Resistance Exercise Program Is Feasible and Effective in Improving Functional Strength in Post-COVID Survivors
Abstract
:1. Introduction
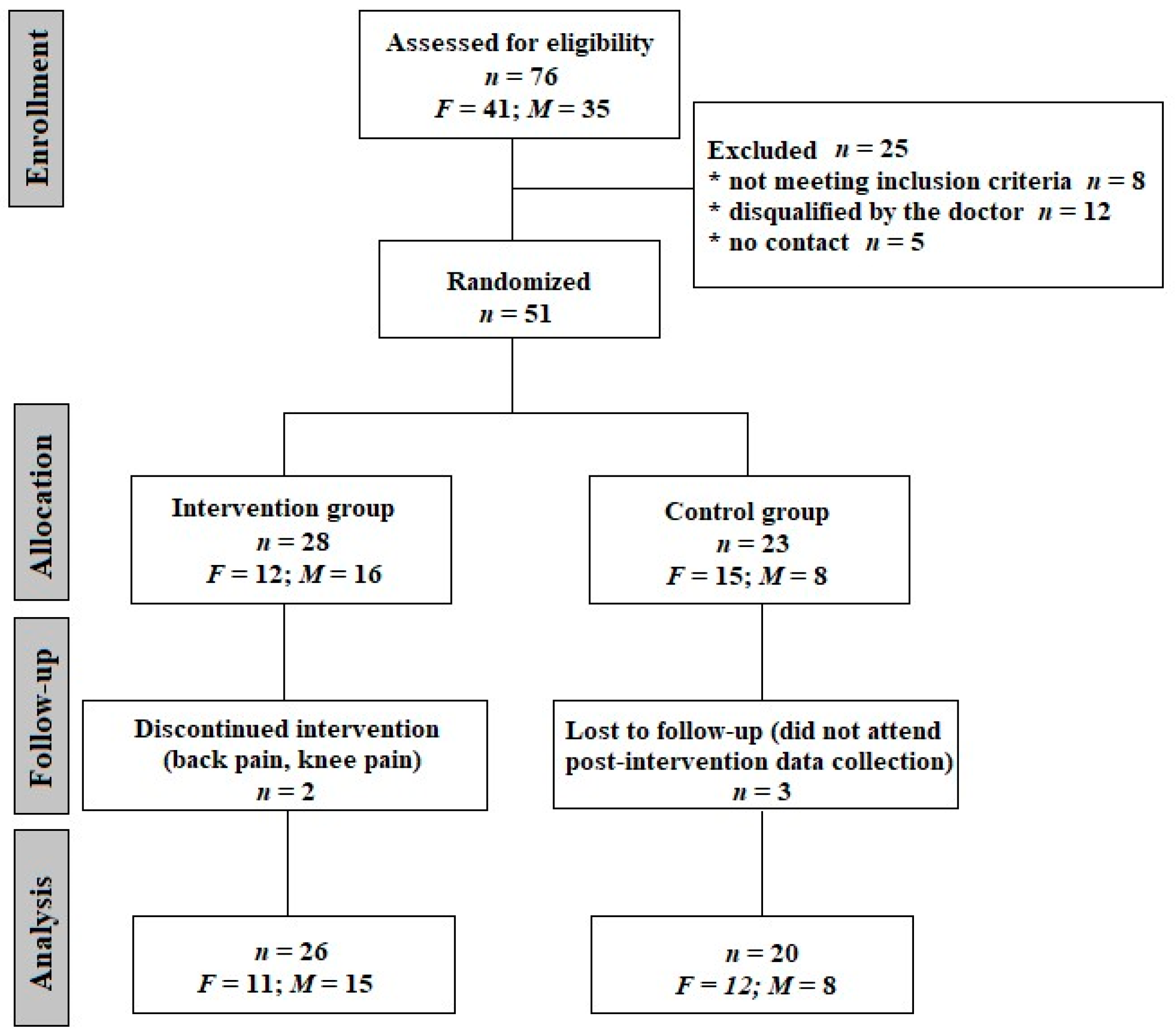
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.2.1. Muscle Strength Tested in Static Conditions
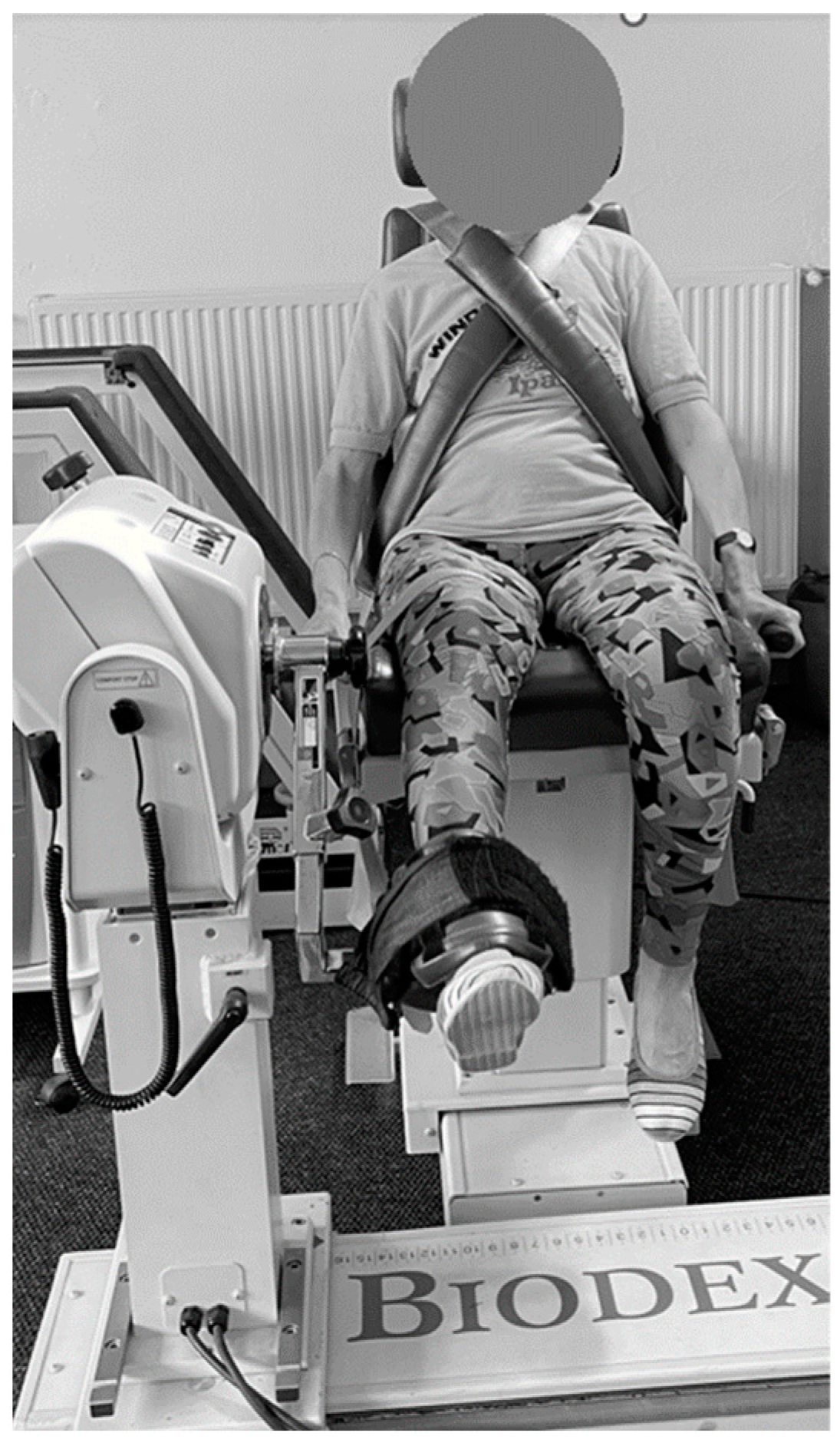
2.2.2. Isokinetic Strength Evaluation
2.2.3. Functional Tests
Time Up and Go test (TUG)
Chair Stand Test
- CS-30 scores—participants were instructed to complete sit-to-stand trials using a 40 cm high seat without using their arms as many times as possible in 30 s [35]. The number of stands was recorded.
- 5STS—participants were asked to sit on a 40 cm high seat without using their arms and then stand repeatedly for five times as quickly as possible [36]. The time was recorded.
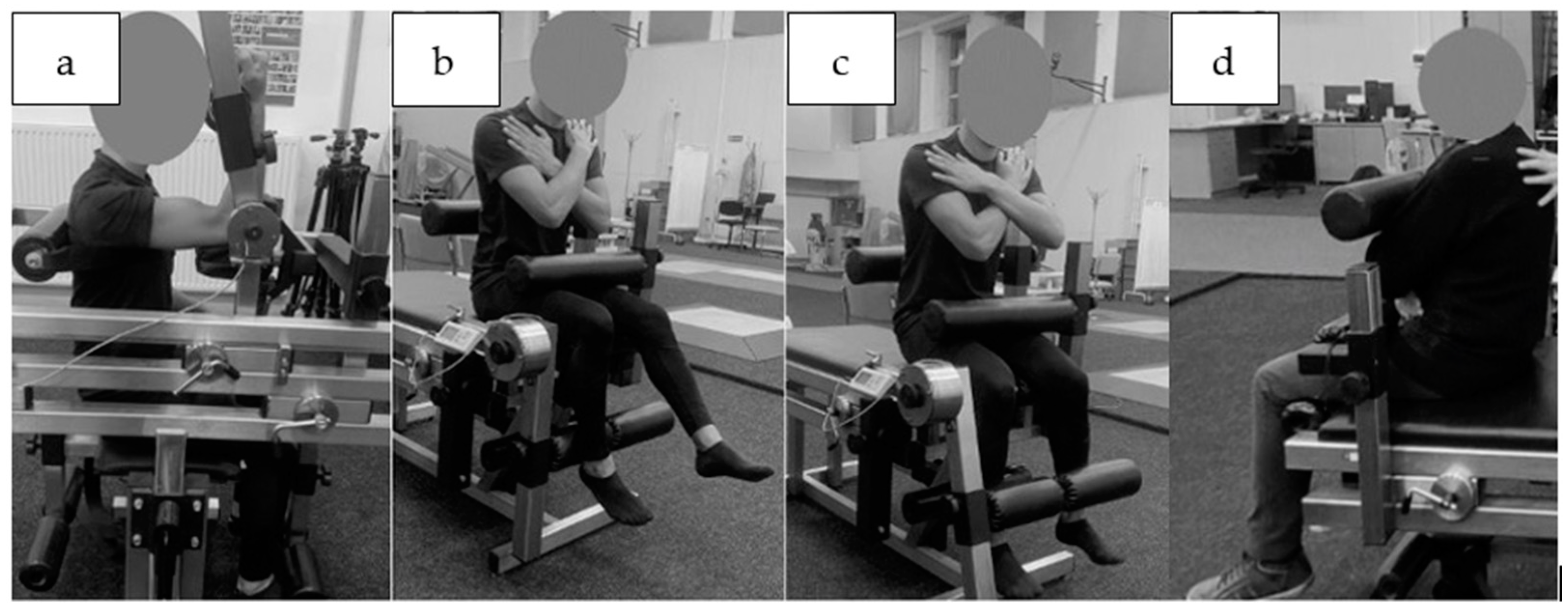
2.3. Intervention
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2022. 2022. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 2 December 2023).
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real-time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. At Least 17 Million People in the WHO European Region Experienced Long COVID in the First Two Years of the Pandemic; Millions May Have to Live with It for Years to Come. 2022. Available online: https://www.who.int/europe/news/item/13-09-2022-at-least-17-million-people-in-the-who-european-region-experienced-long-covid-in-the-first-two-years-of-the-pandemic--millions-may-have-to-live-with-it-for-years-to-come (accessed on 18 December 2023).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. CDC. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 18 December 2023).
- World Health Organization. Post COVID-19 Condition. 2023. Available online: https://www.who.int/teams/health-care-readiness/post-covid-19-condition (accessed on 19 December 2023).
- Baldassano, S.; Alioto, A.; Amato, A.; Rossi, C.; Messina, G.; Bruno, M.R.; Stallone, R.; Proia, P. Fighting the Consequences of the COVID-19 Pandemic: Mindfulness, Exercise, and Nutrition Practices to Reduce Eating Disorders and Promote Sustainability. Sustainability 2023, 15, 2120. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Siso-Almirall, A.; Brito-Zeron, P.; Conangla Ferrin, L.; Kostov, B.; Moragas Moreno, A.; Mestres, J.; Sellarès, J.; Galindo, G.; Morera, R.; Basora, J.; et al. Long COVID-19: Proposed primary care clinical guidelines for diagnosis and disease management. Int. J. Environ. Res. Public Health 2021, 18, 4350. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Brigham, E.; Connolly, B.; McPeake, J.; Agranovich, A.V.; Kenes, M.T.; Casey, K.; Reynolds, C.; Schmidt, K.F.R.; Kim, S.Y.; et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: A multidisciplinary model of care. Lancet Respir. Med. 2021, 9, 1328–1341. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March–June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from the hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Carfi, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- National Institutes of Health. NIH Launches Long COVID Clinical Trials through RECOVER Initiative, Opening Enrollment. NIH. 2023. Available online: https://www.nih.gov/news-events/news-releases/nih-launches-long-covid-clinical-trials-through-recover-initiative-opening-enrollment (accessed on 6 December 2023).
- Cheng, H.; Wang, Y.; Wang, G.-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020, 92, 726–730. [Google Scholar] [CrossRef] [PubMed]
- De Micheli, A.J.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Bobowik, P.; Wiszomirska, I.; Gajewski, J.; Kaczmarczyk, K. Muscle strength and equilibrium-maintaining ability in post-COVID women. Gait Posture 2023, 106, S91–S92. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; van Loon, L.J. Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Kortebein, P.; Symons, T.B.; Ferrando, A.; Paddon-Jones, D.; Ronsen, O.; Protas, E.; Conger, S.; Lombeida, J.; Wolfe, R.; Evans, W.J. Functional impact of 10 days of bed rest in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1076–1081. [Google Scholar] [CrossRef]
- NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. 2021. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 18 December 2023).
- Maley, J.H.; Alba, G.A.; Barry, J.T.; Bartels, M.N.; Fleming, T.K.; Oleson, C.V.; Rydberg, L.; Sampsel, S.; Silver, J.K.; Sipes, S.; et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of breathing discomfort and respiratory sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PMR 2022, 14, 77–95. [Google Scholar] [CrossRef]
- De Souza, Y.; Macedo, J.; Nascimento, R.; Alves, M.A.M.; Medeiros, S.; Leal, L.; Soares, J. Low-Intensity Pulmonary Rehabilitation Through Videoconference for Post-Acute COVID-19 Patients. Am. J. Respir. Crit. Care Med. 2021, 203, A4124. [Google Scholar]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef] [PubMed]
- McNarry, M.A.; Berg, R.M.; Shelley, J.; Hudson, J.; Saynor, Z.L.; Duckers, J.; Lewis, K.; Davies, G.A.; Mackintosh, K.A. Inspiratory muscle training enhances recovery post COVID-19: A randomised controlled trial. Eur. Respir. J. 2022, 60, 2103101. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Nourmahnad, A.; Sinha, I. Optimizing Skeletal Muscle Anabolic Response to Resistance Training in Aging. Front. Physiol. 2020, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Bong, Y.; Song, W. The effects of elastic band exercises and nutritional education on frailty, strength, and nutritional intake in elderly women. Phys. Act. Nutr. 2020, 24, 37–45. [Google Scholar] [CrossRef]
- Akkurt, M.; Ökmen, M.Ş.; Polat, M. Effects of eight-week aerobic exercises combined with resistance training on cardiovascular risk factors in women. Biomed. Hum. Kinet. 2023, 15, 1–8. [Google Scholar] [CrossRef]
- Cotler, J.; Holtzman, C.; Dudun, C.; Jason, L.A. A Brief Questionnaire to Assess Post-Exertional Malaise. Diagnostics 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Buśko, K.; Gajewski, J. Muscle strength and power of elite female and male swimmers. Balt. J. Health Phys. Act. 2011, 3, 13–18. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Brzycki, M. Strength testing—Predicting a one-rep max from reps to fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, S.; Jovell-Fernández, E.; Cuadra-Llopart, L.; Rodríguez-Sanz, J.; Labata-Lezaun, N.; López-de-Celis, C.; Bosch, J.; Pérez-Bellmunt, A. Correlation between Power Elbow Flexion and Physical Performance Test: A Potential Predictor for Assessing Physical Performance in Older Adults. J. Clin. Med. 2023, 12, 5560. [Google Scholar] [CrossRef] [PubMed]
- Harbo, T.; Brincks, J.; Andersen, H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur. J. Appl. Physiol. 2012, 112, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Kuipers, R.; Rombouts, J.A.; Brouwers, K.; Schrauwen-Hinderling, V.B.; Wildberger, J.E.; Verdijk, L.B.; van Loon, L.J. Thigh muscles are more susceptible to age-related muscle loss when compared to lower leg and pelvic muscles. Exp. Gerontol. 2023, 175, 112159. [Google Scholar] [CrossRef] [PubMed]
- Šarabon, N.; Kozinc, Ž.; Perman, M. Establishing Reference Values for Isometric Knee Extension and Flexion Strength. Front. Physiol. 2021, 12, 767941. [Google Scholar] [CrossRef] [PubMed]
- Van Roie, E.; Verschueren, S.M.; Boonen, S.; Bogaerts, A.; Kennis, E.; Coudyzer, W.; Delecluse, C. Force-velocity characteristics of the knee extensors: An indication of the risk for physical frailty in elderly women. Arch. Phys. Med. Rehabil. 2011, 92, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.T.; Jenkins, N.D.M.; Mustad, V.A.; Weir, J.P. Isokinetic Dynamometry in Healthy Versus Sarcopenic and Malnourished Elderly: Beyond Simple Measurements of Muscle Strength. J. Appl. Gerontol. 2017, 36, 709–732. [Google Scholar] [CrossRef]
- Oh, S.L.; Kim, H.J.; Woo, S.; Cho, B.L.; Song, M.; Park, Y.H.; Lim, J.; Song, W. Effects of an integrated health education and elastic band resistance training program on physical function and muscle strength in community-dwelling elderly women: Healthy Aging and Happy Aging II study. Geriatr. Gerontol. Int. 2017, 17, 825–833. [Google Scholar] [CrossRef]
- Kienbacher, T.; Paul, B.; Habenicht, R.; Starek, C.; Wolf, M.; Kollmitzer, J.; Ebenbichler, G. Reliability of isometric trunk moment measurements in healthy persons over 50 years of age. J. Rehabil. Med. 2014, 46, 241–249. [Google Scholar] [CrossRef]
- Sousa, N.; Sampaio, J. Effects of progressive strength training on the performance of the Functional Reach Test and the Timed Get-Up-and-Go Test in an elderly population from the rural north of Portugal. Am. J. Hum. Biol. 2005, 17, 746–751. [Google Scholar] [CrossRef]
- Kurosawa, C.; Shimazu, N.; Yamamoto, S. Where do healthy older adults take more time during the Timed Up and Go test? J. Phys. Ther. Sci. 2020, 32, 663–668. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Reid, N.; Healy, G.N.; Gianoudis, J.; Formica, M.; Gardiner, P.A.; Eakin, E.E.; Nowson, C.A.; Daly, R.M. Association of sitting time and breaks in sitting with muscle mass, strength, function, and inflammation in community-dwelling older adults. Osteoporos. Int. 2018, 29, 1341–1350. [Google Scholar] [CrossRef]
- De Souza Vale, R.G.; Guimarães, A.C.; Cader, S.A.; Wood, R.; André, H.I.O.V.; Pinto de Castro, J.B.; Estélio Henrique Martin Dantas, E.H.D. Balance, physical conditioning, and health perception in elderly women submitted to a 32-week physical exercise program. Biomed. Hum. Kinet. 2022, 14, 33–40. [Google Scholar] [CrossRef]
- Ostchega, Y.; Harris, T.B.; Hirsch, R.; Parsons, V.L.; Kington, R.; Katzoff, M. Reliability and prevalence of physical performance examination assessing mobility and balance in older persons in the US: Data from the Third National Health and Nutrition Examination Survey. J. Am. Geriatr. Soc. 2000, 48, 1136–1141. [Google Scholar] [CrossRef]
- Sato, K.; Kuroki, K.; Saiki, S.; Nagatomi, R. Improving Walking, Muscle Strength, and Balance in the Elderly with an Exergame Using Kinect: A Randomized Controlled Trial. Games Health J. 2015, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]

| Intervention (f = 11, m = 15) | Control (f = 12, m = 8) | |
|---|---|---|
| Female | ||
| Age (years) | 69.3 ± 5.2 | 73.3 ± 7.4 |
| Body mass (kg) | 65.3 ± 10.5 | 72.6 ± 12.1 |
| Height (m) | 163.1 ± 7.6 | 160.9 ± 5.7 |
| BMI (kg/m2) | 24.5 ± 4.8 | 28.1 ± 3.3 |
| Male | ||
| Age (years) | 69.5 ± 4.8 | 75.6 ± 7.1 * |
| Body mass (kg) | 87.7 ± 15.1 | 89.5 ± 20.3 |
| Height (m) | 176.7 ± 6.9 | 179.0 ± 6.1 |
| BMI (kg/m2) | 27.86 ± 3.4 | 27.07 ± 5.4 |
| Post-COVID-19 Symptom | Dizziness and Equilibrium Disorders | Perceived Muscle Weakness | Exercise Intolerance | Memory and Concentration Symptoms |
|---|---|---|---|---|
| Before intervention | 46.2% | 38.5% | 38.5% | 15.3% |
| After intervention | 15.4% * | 7.6% ** | 3.8% ** | 7.6% |
| GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Intervention | |||||||
| TESTING SESSION | GROUP × TESTING SESSION | |||||||
| Male (n = 8) | Female (n = 12) | Male (n = 15) | Female (n = 11) | F1,42 | p | η2 | ||
| EF/m (N·m·kg−1) | Before | 0.71 ± 0.19 | 0.42 ± 0.10 | 0.75 ± 0.14 | 0.49 ± 0.16 | 17.41 | 0.0001 | 0.293 |
| After | 0.62 ± 0.14 | 0.45 ± 0.09 | 0.78 ± 0.13 | 0.59 ± 0.13 ** | ||||
| KF/m (N·m·kg−1) | Before | 0.94 ± 0.22 | 0.69 ± 0.21 | 1.08 ± 0.31 | 0.75 ± 0.22 | 10.15 | 0.0027 | 0.195 |
| After | 0.97 ± 0.27 | 0.69 ± 0.18 | 1.21 ± 0.28 * | 0.94 ± 0.28 ** | ||||
| KE/m (N·m·kg−1) | Before | 1.55 ± 0.39 | 1.23 ± 0.27 | 1.84 ± 0.54 | 1.43 ± 0.43 | 11.35 | 0.0016 | 0.213 |
| After | 1.26 ± 0.38 | 1.28 ± 0.22 | 2.00 ± 0.46 | 1.57 ± 0.45 | ||||
| TF/m (N·m·kg−1) | Before | 1.51 ± 0.40 | 1.05 ± 0.31 | 1.84 ± 0.52 | 1.14 ± 0.33 | 14.21 | 0.0005 | 0.253 |
| After | 1.44 ± 0.38 | 1.13 ± 0.42 | 2.27 ± 0.67 *** | 1.46 ± 0.34 * | ||||
| TE/m (N·m·kg−1) | Before | 2.65 ± 0.77 | 1.64 ± 0.69 | 3.20 ± 1.08 | 2.64 ± 1.23 | 15.75 | 0.0003 | 0.273 |
| After | 2.48 ± 0.76 | 1.84 ± 0.82 | 4.10 ± 0.81 *** | 3.44 ± 1.30 ** | ||||
| GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Intervention | |||||||
| TESTING SESSION | GROUP × TESTING SESSION | |||||||
| Male (n = 8) | Female (n = 12) | Male (n = 15) | Female (n = 11) | F1,42 | p | η2 | ||
| Peak Torque E60 (N·m) | Before | 109.3 ± 34.8 | 78.5 ± 20.0 | 143.5 ± 39.4 | 84.1 ± 15.8 | 1.06 | 0.3095 | 0.025 |
| After | 120.5 ± 24.5 | 80.0 ± 19.0 | 160.0 ± 37.2 * | 90.9 ± 18.6 | ||||
| Peak Torque/m E60 (N·m·kg−1) | Before | 1.29 ± 0.59 | 1.10 ± 0.30 | 1.65 ± 0.36 | 1.31 ± 0.28 | 1.11 | 0.2982 | 0.026 |
| After | 1.47 ± 0.55 | 1.11 ± 0.29 | 1.86 ± 0.40 ** | 1.43 ± 0.31 | ||||
| Peak Torque F60 (N·m) | Before | 68.1 ± 29.4 | 45.4 ± 19.6 | 76.4 ± 31.1 | 46.4 ± 13.5 | 3.73 | 0.0601 | 0.082 |
| After | 69.2 ± 23.0 | 47.2 ± 14.1 | 88.3 ± 27.2 * | 54.3 ± 13.6 | ||||
| Peak Torque/m F60 (N·m·kg−1) | Before | 0.78 ± 0.36 | 0.64 ± 0.30 | 0.88 ± 0.34 | 0.74 ± 0.26 | 3.30 | 0.0766 | 0.073 |
| After | 0.83 ± 0.37 | 0.65 ± 0.20 | 1.02 ± 0.28 | 0.86 ± 0.25 | ||||
| GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Intervention | |||||||
| TESTING SESSION | GROUP × TESTING SESSION | |||||||
| Male (n = 8) | Female (n = 12) | Male (n = 15) | Female (n = 11) | F1,42 | p | η2 | ||
| Peak Torque E180 (N·m) | Before | 65.08 ± 13.36 | 45.00 ± 19.29 | 88.32 ± 25.72 | 48.33 ± 14.62 | 0.16 | 0.6870 | 0.004 |
| After | 78.15 ± 14.81 | 53.16 ± 14.07 | 96.62 ± 20.05 | 58.12 ± 12.33 | ||||
| Peak Torque/m E180 (N·m·kg−1) | Before | 0.76 ± 0.26 | 0.63 ± 0.28 | 1.02 ± 0.27 | 0.76 ± 0.24 | 0.09 | 0.7705 | 0.002 |
| After | 0.94 ± 0.30 * | 0.74 ± 0.21 | 1.12 ± 0.24 | 0.91 ± 0.21 * | ||||
| Peak Torque F180 (N·m) | Before | 47.08 ± 16.67 | 34.14 ± 16.70 | 61.03 ± 27.26 | 35.13 ± 10.89 | 0.10 | 0.7573 | 0.002 |
| After | 57.86 ± 14.98 | 36.90 ± 14.05 | 67.02 ± 20.62 | 45.16 ± 14.68 | ||||
| Peak Torque/m F180 (N·m·kg−1) | Before | 0.56 ± 0.25 | 0.48 ± 0.24 | 0.70 ± 0.28 | 0.56 ± 0.20 | 0.27 | 0.6086 | 0.006 |
| After | 0.70 ± 0.28 | 0.52 ± 0.23 | 0.78 ± 0.23 | 0.71 ± 0.23 | ||||
| GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Intervention | |||||||
| TESTING SESSION | GROUP × TESTING SESSION | |||||||
| Male (n = 8) | Female (n = 12) | Male (n = 15) | Female (n = 11) | F1,42 | p | η2 | ||
| Flexors/Extensors ratio (%) 60 | Before | 62.20 ± 20.79 | 56.30 ± 18.10 | 51.95 ± 10.80 | 54.82 ± 11.06 | 3.44 | 0.0707 | 0.076 |
| After | 55.08 ± 14.85 | 56.85 ± 8.75 | 55.01 ± 8.28 | 59.90 ± 11.76 | ||||
| Flexors/Extensors ratio (%) 180 | Before | 73.16 ± 23.67 | 73.48 ± 12.36 | 67.42 ± 15.50 | 72.91 ± 14.11 | 0.48 | 0.4925 | 0.011 |
| After | 74.29 ± 14.75 | 68.58 ± 13.87 | 69.06 ± 13.51 | 74.49 ± 15.74 | ||||
| GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Intervention | |||||||
| TESTING SESSION | GROUP × TESTING SESSION | |||||||
| Male (n = 8) | Female (n = 12) | Male (n = 15) | Female (n = 11) | F1,42 | p | η2 | ||
| TUG (s) | Before | 6.16 ± 0.91 | 6.83 ± 1.57 | 5.71 ± 0.89 | 5.59 ± 0.69 | 3.06 | 0.0876 | 0.068 |
| After | 6.21 ± 1.18 | 6.65 ± 1.34 | 5.17 ± 0.63 | 5.21 ± 0.77 | ||||
| Chair Test 5STS (s) | Before | 9.10 ± 2.56 | 10.78 ± 4.54 | 9.48 ± 2.00 | 8.78 ± 1.98 | 8.49 | 0.0057 | 0.168 |
| After | 8.72 ± 2.47 | 9.60 ± 3.22 | 6.65 ± 1.15 ### | 7.01 ± 1.35 # | ||||
| Z | p | R | ||||||
| Chair Test CS-30 (n) | Before | 17.5 (13.0–22.5) | 19.0 (13.0–19.5) | 15.0 (14.0–20.0) | 18.0 (14.0–21.0) | 4.65 | 0.0001 | 0.806 |
| After | 17.0 (14.0–24.0) | 16.0 (13.0–19.0) | 24.0 (21.0–28.0) *## | 25.0 (19.0–28.0) **## | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarczyk, K.; Matharu, Y.; Bobowik, P.; Gajewski, J.; Maciejewska-Skrendo, A.; Kulig, K. Resistance Exercise Program Is Feasible and Effective in Improving Functional Strength in Post-COVID Survivors. J. Clin. Med. 2024, 13, 1712. https://doi.org/10.3390/jcm13061712
Kaczmarczyk K, Matharu Y, Bobowik P, Gajewski J, Maciejewska-Skrendo A, Kulig K. Resistance Exercise Program Is Feasible and Effective in Improving Functional Strength in Post-COVID Survivors. Journal of Clinical Medicine. 2024; 13(6):1712. https://doi.org/10.3390/jcm13061712
Chicago/Turabian StyleKaczmarczyk, Katarzyna, Yogi Matharu, Patrycja Bobowik, Jan Gajewski, Agnieszka Maciejewska-Skrendo, and Kornelia Kulig. 2024. "Resistance Exercise Program Is Feasible and Effective in Improving Functional Strength in Post-COVID Survivors" Journal of Clinical Medicine 13, no. 6: 1712. https://doi.org/10.3390/jcm13061712





