the Management of Older Adults with Type 2 Diabetes. Diabetes Ther. 2021, 12, 1227–1247. [Google Scholar] [CrossRef]
- Abd Ghafar, M.Z.A.; O’Donovan, M.; Sezgin, D.; Moloney, E.; Rodríguez-Laso, Á.; Liew, A.; O’Caoimh, R. Frailty and Diabetes in Older Adults: Overview of Current Controversies and Challenges in Clinical Practice. Front. Clin. Diabetes Healthc. 2022, 3, 895313. [Google Scholar] [CrossRef]
- Kondo, T.; Adachi, T.; Kobayashi, K.; Okumura, T.; Izawa, H.; Murohara, T.; McMurray, J.J.V.; Yamada, S. Physical Frailty and Use of Guideline-Recommended Drugs in Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Heart Assoc. 2023, 12, e026844. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Nixon, A.C.; Bampouras, T.M.; Pendleton, N.; Woywodt, A.; Mitra, S.; Dhaygude, A. Frailty and Chronic Kidney Disease: Current Evidence and Continuing Uncertainties. Clin. Kidney J. 2018, 11, 236–245. [Google Scholar] [CrossRef]
- Kleipool, E.E.; Hoogendijk, E.O.; Trappenburg, M.C.; Handoko, M.L.; Huisman, M.; Peters, M.J.; Muller, M. Frailty in Older Adults with Cardiovascular Disease: Cause, Effect or Both? Aging Dis. 2018, 9, 489–497. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Emmerton, D.; Sinclair, A.J. Impact of Frailty Metabolic Phenotypes on the Management of Older People with Type 2 Diabetes Mellitus. Geriatr. Gerontol. Int. 2021, 21, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex Differences in Frailty: A Systematic Review and Meta-Analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; Legault, V.; Li, Q.; Fried, L.P.; Ferrucci, L. Men Sustain Higher Dysregulation Levels Than Women without Becoming Frail. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; O’Neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Habecker, B.A.; Camacho, S.A.; Roberts Davis, M.; Gupta, N.; Hiatt, S.O.; Medysky, M.E.; Purnell, J.Q.; Winters-Stone, K.; Lee, C.S. Characterizing Sex Differences in Physical Frailty Phenotypes in Heart Failure. Circ. Heart Fail. 2021, 14, e008076. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Lee, M.C.; Cheng, Y.H.; Ma, T.; Chen, M.C.; Yang, T.Y.; Jong, G.P. The Association between SGLT2 Inhibitors and New-Onset Acute Coronary Syndrome in the Elderly: A Population-Based Longitudinal Cohort Study. Diabetol. Metab. Syndr. 2023, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joseph, P.; McMurray, J.J.V.; Rouleau, J.; Maggioni, A.P.; Lanas, F.; Sharma, S.K.; Núñez, J.; Mohan, B.; Celik, A.; et al. Frailty and outcomes in heart failure patients from high-, middle-, and low-income countries. Eur. Heart J. 2023, 44, 4435–4444. [Google Scholar] [CrossRef]
- Kutz, A.; Kim, D.H.; Wexler, D.J.; Liu, J.; Schneeweiss, S.; Glynn, R.J.; Patorno, E. Comparative Cardiovascular Effectiveness and Safety of SGLT-2 Inhibitors, GLP-1 Receptor Agonists, and DPP-4 Inhibitors According to Frailty in Type 2 Diabetes. Diabetes Care 2023, 46, 2004–2014. [Google Scholar] [CrossRef]
- Strain, W.; Griffiths, J. A Systematic Review and Meta-Analysis of the Impact of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on Cardiovascular Outcomes in Biologically Healthy Older Adults. Br. J. Diabetes 2021, 21, 30–35. [Google Scholar] [CrossRef]
- Karagiannis, T.; Tsapas, A.; Athanasiadou, E.; Avgerinos, I.; Liakos, A.; Matthews, D.R.; Bekiari, E. GLP-1 Receptor Agonists and SGLT2 Inhibitors for Older People with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2021, 174, 108737. [Google Scholar] [CrossRef]
- Vart, P.; Butt, J.H.; Jongs, N.; Schechter, M.; Chertow, G.M.; Wheeler, D.C.; Pecoits-Filho, R.; Langkilde, A.M.; Correa-Rotter, R.; Rossing, P.; et al. Efficacy and Safety of Dapagliflozin in Patients with Chronic Kidney Disease Across the Spectrum of Frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad181. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Miksza, J.; Zaccardi, F.; Lawson, C.; Nixon, A.C.; Young, H.M.L.; Khunti, K.; Smith, A.C. Associations between frailty trajectories and cardiovascular, renal, and mortality outcomes in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2022, 13, 2426–2435. [Google Scholar] [CrossRef]
- Kennard, A.; Glasgow, N.; Rainsford, S.; Talaulikar, G. Frailty in chronic kidney disease: Challenges in nephrology practice. A review of current literature. Intern. Med. J. 2023, 53, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision medicine in diabetes: A Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 1671–1693. [Google Scholar] [CrossRef]
- Griffin, S. Diabetes precision medicine: Plenty of potential, pitfalls and perils but not yet ready for prime time. Diabetologia 2022, 65, 1913–1921. [Google Scholar] [CrossRef]
- Bosnić, Z.; Yildirim, P.; Babič, F.; Šahinović, I.; Wittlinger, T.; Martinović, I.; Majnaric, L.T. Clustering Inflammatory Markers with Sociodemographic and Clinical Characteristics of Patients with Diabetes Type 2 Can Support Family Physicians’ Clinical Reasoning by Reducing Patients’ Complexity. Healthcare 2021, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R. Precision Medicine in Diabetes, Current Research and Future Perspectives. J. Pers. Med. 2022, 12, 1233. [Google Scholar] [CrossRef]
- Zoungas, S.; Woodward, M.; Li, Q.; Cooper, M.E.; Hamet, P.; Harrap, S.; Heller, S.; Marre, M.; Patel, A.; Poulter, N.; et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014, 57, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kaptoge, S.; Hageman, S.H.J.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Sun, L.; Arnlov, J.; Bozic, M.; et al. Including measures of chronic kidney disease to improve cardiovascular risk prediction by SCORE2 and SCORE2-OP. Eur. J. Prev. Cardiol. 2023, 30, 8–16. [Google Scholar] [CrossRef]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2022, 102, 974–989. [Google Scholar] [CrossRef]
- SCORE2-Diabetes Working Group and the ESC Cardiovascular Risk Collaboration. SCORE2-Diabetes: 10-year cardiovascular risk estimation in type 2 diabetes in Europe. Eur. Heart J. 2023, 44, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Cuenca, A.M.; Mata-Cases, M.; Franch-Nadal, J.; Mauricio, D.; Orozco-Beltrán, D.; Consuegra-Sánchez, L. Half of patients with type 2 diabetes mellitus are at very high cardiovascular risk according to the ESC/EASD: Data from a large Mediterranean population. Eur. J. Prev. Cardiol. 2022, 28, e32–e34. [Google Scholar] [CrossRef] [PubMed]
- Galbete, A.; Tamayo, I.; Librero, J.; Enguita-Germán, M.; Cambra, K.; Ibáñez-Beroiz, B.; CONCEPT Group. Cardiovascular risk in patients with type 2 diabetes: A systematic review of prediction models. Diabetes Res. Clin. Pract. 2022, 184, 109089. [Google Scholar] [CrossRef] [PubMed]
- Dziopa, K.; Asselbergs, F.W.; Gratton, J.; Chaturvedi, N.; Schmidt, A.F. Cardiovascular Risk Prediction in Type 2 Diabetes: A Comparison of 22 Risk Scores in Primary Care Settings. Diabetologia 2022, 65, 644–656. [Google Scholar] [CrossRef]
- Rydén, L.; Ferrannini, G.; Standl, E. Risk prediction in patients with diabetes: Is SCORE 2D the perfect solution? Eur. Heart J. 2023, 44, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, D.; Tang, O.; McEvoy, J.W.; Echouffo-Tcheugui, J.B.; Christenson, R.H.; Selvin, E. Subclinical Cardiovascular Disease in US Adults with and without Diabetes. J. Am. Heart Assoc. 2023, 12, e029083. [Google Scholar] [CrossRef]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front. Endocrinol. 2018, 9, 2. [Google Scholar] [CrossRef]
- van Bussel, E.F.; Richard, E.; Busschers, W.B.; Steyerberg, E.W.; van Gool, W.A.; Moll van Charante, E.P.; Hoevenaar-Blom, M.P. A Cardiovascular Risk Prediction Model for Older People: Development and Validation in a Primary Care Population. J. Clin. Hypertens. 2019, 21, 1145–1152. [Google Scholar] [CrossRef]
- van Bussel, E.F.; Hoevenaar-Blom, M.P.; Poortvliet, R.K.E.; Gussekloo, J.; van Dalen, J.W.; van Gool, W.A.; Richard, E.; Moll van Charante, E.P. Predictive value of traditional risk factors for cardiovascular disease in older people: A systematic review. Prev. Med. 2020, 132, 105986. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Balistreri, C.R.; De Rosa, S.; Muscoli, S.; Selvaggio, S.; Selvaggio, G.; Ferdinandy, P.; De Caterina, R. Impact of Sex Differences and Diabetes on Coronary Atherosclerosis and Ischemic Heart Disease. J. Clin. Med. 2019, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, A., Jr.; Ingham, S.J.; Paes, Â.T.; Tinoco, E.; Greco, A.; Zanata, N.; Pintarelli, V.; Elber, I.; Borges, J.; Camargo Carvalho, A.C. Frailty predictors and outcomes among older patients with cardiovascular disease: Data from Fragicor. Arch. Gerontol. Geriatr. 2015, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Galea, S. Will Precision Medicine Improve Population Health? JAMA 2016, 316, 1357–1358. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.R.; Angrisani, M.; Troxel, W.M.; Gutsche, T.; Ortega, E.; Jain, M.; Boch, A.; Kapteyn, A. American Life in Realtime: A benchmark registry of health data for equitable precision health. Nat. Med. 2023, 29, 283–286. [Google Scholar] [CrossRef]
- Majnarić, L.T.; Babič, F.; O’Sullivan, S.; Holzinger, A. AI and Big Data in Healthcare: Towards a More Comprehensive Research Framework for Multimorbidity. J. Clin. Med. 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, N.; Marston, L.; Walters, K.; Wood, J.; Reynolds, M.R.; Petersen, I. Making inferences on treatment effects from real world data: Propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013, 347, f6409. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Wyss, R.; Layton, J.B.; Stürmer, T. Propensity score methods for confounding control in nonexperimental research. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 604–611. [Google Scholar] [CrossRef]
- Stukel, T.A.; Fisher, E.S.; Wennberg, D.E.; Alter, D.A.; Gottlieb, D.J.; Vermeulen, M.J. Analysis of Observational Studies in the Presence of Treatment Selection Bias: Effects of Invasive Cardiac Management on AMI Survival Using Propensity Score and Instrumental Variable Methods. JAMA 2007, 297, 278–285. [Google Scholar] [CrossRef]
- Lévesque, L.E.; Hanley, J.A.; Kezouh, A.; Suissa, S. Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ 2010, 340, b5087. [Google Scholar] [CrossRef] [PubMed]
- FDA. U.S. Food & Drug Administration. Framework for FDA’s Real-World Evidence Program. Issued: December 2018. Available online: https://www.fda.gov/media/120060/download (accessed on 3 March 2024).

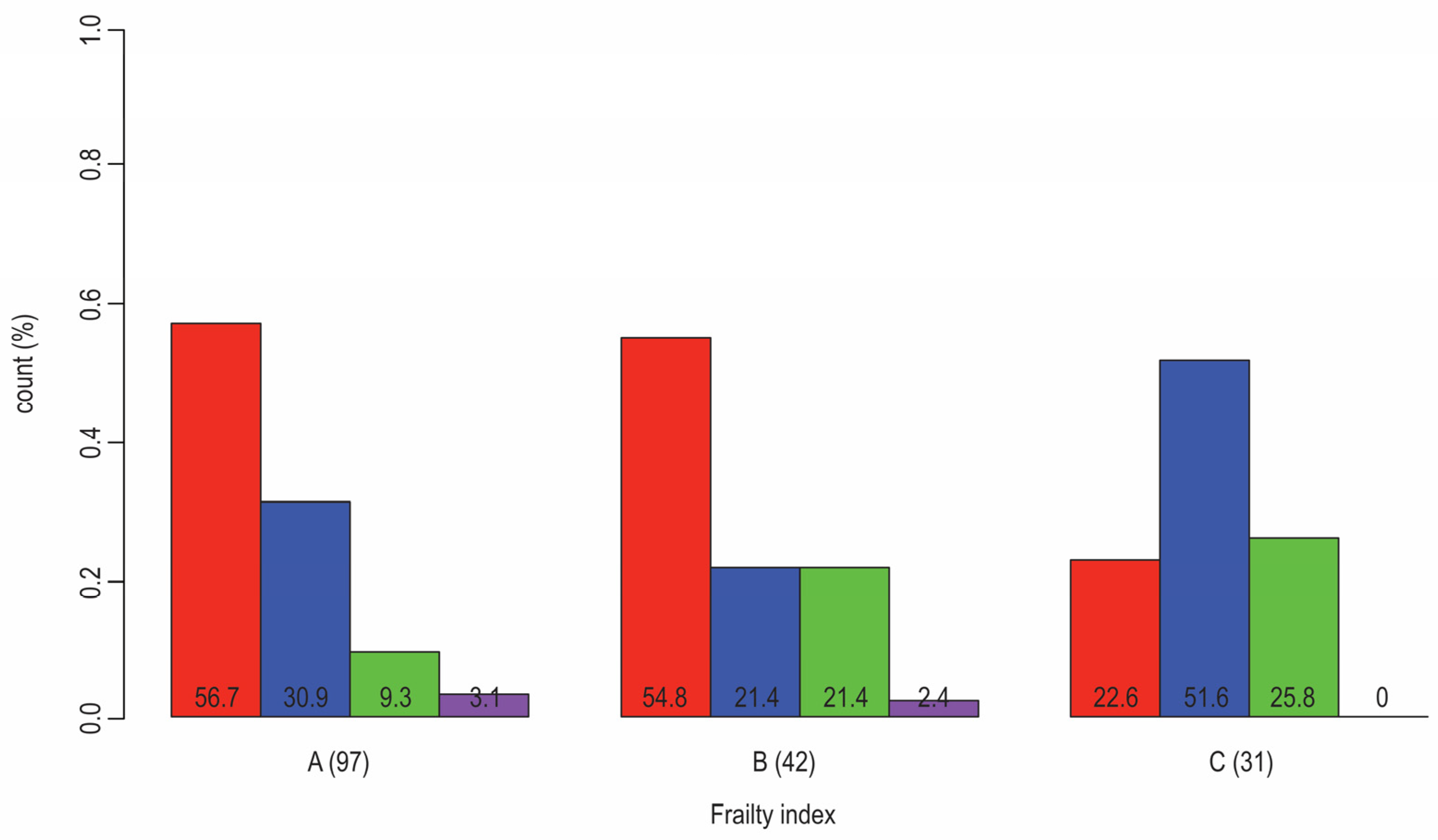
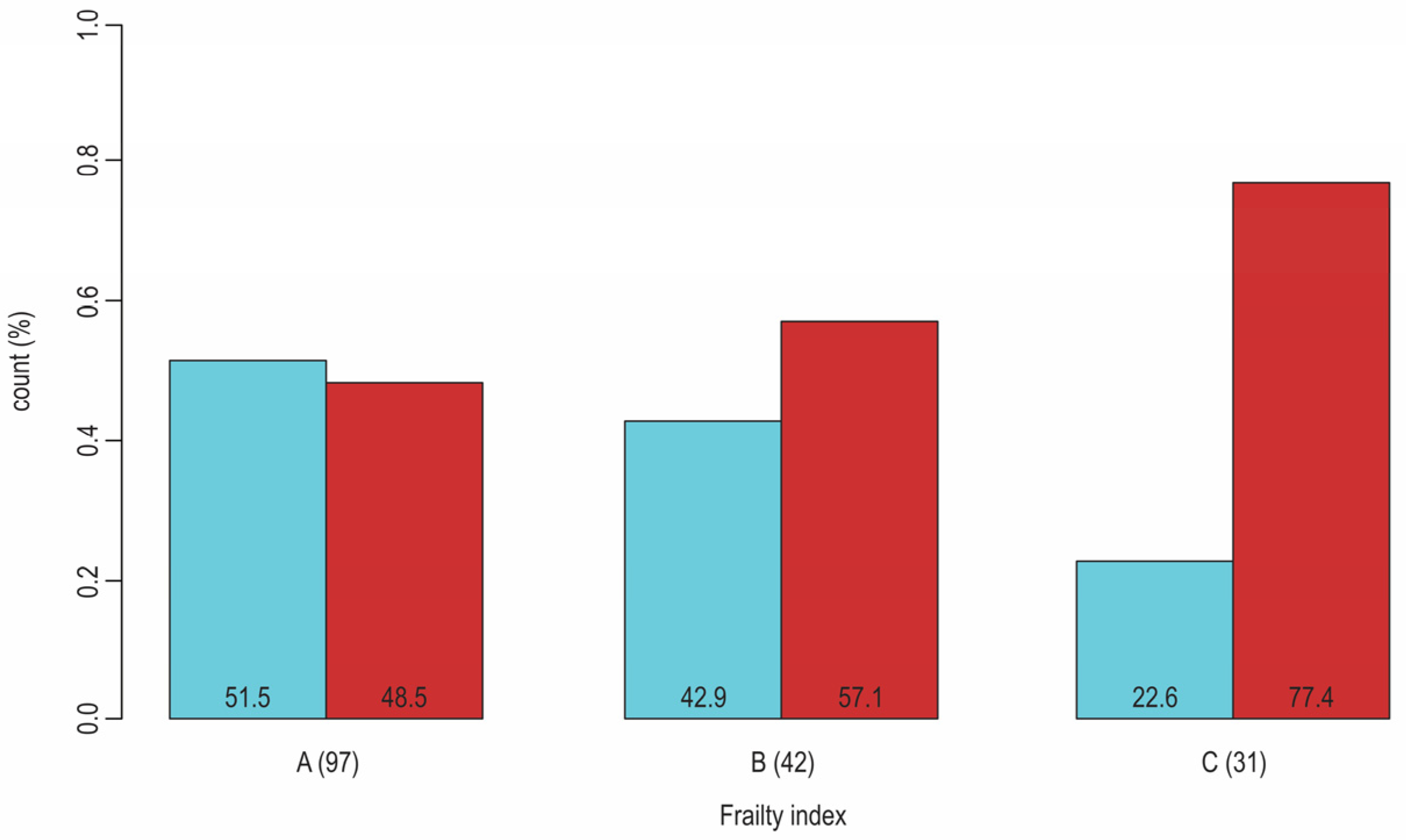
| Physician-Related Factors: | Patient-Related Factors: | Healthcare-Related Factors: |
|---|---|---|
| Lack of knowledge about the disease Overlooking the seriousness of the symptoms Not starting the treatment Inability to identify clinical outcomes Not adjusting the treatment until the intended result has been attained Inability to recognize and address comorbid conditions An assumption that a patient will not follow recommendations to intensify the therapy Lack of knowledge about the guidelines Reactive care as opposed to a proactive treatment strategy | Refusal to accept having the illness Non-existence of symptoms Inadequate knowledge of the health Considering that the illness is not severe Taking too many medications Financial limitation (cost of medications) The negative effects of medications Aspects of lifestyle Ineffective patient–doctor communication (lack of trust) | The absence of clinical guidelines No database of morbidity or disease registries No visit strategy (not scheduling appointments) The absence of decision assistance and team-based care Insufficient funds and time Technical deficiency |
| Heterogeneous Treatment Effects | Possible Side Effects |
|---|---|
| Higher baseline HbA1c values are associated with greater glycemic responses. GLP-1ra: There is no proof that renal function affects the response to glucose. SGLT2-in: Renal function needs to be regularly monitored due to a possible reduction in GFR. There is not any reliable proof that one’s body mass index significantly modifies the glycemic response. GLP-1ra: Less of a glycemic response is linked to a longer duration of diabetes. SGLT2-in: The length of diabetes does not consistently affect the glycemic response. GLP-1ra: No proof that aging affects the glycemic response. SGLT2-in: Elderly patients and those with renal impairment should use much more caution. No reliable proof that gender or ethnicity significantly modifies the glycemic response. SGLT2-in: No indication of variations in the reaction to treatment for patients with obstructive sleep apnea or insulin secretion and insulin. GLP-1ra: Studies indicate that lower fasting C-peptide- and urine C-peptide-to-creatinine ratios are linked to a reduced glycemic response. | Concerns regarding the start of SGLT2-in in patients with CVD or kidney illness as well as the necessity of the close observation of diabetic ketoacidosis (DKA) in cases of COVID-19 or other acute conditions requiring hospitalization. There is a higher risk of genital and urinary tract infections, which can be attributed to prolonged and increased glycosuria. Raised concerns about gliflozin-induced cancers (bladder and breast cancer), even though causality cannot be established. More research is required. Gliflozins cannot rule out drug-induced liver damage. Due to SGLT1 transporters’ reduced inhibition, there may be a lower risk of severe hypoglycemia when using gliflozins. Hypovolemic events are frequent with gliflozins, and DKA can appear infrequently and be more challenging to diagnose. Gliflozins-associated fracture risk is likely influenced by several variables that require additional research. Renal function needs to be regularly monitored due to a possible reduction in GFR. Elderly patients and those with renal impairment should use much more caution. Although evidence indicates that SGLT2 inhibition increases total serum cholesterol, this effect relates to a greater rise in the less atherogenic LDL-cholesterol subfraction. In addition, the ratio of LDL- to HDL-cholesterol remains nearly unaltered because of a concurrent rise in HDL-cholesterol. |
| Study (Design) | Findings Summary |
|---|---|
| Lin TK, et al., 2023. (population-based longitudinal cohort study) ref. [78] | SGLT2-in use was associated with a non-significantly decreased risk of ACS. No difference in the SGLT2-in subtype was observed in subgroup analyses. The results indicated an increased risk for the incidence of ACS in male and older (>70 years) patients. |
| Leong DP, et al., 2023. (population-based longitudinal cohort study) ref. [79] | Frailty confers substantial incremental prognostic information to prognostic variables for predicting death and hospitalization in heart failure patients. The relationship between frailty and these outcomes is consistent across countries at all income levels. |
| Kutz A, et al., 2023. (1:1 propensity score-matched cohort studies) ref. [80] | SGLT2-in and GLP-1ra safely improved CV outcomes and all-cause mortality, with the largest absolute benefits among frail people. |
| Young KG, et al., 2023. (a systematic review) ref. [53] | Current evidence on treatment effect heterogeneity for SGLT2-in and GLP-1ra therapies is limited, likely reflecting the methodological limitations of published studies. Robust and appropriately powered studies are required to understand T2D treatment effect heterogeneity. |
| Strain WD, Griffiths J, 2021. (a systematic review and meta-analysis) ref. [81] | GLP-1ra and SGLT2-in reduced MACE outcomes in older adults who were eligible to participate in clinical trials. Whereas this is reassuring for the biologically robust, it should not be extrapolated to frail older adults without further investigation. |
| Karagiannis T, et al., 2021. (a systematic review and meta-analysis) ref. [82] Vart P, et al., 2023. (a randomized controlled trial) ref. [83] | In older adults (≥65) with T2D, GLP-1ra reduced MACE and its components. SGLT2-in reduced MACE, heart failure and renal outcomes. The relative benefit of dapagliflozin in patients with chronic kidney disease (with/without T2D) for all outcomes was consistent across all frailty categories, with no difference in associated safety. |
| Research | Guidelines Development | Knowledge Implementation |
|---|---|---|
| More comprehensive patient profiling for CVOTs Real-world data-driven research Patient clustering based on multiple descriptors (including comorbidity patterns) Research intensification on the treatment effects of non-pharmacological interventions, particularly including psychological factors New biomarkers exploration Integrated models of care Qualitative research—assessing family doctors’ attitudes Translation research intensification | Clear division of areas where there is no support by evidence Case report Examples dealing with real-life experience Monitoring for the side effects of the treatment regimens The effects of comorbidity and frailty on treatment outcomes Evidence provided by cluster-analyses Clear recommendations on when to intensify and when to de-escalate therapy Clear statements on when to start with the cardio-protective therapy in the course of the disease The effects on outcomes of non-pharmacological interventions, particularly concerning psychological factors Problem-solving approach Guidelines adaptation for use in primary care | Continuous knowledge translation Educational training modules for healthcare providers, health policy officials and patients Learning on case reports Audit analysis of real-world data with the support of information technology Implementation of care protocols and workflows Empowerment of family doctors in life science knowledge The establishment of the platform for data collection and curation from electronic health records, smartphones and wearables Data selection for data-driven modeling The results of data modeling become visible in the workplace by using AI visual techniques |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurevija, T.; Šojat, D.; Bosnić, Z.; Mujaj, B.; Canecki Varžić, S.; Majnarić Trtica, L. The Reasons for the Low Uptake of New Antidiabetic Drugs with Cardiovascular Effects—A Family Doctor Perspective. J. Clin. Med. 2024, 13, 1617. https://doi.org/10.3390/jcm13061617
Kurevija T, Šojat D, Bosnić Z, Mujaj B, Canecki Varžić S, Majnarić Trtica L. The Reasons for the Low Uptake of New Antidiabetic Drugs with Cardiovascular Effects—A Family Doctor Perspective. Journal of Clinical Medicine. 2024; 13(6):1617. https://doi.org/10.3390/jcm13061617
Chicago/Turabian StyleKurevija, Tomislav, Dunja Šojat, Zvonimir Bosnić, Blerim Mujaj, Silvija Canecki Varžić, and Ljiljana Majnarić Trtica. 2024. "The Reasons for the Low Uptake of New Antidiabetic Drugs with Cardiovascular Effects—A Family Doctor Perspective" Journal of Clinical Medicine 13, no. 6: 1617. https://doi.org/10.3390/jcm13061617





