Implementation of the Care Bundle for the Management of Chronic Obstructive Pulmonary Disease with/without Heart Failure
Abstract
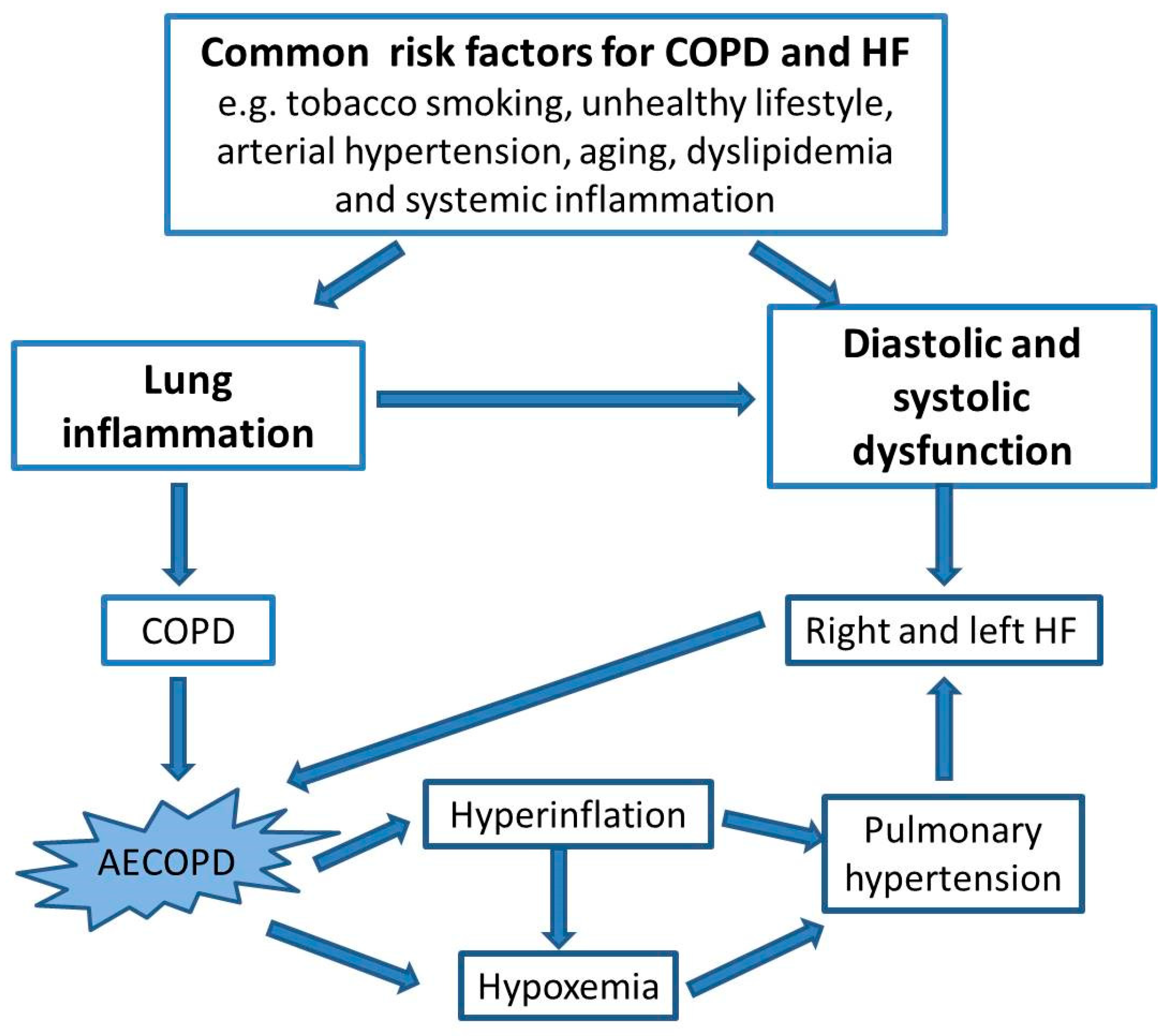
:1. Introduction
2. Differential Diagnosis and Paired Diagnosis
2.1. The Importance of a Medical Interview in the Differential Diagnosis
- genetics (i.e., serpin family A member 1 [SERPINA1] gene mutation leading to α-1 antitrypsin deficiency, telomerase reverse transcriptase mutations, or other epigenetic causes still remaining to be defined);
- early life events (i.e., prematurity, low weight at birth, childhood asthma);
- infections (i.e., childhood infections such as with pneumonia and respiratory syncytial virus or adulthood infections such as with tuberculosis and human immunodeficiency virus [HIV]);
- inhalation of tobacco, drugs, and other combustible substances;
- environmental exposures (i.e., indoor pollutants, ambient air pollution, occupational exposures).
2.2. Differential Diagnosis in the Acute Care Setting: AECOPD and/or HF?
3. Acute Care Therapy
4. Risk Stratification and Correct Allocation
5. Hospital Stay: Definitive Diagnosis
6. Hospital Discharge and Follow-Up
7. Post-Discharge COPD and COPD/HF Therapy
- Patients with COPD who present with occasional symptoms or are already under treatment with long-acting bronchodilators but need immediate relief of symptoms are treated with short-acting bronchodilators: either a short-acting muscarinic antagonist or a short-acting β2-agonist or their combination [11].
- Patients with COPD with dyspnea and two or more moderate AECOPDs or with one or more severe AECOPDs requiring hospitalization are treated with a long-acting β2-agonist (LABA) or a long-acting muscarinic antagonist (LAMA) or their combination; triple therapy with LABA + LAMA + inhaled corticosteroids (ICSs) is recommended if the eosinophil count is above the threshold of ≥300 eosinophils/μL [11]. However, eosinophil levels should be combined with clinical assessment as certain patients with eosinophil counts below 300 eosinophils/μL could also benefit from triple therapy [11].
- Patients with COPD with no or one moderate AECOPD and without the need for hospitalization should be treated with a single bronchodilator (mMRC score 0–1, CAT score < 10) or with LABA + LAMA (mMRC score ≥ 2, CAT score > 10). When patients experience recurrent exacerbations despite treatment with a dual bronchodilator (LABA + LAMA), the GOLD guidelines recommends switching to triple inhaled therapy with LABA + LAMA + ICSs [11].
8. Outpatient Care
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshabanat, A.; Otterstatter, M.C.; Sin, D.D.; Road, J.; Rempel, C.; Burns, J.; van Eeden, S.F.; FitzGerald, J.M. Impact of a COPD comprehensive case management program on hospital length of stay and readmission rates. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 961–971. [Google Scholar] [CrossRef]
- Mulpuru, S.; McKay, J.; Ronksley, P.E.; Thavorn, K.; Kobewka, D.M.; Forster, A.J. Factors contributing to high-cost hospital care for patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 989–995. [Google Scholar] [CrossRef]
- Miravitlles, M.; Murio, C.; Guerrero, T.; Gisbert, R.; DAFNE Study Group. Decisiones sobre Antibioticoterapia y Farmacoeconomía en la EPOC, Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 2002, 121, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Press, V.G.; Konetzka, R.T.; White, S.R. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr. Opin. Pulm. Med. 2018, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- McIlvennan, C.K.; Eapen, Z.J.; Allen, L.A. Hospital readmissions reduction program. Circulation 2015, 131, 1796–1803. [Google Scholar] [CrossRef]
- Criner, G.J.; Bourbeau, J.; Diekemper, R.L.; Ouellette, D.R.; Goodridge, D.; Hernandez, P.; Curren, K.; Balter, M.S.; Bhutani, M.; Camp, P.G.; et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015, 147, 894–942. [Google Scholar] [CrossRef]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef]
- Green, S.A.; Bell, D.; Mays, N. Identification of factors that support successful implementation of care bundles in the acute medical setting: A qualitative study. BMC Health Serv. Res. 2017, 17, 120. [Google Scholar] [CrossRef]
- Dhalla, I.A.; O’Brien, T.; Ko, F.; Laupacis, A. Toward safer transitions: How can we reduce post-discharge adverse events? Healthc. Q. 2012, 15, 63–67. [Google Scholar] [CrossRef]
- Hussey, P.S.; Schneider, E.C.; Rudin, R.S.; Fox, D.S.; Lai, J.; Pollack, C.E. Continuity and the costs of care for chronic disease. JAMA Intern. Med. 2014, 174, 742–748. [Google Scholar] [CrossRef]
- 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease—GOLD (n.d.). Available online: https://goldcopd.org/2024-gold-report/ (accessed on 22 January 2024).
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef]
- Seemungal, T.; Harper-Owen, R.; Bhowmik, A.; Moric, I.; Sanderson, G.; Message, S.; Maccallum, P.; Meade, T.W.; Jeffries, D.J.; Johnston, S.L.; et al. Respiratory viruses; symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1618–1623. [Google Scholar] [CrossRef]
- Sethi, S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest 2000, 117, 380S–385S. [Google Scholar] [CrossRef]
- Wedzicha, J.A. Exacerbations: Etiology and pathophysiologic mechanisms. Chest 2002, 121, 136S–141S. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Huchon, G.; Ieven, M.; Leven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. European Society of Clinical Microbiology and Infectious Diseases, Guidelines for the management of adult lower respiratory tract infections. Eur. Respir. J. 2005, 26, 1138–1180. [Google Scholar] [CrossRef]
- Hurst, J.R.; Wedzicha, J.A. The biology of a chronic obstructive pulmonary disease exacerbation. Clin. Chest Med. 2007, 28, 525–536. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Donaldson, G.C. Exacerbations of chronic obstructive pulmonary disease. Respir. Care 2003, 48, 1204–1213; discussion 1213–1215. [Google Scholar] [PubMed]
- Celli, B.R.; Barnes, P.J. Exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 29, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.; Hershfield, E.S.; Harding, G.K.; Nelson, N.A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Stockley, R.A.; O’Brien, C.; Pye, A.; Hill, S.L. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2000, 117, 1638–1645. [Google Scholar] [CrossRef]
- Bozinovski, S.; Hutchinson, A.; Thompson, M.; Macgregor, L.; Black, J.; Giannakis, E.; Karlsson, A.-S.; Silvestrini, R.; Smallwood, D.; Vlahos, R.; et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 269–278. [Google Scholar] [CrossRef]
- Gallego, M.; Pomares, X.; Capilla, S.; Marcos, M.A.; Suárez, D.; Monsó, E.; Montón, C. C-reactive protein in outpatients with acute exacerbation of COPD: Its relationship with microbial etiology and severity. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2633–2640. [Google Scholar] [CrossRef]
- Hurst, J.R.; Donaldson, G.C.; Perera, W.R.; Wilkinson, T.M.A.; Bilello, J.A.; Hagan, G.W.; Vessey, R.S.; Wedzicha, J.A. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 174, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, S.; Tang, R.; Qiu, H.; Huang, Q.; Mason, T.G.; Tian, L. Major air pollutants and risk of COPD exacerbations: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 3079–3091. [Google Scholar] [CrossRef]
- Miravitlles, M.; Moragas, A.; Hernández, S.; Bayona, C.; Llor, C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest 2013, 144, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.R.; Hurst, J.R.; Wilkinson, T.M.A.; Sapsford, R.J.; Müllerova, H.; Donaldson, G.C.; Wedzicha, J.A. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur. Respir. J. 2007, 29, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Evans, N.; Grant, B.J.B.; Murphy, T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002, 347, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Bakke, P.S.; Baste, V.; Hanoa, R.; Gulsvik, A. Prevalence of obstructive lung disease in a general population: Relation to occupational title and exposure to some airborne agents. Thorax 1991, 46, 863–870. [Google Scholar] [CrossRef]
- Tan, W.C.; Sin, D.D.; Bourbeau, J.; Hernandez, P.; Chapman, K.R.; Cowie, R.; FitzGerald, J.M.; Marciniuk, D.D.; Maltais, F.; Buist, A.S.; et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: Results from the CanCOLD study. Thorax 2015, 70, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Chisholm, R.S.; Brennenstuhl, S. COPD in a Population-Based Sample of Never-Smokers: Interactions among Sex, Gender, and Race. Int. J. Chronic Dis. 2016, 2016, 5862026. [Google Scholar] [CrossRef]
- Smith, B.M.; Kirby, M.; Hoffman, E.A.; Kronmal, R.A.; Aaron, S.D.; Allen, N.B.; Bertoni, A.; Coxson, H.O.; Cooper, C.; Couper, D.J.; et al. Association of Dysanapsis With Chronic Obstructive Pulmonary Disease Among Older Adults. JAMA 2020, 323, 2268–2280. [Google Scholar] [CrossRef]
- Moran-Mendoza, O.; Pérez-Padilla, J.R.; Salazar-Flores, M.; Vazquez-Alfaro, F. Wood smoke-associated lung disease: A clinical; functional, radiological and pathological description. Int. J. Tuberc. Lung Dis. 2008, 12, 1092–1098. [Google Scholar]
- Lee, S.H.; Hwang, E.D.; Lim, J.E.; Moon, S.; Kang, Y.A.; Jung, J.Y.; Park, M.S.; Kim, S.K.; Chang, J.; Kim, Y.S.; et al. The Risk Factors and Characteristics of COPD Among Nonsmokers in Korea: An Analysis of KNHANES IV and V. Lung 2016, 194, 353–361. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Yao, W.; Chen, P.; Kang, J.; Huang, S.; Chen, B.; Wang, C.; Ni, D.; Wang, X.; et al. COPD in Chinese nonsmokers. Eur. Respir. J. 2009, 33, 509–518. [Google Scholar] [CrossRef]
- Thomsen, M.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: A prospective population study. Lancet Respir. Med. 2013, 1, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Golpe, R.; López, P.S.; Jiménez, E.C.; Añón, O.C.; de Llano, L.A.P. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Arch. Bronconeumol. 2014, 50, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Brashier, B.B.; Londhe, J.; Pyasi, K.; Vincent, V.; Kajale, S.S.; Tambe, S.; Mandani, K.; Nair, A.; Mak, S.M.; et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, X.; Bai, C. Comparison of clinical features between non-smokers with COPD and smokers with COPD: A retrospective observational study. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhou, Y.; Jiang, C.; Zhao, Z.; He, F.; Ran, P. Small airway disease: A different phenotype of early stage COPD associated with biomass smoke exposure. Respirology 2018, 23, 198–205. [Google Scholar] [CrossRef]
- Krimmer, D.; Ichimaru, Y.; Burgess, J.; Black, J.; Oliver, B. Exposure to biomass smoke extract enhances fibronectin release from fibroblasts. PLoS ONE 2013, 8, e83938. [Google Scholar] [CrossRef]
- Bhome, A.B.; Brashier, B. Profiles of chronic obstructive lung disease: Characteristics of stable chronic obstructive lung disease in different parts of Asia. Curr. Opin. Pulm. Med. 2014, 20, 165–172. [Google Scholar] [CrossRef]
- Almagro, P.; Barreiro, B.; de Echaguen, A.O.; Quintana, S.; Carballeira, M.R.; Heredia, J.L.; Garau, J. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration 2006, 73, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Farrero, E.; Félez, M.A.; Izquierdo, J.; Marrades, R.M.; Antó, J.M. Estudi del Factors de Risc d’Agudització de la MPOC investigators, Risk factors of readmission to hospital for a COPD exacerbation: A prospective study. Thorax 2003, 58, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, G.; Gislason, T.; Janson, C.; Lindberg, E.; Hallin, R.; Ulrik, C.S.; Brøndum, E.; Nieminen, M.M.; Aine, T.; Bakke, P. Risk factors for rehospitalisation in COPD: Role of health status, anxiety and depression. Eur. Respir. J. 2005, 26, 414–419. [Google Scholar] [CrossRef]
- Mercer, P.; Shute, J.; Bhowmik, A.; Donaldson, G.; Wedzicha, J.; Warner, J. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir. Res. 2005, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Seemungal, T.A.R.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef] [PubMed]
- McGhan, R.; Radcliff, T.; Fish, R.; Sutherland, E.R.; Welsh, C.; Make, B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007, 132, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; YAM, L.; Poon, E. Hospital re-admission in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir. Med. 2001, 95, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Nantsupawat, T.; Limsuwat, C.; Nugent, K. Factors affecting chronic obstructive pulmonary disease early rehospitalization. Chronic Respir. Dis. 2012, 9, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.I.; Bartziokas, K.; Tsikrika, S.; Karakontaki, F.; Kastanakis, E.; Banya, W.; Haniotou, A.; Papiris, S.; Loukides, S.; Polychronopoulos, V.; et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur. Respir. J. 2013, 41, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Criner, G.J.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Kalhan, R.; Lange, P.; Lipson, D.A.; Martinez, F.J.; Midwinter, D.; et al. Time-Dependent Risk of Cardiovascular Events following an Exacerbation in Patients with Chronic Obstructive Pulmonary Disease: Post Hoc Analysis from the IMPACT Trial. J. Am. Heart Assoc. 2022, 11, e024350. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Li, S.; Xu, S. Association of Chronic Obstructive Pulmonary Disease With Arrhythmia Risks: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 732349. [Google Scholar] [CrossRef]
- Terzano, C.; Romani, S.; Conti, V.; Paone, G.; Oriolo, F.; Vitarelli, A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2908–2917. [Google Scholar] [PubMed]
- Houben-Wilke, S.; Jörres, R.A.; Bals, R.; Franssen, F.M.E.; Gläser, S.; Holle, R.; Karch, A.; Koch, A.; Magnussen, H.; Obst, A.; et al. Peripheral Artery Disease and Its Clinical Relevance in Patients with Chronic Obstructive Pulmonary Disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am. J. Respir. Crit. Care Med. 2017, 195, 189–197. [Google Scholar] [CrossRef]
- Le Jemtel, T.H.; Padeletti, M.; Jelic, S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 171–180. [Google Scholar] [CrossRef]
- Rutten, F.H.; Cramer, M.-J.M.; Grobbee, D.E.; Sachs, A.P.E.; Kirkels, J.H.; Lammers, J.-W.J.; Hoes, A.W. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur. Heart J. 2005, 26, 1887–1894. [Google Scholar] [CrossRef]
- Canepa, M.; Temporelli, P.L.; Rossi, A.; Rossi, A.; Gonzini, L.; Nicolosi, G.L.; Staszewsky, L.; Marchioli, R.; Maggioni, A.P.; Tavazzi, L. Prevalence and Prognostic Impact of Chronic Obstructive Pulmonary Disease in Patients with Chronic Heart Failure: Data from the GISSI-HF Trial. Cardiology 2017, 136, 128–137. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Petrie, M.C.; Jhund, P.S.; Chalmers, G.W.; Dunn, F.G.; McMurray, J.J.V. Heart failure and chronic obstructive pulmonary disease: Diagnostic pitfalls and epidemiology. Eur. J. Heart Fail. 2009, 11, 130–139. [Google Scholar] [CrossRef]
- Güder, G.; Brenner, S.; Störk, S.; Hoes, A.; Rutten, F.H. Chronic obstructive pulmonary disease in heart failure: Accurate diagnosis and treatment. Eur. J. Heart Fail. 2014, 16, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Torp-Pedersen, C.; Brendorp, B.; Seibaek, M.; Burchardt, H.; Køber, L. Long-term survival in patients hospitalized with congestive heart failure: Relation to preserved and reduced left ventricular systolic function. Eur. Heart J. 2003, 24, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, L.; Rosengren, A.; Eriksson, H.; Lappas, G. Heart failure in the general population of men—Morbidity, risk factors and prognosis. J. Intern. Med. 2001, 249, 253–261. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Man, S.F.P. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003, 107, 1514–1519. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Man, S.F.P.; Connett, J.E.; Anthonisen, N.R.; Wise, R.A.; Tashkin, D.P.; Sin, D.D. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006, 61, 849–853. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; Maaten, J.M.T.; et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017, 38, 1883–1890. [Google Scholar] [CrossRef]
- Staszewsky, L.; Wong, M.; Masson, S.; Barlera, S.; Carretta, E.; Maggioni, A.P.; Anand, I.S.; Cohn, J.N.; Tognoni, G.; Latini, R. Valsartan Heart Failure Trial Investigators, Clinical, neurohormonal, and inflammatory markers and overall prognostic role of chronic obstructive pulmonary disease in patients with heart failure: Data from the Val-HeFT heart failure trial. J. Card. Fail. 2007, 13, 797–804. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Virani, S.; Ceconi, C. Heart failure and chronic obstructive pulmonary disease: The challenges facing physicians and health services. Eur. Heart J. 2013, 34, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Müllerova, H.; Agusti, A.; Erqou, S.; Mapel, D.W. Cardiovascular comorbidity in COPD: Systematic literature review. Chest 2013, 144, 1163–1178. [Google Scholar] [CrossRef]
- Axson, E.L.; Ragutheeswaran, K.; Sundaram, V.; Bloom, C.I.; Bottle, A.; Cowie, M.R.; Quint, J.K. Hospitalisation and mortality in patients with comorbid COPD and heart failure: A systematic review and meta-analysis. Respir. Res. 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Canepa, M.; Straburzynska-Migaj, E.; Drozdz, J.; Fernandez-Vivancos, C.; Pinilla, J.M.G.; Nyolczas, N.; Temporelli, P.L.; Mebazaa, A.; Lainscak, M.; Laroche, C.; et al. ESC-HFA Heart Failure Long-Term Registry Investigators, Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2018, 20, 100–110. [Google Scholar] [CrossRef]
- Fisher, K.A.; Stefan, M.S.; Darling, C.; Lessard, D.; Goldberg, R.J. Impact of COPD on the mortality and treatment of patients hospitalized with acute decompensated heart failure: The Worcester Heart Failure Study. Chest 2015, 147, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Roversi, S.; Fabbri, L.; Sin, D.; Hawkins, N.; Agustì, A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgen. Need for Integrated Care. Am. J. Respir. Crit. Care Med. 2016, 194, 1319–1336. [Google Scholar] [CrossRef]
- Braunstein, J.B.; Anderson, G.F.; Gerstenblith, G.; Weller, W.; Niefeld, M.; Herbert, R.; Wu, A.W. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 1226–1233. [Google Scholar] [CrossRef]
- Rusinaru, D.; Saaidi, I.; Godard, S.; Mahjoub, H.; Battle, C.; Tribouilloy, C. Impact of chronic obstructive pulmonary disease on long-term outcome of patients hospitalized for heart failure. Am. J. Cardiol. 2008, 101, 353–358. [Google Scholar] [CrossRef]
- Mentz, R.J.; Fiuzat, M.; Wojdyla, D.M.; Chiswell, K.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: Findings from OPTIMIZE-HF. Eur. J. Heart Fail. 2012, 14, 395–403. [Google Scholar] [CrossRef]
- Jacob, J.; Tost, J.; Miró, Ò.; Herrero, P.; Martín-Sánchez, F.J.; Llorens, P. Impact of chronic obstructive pulmonary disease on clinical course after an episode of acute heart failure. EAHFE–COPD study. Int. J. Cardiol. 2017, 227, 450–456. [Google Scholar] [CrossRef]
- Tavazzi, L.; Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Lainscak, M.; Robertson, M.; Ford, I.; Investigators, S.H.F. Clinical profiles and outcomes in patients with chronic heart failure and chronic obstructive pulmonary disease: An efficacy and safety analysis of SHIFT study. Int. J. Cardiol. 2013, 170, 182–188. [Google Scholar] [CrossRef]
- De Blois, J.; Simard, S.; Atar, D.; Agewall, S. Norwegian Heart Failure Registry, COPD predicts mortality in HF: The Norwegian Heart Failure Registry. J. Card. Fail. 2010, 16, 225–229. [Google Scholar] [CrossRef]
- Walke, L.M.; Byers, A.L.; Tinetti, M.E.; Dubin, J.A.; McCorkle, R.; Fried, T.R. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch. Intern. Med. 2007, 167, 2503–2508. [Google Scholar] [CrossRef]
- de Souza Caroci, A.; Lareau, S.C. Descriptors of dyspnea by patients with chronic obstructive pulmonary disease versus congestive heart failure. Heart Lung 2004, 33, 102–110. [Google Scholar] [CrossRef]
- Zvezdin, B.; Milutinov, S.; Kojicic, M.; Hadnadjev, M.; Hromis, S.; Markovic, M.; Gajic, O. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest 2009, 136, 376–380. [Google Scholar] [CrossRef]
- Mannino, D.M.; Doherty, D.E.; Buist, A.S. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir. Med. 2006, 100, 115–122. [Google Scholar] [CrossRef]
- Masa, J.F.; Utrabo, I.; de Terreros, J.G.; Aburto, M.; Esteban, C.; Prats, E.; Núñez, B.; Ortega-González, Á.; Jara-Palomares, L.; Martin-Vicente, M.J.; et al. Noninvasive ventilation for severely acidotic patients in respiratory intermediate care units: Precision medicine in intermediate care units. BMC Pulm. Med. 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Voelker, H.; Bhatt, S.P.; Brenner, K.; Casaburi, R.; Come, C.E.; Cooper, J.A.D.; Criner, G.J.; Curtis, J.L.; Han, M.K.; et al. Metoprolol for the Prevention of Acute Exacerbations of COPD. N. Engl. J. Med. 2019, 381, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Canepa, M.; Franssen, F.M.E.; Olschewski, H.; Lainscak, M.; Böhm, M.; Tavazzi, L.; Rosenkranz, S. Diagnostic and Therapeutic Gaps in Patients With Heart Failure and Chronic Obstructive Pulmonary Disease. JACC Heart Fail. 2019, 7, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.W.; Wilkinson, T.M.A. Predicting and preventing hospital readmission for exacerbations of COPD. ERJ Open Res. 2020, 6, 00325-2019. [Google Scholar] [CrossRef]
- Press, V.G.; Myers, L.C.; Feemster, L.C. Preventing COPD Readmissions Under the Hospital Readmissions Reduction Program. Chest 2021, 159, 996–1006. [Google Scholar] [CrossRef]
- Sharpe, I.; Bowman, M.; Kim, A.; Srivastava, S.; Jalink, M.; Wijeratne, D.T. Strategies to Prevent Readmissions to Hospital for COPD: A Systematic Review. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 456–468. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Ezeana, C.F.; Ogunti, R.; Puppala, M.; He, T.; Yu, X.; Wong, S.S.Y.; Yin, Z.; Roberts, A.W.; et al. An AI-driven clinical care pathway to reduce 30-day readmission for chronic obstructive pulmonary disease (COPD) patients. Sci. Rep. 2022, 12, 20633. [Google Scholar] [CrossRef]
- Bamforth, R.J.; Chhibba, R.; Ferguson, T.W.; Sabourin, J.; Pieroni, D.; Askin, N.; Tangri, N.; Komenda, P.; Rigatto, C. Strategies to prevent hospital readmission and death in patients with chronic heart failure, chronic obstructive pulmonary disease, and chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249542. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Lim, K.; Larson, M.; Helfinstine, K.; Homan, J.; Schwarz, R.; Dankbar, G. A Practice Redesign Collaborative for Reducing Hospital Readmission for Chronic Obstructive Pulmonary Disease in an Affiliated Network of Health Care Organizations. Jt. Comm. J. Qual. Patient Saf. 2021, 47, 412–421. [Google Scholar] [CrossRef]
- Kalhan, R.; Mutharasan, R.K. Reducing Readmissions in Patients With Both Heart Failure and COPD. Chest 2018, 154, 1230–1238. [Google Scholar] [CrossRef]
- Celli, B.R.; Fabbri, L.M.; Aaron, S.D.; Agusti, A.; Brook, R.D.; Criner, G.J.; Franssen, F.M.E.; Humbert, M.; Hurst, J.R.; de Oca, M.M.; et al. Differential Diagnosis of Suspected COPD Exacerbations in the Acute Care Setting: Best Practice. Am. J. Respir. Crit. Care Med. 2023, 207, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Using Care Bundles to Improve Health Care Quality|IHI—Institute for Healthcare Improvement, (n.d.). Available online: https://www.ihi.org:443/resources/Pages/IHIWhitePapers/UsingCareBundles.aspx (accessed on 17 December 2022).
- Turner, A.M.; Lim, W.S.; Rodrigo, C.; Welham, S.A.; Calvert, J.M. A care-bundles approach to improving standard of care in AECOPD admissions: Results of a national project. Thorax 2015, 70, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W.; Adamson, A.; Hurst, J.R.; Roberts, C.M.; Quint, J.K. Does pay-for-performance improve patient outcomes in acute exacerbation of COPD admissions? Thorax 2022, 77, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Hurst, J.R.; Miravitlles, M.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Prevention of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 50, 1602265. [Google Scholar] [CrossRef] [PubMed]
- Laennec, R.; Forbes, J.; Fisher, J.; Andral, G. A Treatise on the Diseases of the Chest, and on Mediate Auscultation—Digital Collections—National Library of Medicine, Medicine in the Americas, 1610–1920 (n.d.). Available online: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-9308216-bk (accessed on 21 November 2022).
- Calverley, P.M.A. Minimal clinically important difference—Exacerbations of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2005, 2, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Effing, T.W.; Kerstjens, H.A.M.; Monninkhof, E.M.; van der Valk, P.D.L.P.M.; Wouters, E.F.M.; Postma, D.S.; Zielhuis, G.A.; van der Palen, J. Definitions of exacerbations: Does it really matter in clinical trials on COPD? Chest 2009, 136, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.; Mkorombindo, T.; Schumann, D.M.; Agusti, A.; Ash, S.Y.; Bafadhel, M.; Bai, C.; Chalmers, J.D.; Criner, G.J.; Dharmage, S.C.; et al. Towards the elimination of chronic obstructive pulmonary disease: A Lancet Commission. Lancet 2022, 400, 921–972. [Google Scholar] [CrossRef]
- Rennard, S.I. Overview of causes of COPD. New understanding of pathogenesis and mechanisms can guide future therapy. Postgrad. Med. 2002, 111, 28–30, 33–34, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Renier, W.; Winckelmann, K.H.; Verbakel, J.Y.; Aertgeerts, B.; Buntinx, F. Signs and symptoms in adult patients with acute dyspnea: A systematic review and meta-analysis. Eur. J. Emerg. Med. 2018, 25, 3–11. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. Engl. Ed. 2021, 74, 544. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Peterson, S.; Ezzat, A.M.; Vijh, R.; Virani, S.A.; Gibb, A.; Mancini, G.B.J.; Wong, S.T. Control of Cardiovascular Risk Factors in Patients with Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2022, 19, 1102–1111. [Google Scholar] [CrossRef]
- Crisafulli, E.; Manco, A.; Ferrer, M.; Huerta, A.; Micheletto, C.; Girelli, D.; Clini, E.; Torres, A. Pneumonic versus Nonpneumonic Exacerbations of Chronic Obstructive Pulmonary Disease. Semin. Respir. Crit. Care Med. 2020, 41, 817–829. [Google Scholar] [CrossRef]
- Torres, A.; Blasi, F.; Dartois, N.; Akova, M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015, 70, 984–989. [Google Scholar] [CrossRef]
- Davie, A.P.; Francis, C.M.; Love, M.P.; Caruana, L.; Starkey, I.R.; Shaw, T.R.; Sutherland, G.R.; McMurray, J.J. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996, 312, 222. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Ferry, O.R.; Huang, Y.C.; Masel, P.J.; Hamilton, M.; Fong, K.M.; Bowman, R.V.; McKenzie, S.C.; Yang, I.A. Diagnostic approach to chronic dyspnoea in adults. J. Thorac. Dis. 2019, 11, S2117–S2128. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.J.; Guha, K.; Sharma, R. How to Improve Time to Diagnosis in Acute Heart Failure—Clinical Signs and Chest X-ray. Card. Fail. Rev. 2015, 1, 69–74. [Google Scholar] [CrossRef]
- Debray, M.P.; Carette, M.F.; Loubet, P.; Pasquet, B.; Fidouh, N.H.; Benjoar, M.; Varon, E.; Brun, A.L.; Claessens, Y.E.; Duval, X.; et al. CT features of community-acquired pneumonia at the emergency department. Respir. Med. Res. 2022, 81, 100892. [Google Scholar] [CrossRef]
- Bafadhel, M.; Clark, T.W.; Reid, C.; Medina, M.-J.; Batham, S.; Barer, M.R.; Nicholson, K.G.; Brightling, C.E. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest 2011, 139, 1410–1418. [Google Scholar] [CrossRef]
- Elmenawi, K.A.; Anil, V.; Gosal, H.; Kaur, H.; Ngassa, H.C.; Mohammed, L. The Importance of Measuring Troponin in Chronic Obstructive Pulmonary Disease Exacerbations: A Systematic Review. Cureus 2021, 13, e17451. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction, Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Silver, M.A.; Maisel, A.; Yancy, C.W.; McCullough, P.A.; Burnett, J.C.; Francis, G.S.; Mehra, M.R.; Peacock, W.F.; Fonarow, G.; Gibler, W.B.; et al. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest. Heart Fail. 2004, 10, 1–30. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.B.; et al. Breathing Not Properly Multinational Study Investigators, Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An. international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Sarzani, R.; Allevi, M.; Di Pentima, C.; Schiavi, P.; Spannella, F.; Giulietti, F. Role of Cardiac Natriuretic Peptides in Heart Structure and Function. Int. J. Mol. Sci. 2022, 23, 14415. [Google Scholar] [CrossRef]
- McCullough, P.A.; Hollander, J.E.; Nowak, R.M.; Storrow, A.B.; Duc, P.; Omland, T.; McCord, J.; Herrmann, H.C.; Steg, P.G.; Westheim, A.; et al. BNP Multinational Study Investigators, Uncovering heart failure in patients with a history of pulmonary disease: Rationale for the early use of B-type natriuretic peptide in the emergency department. Acad. Emerg. Med. 2003, 10, 198–204. [Google Scholar] [CrossRef]
- Morrison, L.K.; Harrison, A.; Krishnaswamy, P.; Kazanegra, R.; Clopton, P.; Maisel, A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J. Am. Coll. Cardiol. 2002, 39, 202–209. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Khosla, A.; Virani, S.A.; McMurray, J.J.V.; FitzGerald, J.M. B-type natriuretic peptides in chronic obstructive pulmonary disease: A systematic review. BMC Pulm. Med. 2017, 17, 11. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.; Breidthardt, T.; Christ-Crain, M.; Bingisser, R.; Miedinger, D.; Leuppi, J.; Mueller, B.; Tamm, M.; Mueller, C. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest 2008, 133, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Fedecostante, M.; Giordano, P.; Gattafoni, P.; Espinosa, E.; Busco, F.; Piccinini, G.; Dessì-Fulgheri, P. NT-proBNP and Its Correlation with In-Hospital Mortality in the Very Elderly without an Admission Diagnosis of Heart Failure. PLoS ONE 2016, 11, e0153759. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Chen-Tournoux, A.A.; Moe, G. Amino-terminal pro-B-type natriuretic peptide testing for the diagnosis or exclusion of heart failure in patients with acute symptoms. Am. J. Cardiol. 2008, 101, 29–38. [Google Scholar] [CrossRef]
- Di Somma, S.; Magrini, L.; Pittoni, V.; Marino, R.; Mastrantuono, A.; Ferri, E.; Ballarino, P.; Semplicini, A.; Bertazzoni, G.; Carpinteri, G.; et al. In-hospital percentage BNP reduction is highly predictive for adverse events in patients admitted for acute heart failure: The Italian RED Study. Crit. Care 2010, 14, R116. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Marino, R.; Zampini, G.; Magrini, L.; Ferri, E.; Shah, K.; Clopton, P.; Maisel, A.S. Predictive value for death and rehospitalization of 30-day postdischarge B-type natriuretic peptide (BNP) in elderly patients with heart failure. Sub-analysis of Italian RED Study. Clin. Chem. Lab. Med. 2015, 53, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Magrini, L.; Tabacco, F.; Marino, R.; Talucci, V.; Marrocco, F.; Cardelli, P.; Ferri, E.; Pittoni, V. Brain natriuretic peptide and N-terminal pro-B-type natriuretic peptide show a different profile in response to acute decompensated heart failure treatment. Congest. Heart Fail. 2008, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, I.; Mosterd, A.; Bloemen, J.A.; Valk, M.J.; van Velzen, E.; Wielders, J.P.; Zuithoff, N.P.; Rutten, F.H.; Hoes, A.W. Clinical evaluation of geriatric outpatients with suspected heart failure: Value of symptoms, signs, and additional tests. Eur. J. Heart Fail. 2011, 13, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Vaes, B.; Delgado, V.; Bax, J.; Degryse, J.; Westendorp, R.G.J.; Gussekloo, J. Diagnostic accuracy of plasma NT-proBNP levels for excluding cardiac abnormalities in the very elderly. BMC Geriatr. 2010, 10, 85. [Google Scholar] [CrossRef]
- Vaes, B.; de Ruijter, W.; Degryse, J.; Westendorp, R.G.J.; Gussekloo, J. Clinical relevance of a raised plasma N-terminal pro-brain natriuretic peptide level in a population-based cohort of nonagenarians. J. Am. Geriatr. Soc. 2009, 57, 823–829. [Google Scholar] [CrossRef]
- Richards, M.; Di Somma, S.; Mueller, C.; Nowak, R.; Peacock, W.F.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.B.; Clopton, P.; et al. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: Results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail. 2013, 1, 192–199. [Google Scholar] [CrossRef]
- Leuchte, H.H.; Baumgartner, R.A.; Nounou, M.E.; Vogeser, M.; Neurohr, C.; Trautnitz, M.; Behr, J. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am. J. Respir. Crit. Care Med. 2006, 173, 744–750. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Docherty, K.F.; Petrie, M.C.; Januzzi, J.L.; Mueller, C.; Anderson, L.; Bozkurt, B.; Butler, J.; Chioncel, O.; Cleland, J.G.F.; et al. Practical algorithms for early diagnosis of heart failure and heart stress using NT-proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1891–1898. [Google Scholar] [CrossRef]
- COPD & Spirometry|British Thoracic Society|Better Lung Health for All, (n.d.). Available online: https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/copd-spirometry/ (accessed on 17 December 2022).
- Evans, K.A.; Pollack, M.; Portillo, E.; Strange, C.; Touchette, D.R.; Staresinic, A.; Patel, S.; Tkacz, J.; Feigler, N. Prompt initiation of triple therapy following hospitalization for a chronic obstructive pulmonary disease exacerbation in the United States: An analysis of the PRIMUS study. J. Manag. Care Spec. Pharm. 2022, 28, 1366–1377. [Google Scholar] [CrossRef]
- Halpin, D.M.G.; Dickens, A.P.; Skinner, D.; Murray, R.; Singh, M.; Hickman, K.; Carter, V.; Couper, A.; Evans, A.; Pullen, R.; et al. Identification of key opportunities for optimising the management of high-risk COPD patients in the UK using the CONQUEST quality standards: An observational longitudinal study. Lancet Reg. Health Eur. 2023, 29, 100619. [Google Scholar] [CrossRef]
- Light, R.W.; George, R.B. Serial pulmonary function in patients with acute heart failure. Arch. Intern. Med. 1983, 143, 429–433. [Google Scholar] [CrossRef]
- Brenner, S.; Güder, G.; Berliner, D.; Deubner, N.; Fröhlich, K.; Ertl, G.; Jany, B.; Angermann, C.E.; Störk, S. Airway obstruction in systolic heart failure—COPD or congestion? Int. J. Cardiol. 2013, 168, 1910–1916. [Google Scholar] [CrossRef]
- Kostikas, K.; Rhee, C.K.; Hurst, J.R.; Agostoni, P.; Cao, H.; Fogel, R.; Jones, R.; Kocks, J.W.H.; Mezzi, K.; Ming, S.W.Y.; et al. Adequacy of Therapy for People with Both COPD and Heart Failure in the UK: Historical Cohort Study. Pragmat. Obs. Res. 2020, 11, 55–66. [Google Scholar] [CrossRef]
- Ansari, M.; Alexander, M.; Tutar, A.; Bello, D.; Massie, B.M. Cardiology participation improves outcomes in patients with new-onset heart failure in the outpatient setting. J. Am. Coll. Cardiol. 2003, 41, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, A.D.; Hamel, M.B.; Davis, R.B.; Connors, A.F.; Regueiro, C.; Desbiens, N.; Goldman, L.; Califf, R.M.; Dawson, N.V.; Wenger, N.; et al. Resource use and survival of patients hospitalized with congestive heart failure: Differences in care by specialty of the attending physician. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann. Intern. Med. 2000, 132, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Philbin, E.F.; Jenkins, P.L. Differences between patients with heart failure treated by cardiologists, internists, family physicians, and other physicians: Analysis of a large, statewide database. Am. Heart J. 2000, 139, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Jong, P.; Gong, Y.; Liu, P.P.; Austin, P.C.; Lee, D.S.; Tu, J.V. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation 2003, 108, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Engström, G. The restrictive-obstructive continuum and the failing heart. Thorax 2016, 71, 487–488. [Google Scholar] [CrossRef]
- Hawkins, N.M. Chronic obstructive pulmonary disease and heart failure in Europe-further evidence of the need for integrated care. Eur. J. Heart Fail. 2018, 20, 111–113. [Google Scholar] [CrossRef]
- Magnussen, H.; Canepa, M.; Zambito, P.E.; Brusasco, V.; Meinertz, T.; Rosenkranz, S. What can we learn from pulmonary function testing in heart failure? Eur. J. Heart Fail. 2017, 19, 1222–1229. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; MacNee, W.; Force, E.R.T. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur. Respir. J. 2004, 23, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Buist, A.S.; Vollmer, W.M. Chronic obstructive pulmonary disease in the older adult: What defines abnormal lung function? Thorax 2007, 62, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Barrecheguren, M.; Pinto, L.; Mostafavi-Pour-Manshadi, S.-M.-Y.; Tan, W.C.; Li, P.Z.; Aaron, S.D.; Benedetti, A.; Chapman, K.R.; Walker, B.; Fitzgerald, J.M.; et al. Identification and definition of asthma-COPD overlap: The CanCOLD study. Respirology 2020, 25, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bateman, E.D.; Becker, A.; Boulet, L.-P.; Cruz, A.A.; Drazen, J.M.; Haahtela, T.; Hurd, S.S.; Inoue, H.; de Jongste, J.C.; et al. A summary of the new GINA strategy: A roadmap to asthma control. Eur. Respir. J. 2015, 46, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Simpson, J.L. The overlap syndrome of asthma and COPD: What are its features and how important is it? Thorax 2009, 64, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Sin, D.D. Asthma-COPD overlap syndrome: Pathogenesis; clinical features; therapeutic targets. BMJ 2017, 358, j3772. [Google Scholar] [CrossRef]
- Carolan, B.J.; Sutherland, E.R. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: Recent advances. J. Allergy Clin. Immunol. 2013, 131, 627–634; quiz 635. [Google Scholar] [CrossRef]
- Sharma, G.; Kuo, Y.-F.; Freeman, J.L.; Zhang, D.D.; Goodwin, J.S. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch. Intern. Med. 2010, 170, 1664–1670. [Google Scholar] [CrossRef]
- Sin, D.D.; Bell, N.R.; Svenson, L.W.; Man, S.F.P. The impact of follow-up physician visits on emergency readmissions for patients with asthma and chronic obstructive pulmonary disease: A population-based study. Am. J. Med. 2002, 112, 120–125. [Google Scholar] [CrossRef]
- Atwood, C.E.; Bhutani, M.; Ospina, M.B.; Rowe, B.H.; Leigh, R.; Deuchar, L.; Faris, P.; Michas, M.; Mrklas, K.J.; Graham, J.; et al. Optimizing COPD Acute Care Patient Outcomes Using a Standardized Transition Bundle and Care Coordinator: A Randomized Clinical Trial. Chest 2022, 162, 321–330. [Google Scholar] [CrossRef]
- Bourbeau, J.; Echevarria, C. Models of care across the continuum of exacerbations for patients with chronic obstructive pulmonary disease. Chronic Respir. Dis. 2020, 17, 1479973119895457. [Google Scholar] [CrossRef]
- Lenferink, A.; Brusse-Keizer, M.; van der Valk, P.D.; Frith, P.A.; Zwerink, M.; Monninkhof, E.M.; van der Palen, J.; Effing, T.W. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 8, CD011682. [Google Scholar] [CrossRef] [PubMed]
- Benzo, R.; Vickers, K.; Novotny, P.J.; Tucker, S.; Hoult, J.; Neuenfeldt, P.; Connett, J.; Lorig, K.; McEvoy, C. Health Coaching and Chronic Obstructive Pulmonary Disease Rehospitalization. A Randomized Study. Am. J. Respir. Crit. Care Med. 2016, 194, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Gavish, R.; Levy, A.; Dekel, O.K.; Karp, E.; Maimon, N. The Association Between Hospital Readmission and Pulmonologist Follow-up Visits in Patients With COPD. Chest 2015, 148, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Churpek, M.M.; Perraillon, M.C.; Konetzka, R.T. Understanding why patients with COPD get readmitted: A large national study to delineate the Medicare population for the readmissions penalty expansion. Chest 2015, 147, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.L.; Dewan, N.; Bloomfield, H.E.; Grill, J.; Schult, T.M.; Nelson, D.B.; Kumari, S.; Thomas, M.; Geist, L.J.; Beaner, C.; et al. Disease management program for chronic obstructive pulmonary disease: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Rea, H.; McAuley, S.; Stewart, A.; Lamont, C.; Roseman, P.; Didsbury, P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern. Med. J. 2004, 34, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bourbeau, J.; Julien, M.; Maltais, F.; Rouleau, M.; Beaupré, A.; Bégin, R.; Renzi, P.; Nault, D.; Borycki, E.; Schwartzman, K.; et al. Chronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du Québec, Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: A disease-specific self-management intervention. Arch. Intern. Med. 2003, 163, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Zwerink, M.; Brusse-Keizer, M.; van der Valk, P.D.L.P.M.; Zielhuis, G.A.; Monninkhof, E.M.; van der Palen, J.; Frith, P.A.; Effing, T. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014, 3, CD002990. [Google Scholar] [CrossRef]
- Kessler, R.; Casan-Clara, P.; Koehler, D.; Tognella, S.; Viejo, J.L.; Negro, R.W.D.; Díaz-Lobato, S.; Reissig, K.; González-Moro, J.M.R.; Devouassoux, G.; et al. COMET: A multicomponent home-based disease-management programme versus routine care in severe COPD. Eur. Respir. J. 2018, 51, 1701612. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Patel, S.B.; Anderson, E.M.; Baugh, D.; Givens, T.; Schumann, C.; Sanders, J.G.; Windham, S.T.; Cutter, G.R.; Dransfield, M.T. Video Telehealth Pulmonary Rehabilitation Intervention in Chronic Obstructive Pulmonary Disease Reduces 30-Day Readmissions. Am. J. Respir. Crit. Care Med. 2019, 200, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Marcos, P.J.; Represas, C.R.; Ramos, C.; Álvarez, B.C.; Villar, A.F.; Liste, A.F.; Nocelo, S.F.; del Río, J.Q.; Sanz, C.Z.; Golpe, R.; et al. Impact of a Home Telehealth Program After a Hospitalized COPD Exacerbation: A Propensity Score Analysis. Arch. Bronconeumol. 2022, 58, 474–481. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.; Kim, S.; Moon, D.; Jo, H. Effects of Transitional Care after Hospital Discharge in Patients with Chronic Obstructive Pulmonary Disease: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6053. [Google Scholar] [CrossRef]
- Hartman, M.; Mináriková, J.; Batalik, L.; Pepera, G.; Su, J.J.; Formiga, M.F.; Cahalin, L.; Dosbaba, F. Effects of Home-Based Training with Internet Telehealth Guidance in COPD Patients Entering Pulmonary Rehabilitation: A Systematic Review. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2305–2319. [Google Scholar] [CrossRef]
- Sculley, J.A.; Musick, H.; Krishnan, J.A. Telehealth in chronic obstructive pulmonary disease: Before, during, and after the coronavirus disease 2019 pandemic. Curr. Opin. Pulm. Med. 2022, 28, 93–98. [Google Scholar] [CrossRef]
- Barbosa, M.T.; Sousa, C.S.; Morais-Almeida, M.; Simões, M.J.; Mendes, P. Telemedicine in COPD: An Overview by Topics. COPD 2020, 17, 601–617. [Google Scholar] [CrossRef]
- Janjua, S.; Carter, D.; Threapleton, C.J.; Prigmore, S.; Disler, R.T. Telehealth interventions: Remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2021, 7, CD013196. [Google Scholar] [CrossRef]
- Casas, A.; Troosters, T.; Garcia-Aymerich, J.; Roca, J.; Hernández, C.; Alonso, A.; del Pozo, F.; de Toledo, P.; Antó, J.M.; Rodríguez-Roisín, R.; et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur. Respir. J. 2006, 28, 123–130. [Google Scholar] [CrossRef]
- Annandale, J.; Hurlin, C.; Lewis, K. Reducing COPD admissions with a specialist chronic disease management team. Nurs. Times 2009, 105, 25. [Google Scholar] [PubMed]
- Vestbo, J.; Anderson, J.A.; Calverley, P.M.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Knobil, K.; Willits, L.R.; Yates, J.C.; Jones, P.W. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009, 64, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.M.; Kuykendall, D.H.; Johnson, M.L.; Wray, N.P.; Wu, L. The association between the quality of inpatient care and early readmission. Ann. Intern. Med. 1995, 122, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.A.; Donaldson, G.C.; Hurst, J.R.; Seemungal, T.A.R.; Wedzicha, J.A. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 169, 1298–1303. [Google Scholar] [CrossRef]
- Oancea, C.; Fira-Mladinescu, O.; Timar, B.; Tudorache, V. Impact of medical education program on COPD patients: A cohort prospective study. Wien. Klin. Wochenschr. 2015, 127, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Miravitlles, M.; Hurst, J.R.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Management of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 49, 1600791. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Chu, J.; Yurkovich, M.; Harriman, D.; Taraboanta, C.; Fitzgerald, J.M. Variations in the management of acute exacerbations of chronic obstructive pulmonary disease. Can. Respir. J. 2013, 20, 175–179. [Google Scholar] [CrossRef]
- Boulet, L.-P.; Bourbeau, J.; Skomro, R.; Gupta, S. Major care gaps in asthma, sleep and chronic obstructive pulmonary disease: A road map for knowledge translation. Can. Respir. J. 2013, 20, 265–269. [Google Scholar] [CrossRef]
- Kripalani, S.; Jackson, A.T.; Schnipper, J.L.; Coleman, E.A. Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists. J. Hosp. Med. 2007, 2, 314–323. [Google Scholar] [CrossRef]
- van Walraven, C.; Oake, N.; Jennings, A.; Forster, A.J. The association between continuity of care and outcomes: A systematic and critical review. J. Eval. Clin. Pract. 2010, 16, 947–956. [Google Scholar] [CrossRef]
- Langsetmo, L.; Platt, R.W.; Ernst, P.; Bourbeau, J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am. J. Respir. Crit. Care Med. 2008, 177, 396–401. [Google Scholar] [CrossRef]
- Pullen, R.; Miravitlles, M.; Sharma, A.; Singh, D.; Martinez, F.; Hurst, J.R.; Alves, L.; Dransfield, M.; Chen, R.; Muro, S.; et al. CONQUEST Quality Standards: For the Collaboration on Quality Improvement Initiative for Achieving Excellence in Standards of COPD Care. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2301–2322. [Google Scholar] [CrossRef] [PubMed]
- Fidahussein, S.S.; Croghan, I.T.; Cha, S.S.; Klocke, D.L. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag. Healthc. Policy 2014, 7, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; Rose, E.S.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Lipworth, B.; Skinner, D.; Devereux, G.; Thomas, V.; Jie, J.L.Z.; Martin, J.; Carter, V.; Price, D.B. Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease. Heart 2016, 102, 1909–1914. [Google Scholar] [CrossRef]
- Du, Q.; Sun, Y.; Ding, N.; Lu, L.; Chen, Y. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: A meta-analysis of observational studies. PLoS ONE 2014, 9, e113048. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Salpeter, S.; Ormiston, T.; Salpeter, E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2005, 4, CD003566. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Anker, S.D.; Dahlström, U.; Filippatos, G.; Ponikowski, P.; Zannad, F.; Amir, O.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; et al. Heart Failure Association of the ESC, Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2013, 15, 1173–1184. [Google Scholar] [CrossRef]
- Dewan, P.; Docherty, K.F.; Bengtsson, O.; de Boer, R.A.; Desai, A.S.; Drozdz, J.; Hawkins, N.M.; Inzucchi, S.E.; Kitakaze, M.; Køber, L.; et al. Effects of dapagliflozin in heart failure with reduced ejection fraction and chronic obstructive pulmonary disease: An analysis of DAPA-HF. Eur. J. Heart Fail. 2021, 23, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Canepa, M.; Ameri, P.; Lainscak, M. Chronic obstructive pulmonary disease and comorbidities in heart failure: The next frontier of sodium-glucose co-transporter 2 inhibitors? Eur. J. Heart Fail. 2021, 23, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Pascual-Figal, D.; Fabregat, J.; Domingo, M.; Planas, F.; Casas, T.; Ordoñez-Llanos, J.; Valdes, M.; Cinca, J. Serial NT-proBNP monitoring and outcomes in outpatients with decompensation of heart failure. Int. J. Cardiol. 2007, 120, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Domingo, M.; Casas, T.; Gich, I.; Ordoñez-Llanos, J.; Martínez, P.; Cinca, J.; Valdés, M.; Januzzi, J.L.; Bayes-Genis, A. Usefulness of clinical and NT-proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur. Heart J. 2008, 29, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Asano, T.; Takahashi, O.; Komiyama, N.; Ohde, S. The minimal informative monitoring interval of N-terminal pro-B-type natriuretic peptide in patients with stable heart failure. BMC Cardiovasc. Disord. 2020, 20, 262. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax 2012, 67, 957–963. [Google Scholar] [CrossRef]
- Whittaker, H.; Rubino, A.; Müllerová, H.; Morris, T.; Varghese, P.; Xu, Y.; De Nigris, E.; Quint, J.K. Frequency and Severity of Exacerbations of COPD Associated with Future Risk of Exacerbations and Mortality: A UK Routine Health Care Data Study. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 427–437. [Google Scholar] [CrossRef]
- Rothnie, K.J.; Müllerová, H.; Smeeth, L.; Quint, J.K. Natural History of Chronic Obstructive Pulmonary Disease Exacerbations in a General Practice-based Population with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 464–471. [Google Scholar] [CrossRef]
- Sethi, S.; Make, B.J.; Robinson, S.B.; Kumar, S.; Pollack, M.; Moretz, C.; Dreyfus, J.; Xi, A.; Powell, D.; Feigler, N. Relationship of COPD Exacerbation Severity and Frequency on Risks for Future Events and Economic Burden in the Medicare Fee-For-Service Population. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.; Tkacz, J.; Schinkel, J. Exacerbations and real-world outcomes (EROS) among patients with COPD receiving single inhaler triple therapy of budesonide/glycopyrrolate/formoterol fumarate [Poster Discussion]. In Proceedings of the American Thoracic Society International Conference, Washington, DC, USA, 19–24 May 2023. [Google Scholar]
- Donaldson, G.C.; Hurst, J.R.; Smith, C.J.; Hubbard, R.B.; Wedzicha, J.A. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010, 137, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.; Tave, A.; Neikirk, A. Incidence of acute cardiovascular events following acute exacerbation of chronic obstructive pulmonary disease in a large US claims database [Thematic Poster Session]. In Proceedings of the American Thoracic Society International Conference, Washington, DC, USA, 19–24 May 2023. [Google Scholar]
- Dransfield, M.; Halpin, D.; Han, M.L.; Hartley, B.; Jones, C.E.; Kalhan, R.; Kilbride, S.; Kunisaki, K.; Lange, P.; Lipson, D.; et al. Time-Dependent Risk Of Cardiovascular Events Following An Exacerbation In Patients With Copd: Post Hoc Analysis From The Impact Triall. Chest 2020, 158, A1722–A1726. [Google Scholar] [CrossRef]


| Laboratory Tests | Imaging |
| Blood cell count Plasma electrolytes | Chest radiography |
| ESR | Lung sonography with assessment of diaphragmatic excursion and pleural effusion |
| CRP | Chest computed tomography scan |
| D-dimer | Diagnostic tests |
| Electrocardiography | |
| hs-cardiac troponin | Pulse oximetry |
| Glycemia | Blood gas analysis |
| Eosinophilic and neutrophilic pattern | |
| NT-proBNP (recommended both as a diagnostic/prognostic test for heart overload or HF or to exclude them) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, A.; Canepa, M.; Catapano, G.A.; Marvisi, M.; Oliva, F.; Passantino, A.; Sarzani, R.; Tarsia, P.; Versace, A.G. Implementation of the Care Bundle for the Management of Chronic Obstructive Pulmonary Disease with/without Heart Failure. J. Clin. Med. 2024, 13, 1621. https://doi.org/10.3390/jcm13061621
Bianco A, Canepa M, Catapano GA, Marvisi M, Oliva F, Passantino A, Sarzani R, Tarsia P, Versace AG. Implementation of the Care Bundle for the Management of Chronic Obstructive Pulmonary Disease with/without Heart Failure. Journal of Clinical Medicine. 2024; 13(6):1621. https://doi.org/10.3390/jcm13061621
Chicago/Turabian StyleBianco, Andrea, Marco Canepa, Giosuè Angelo Catapano, Maurizio Marvisi, Fabrizio Oliva, Andrea Passantino, Riccardo Sarzani, Paolo Tarsia, and Antonio Giovanni Versace. 2024. "Implementation of the Care Bundle for the Management of Chronic Obstructive Pulmonary Disease with/without Heart Failure" Journal of Clinical Medicine 13, no. 6: 1621. https://doi.org/10.3390/jcm13061621






