Reconstructive Paradigms: A Problem-Solving Approach in Complex Tissue Defects
Abstract
:1. Introduction
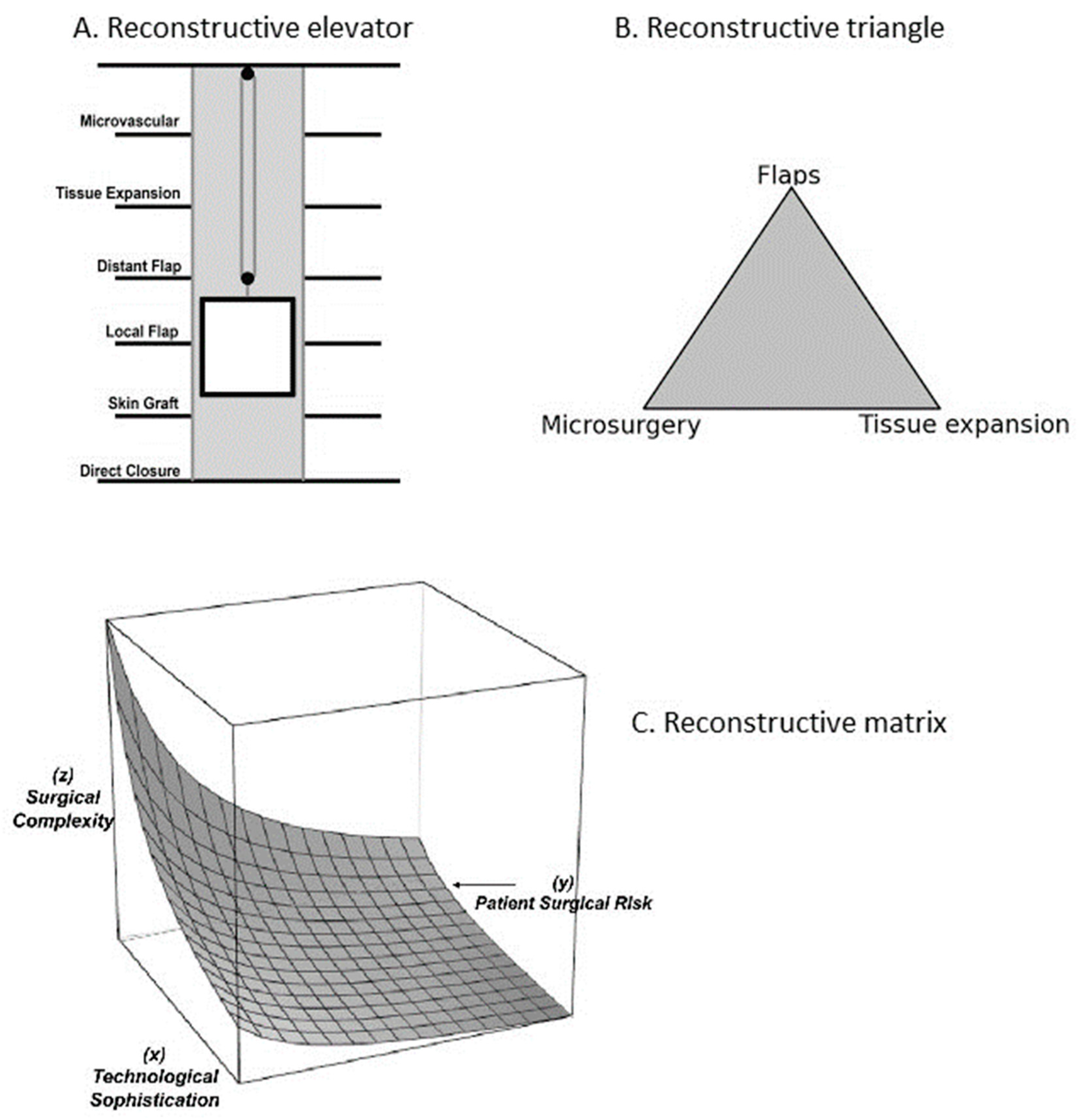
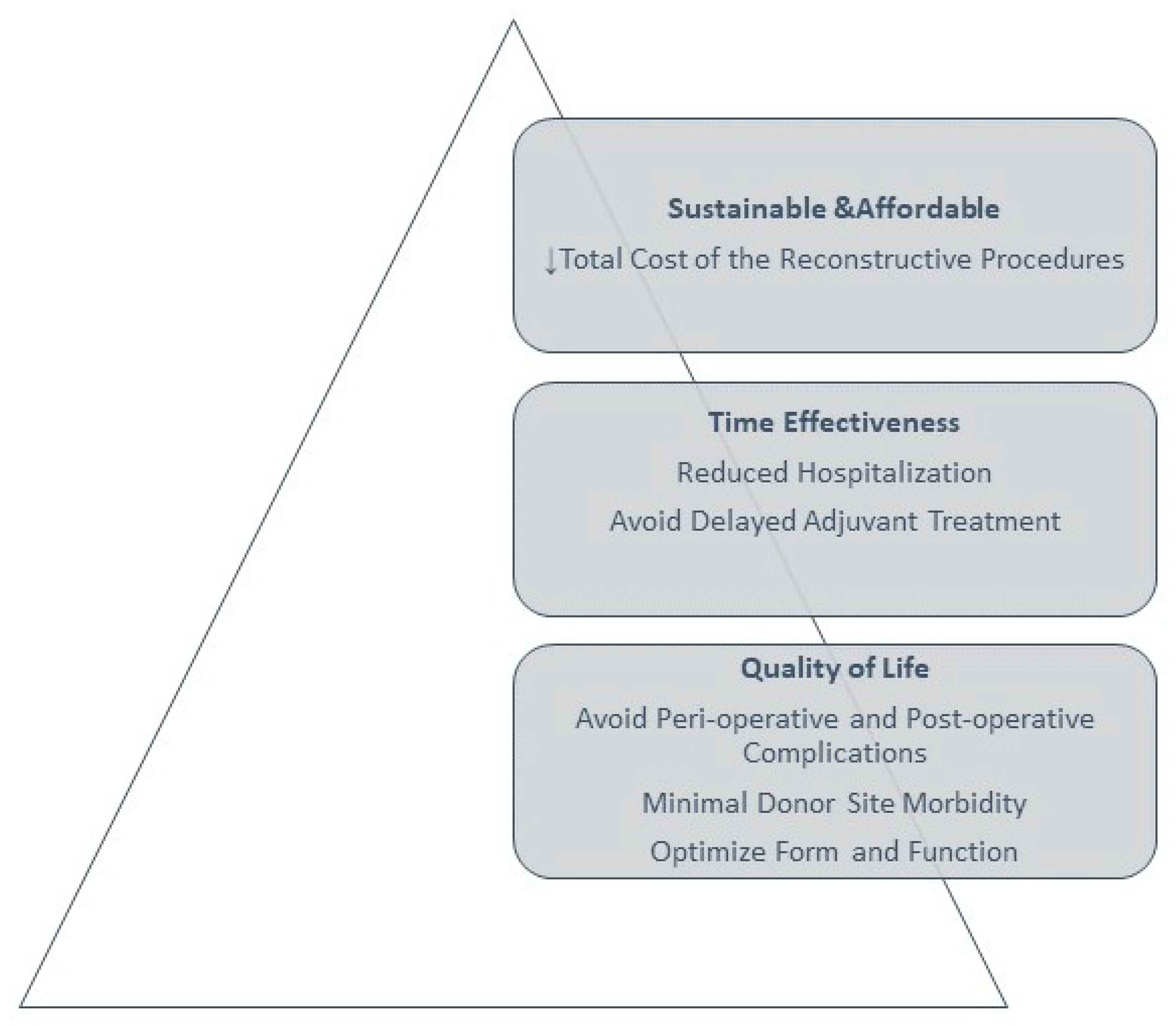
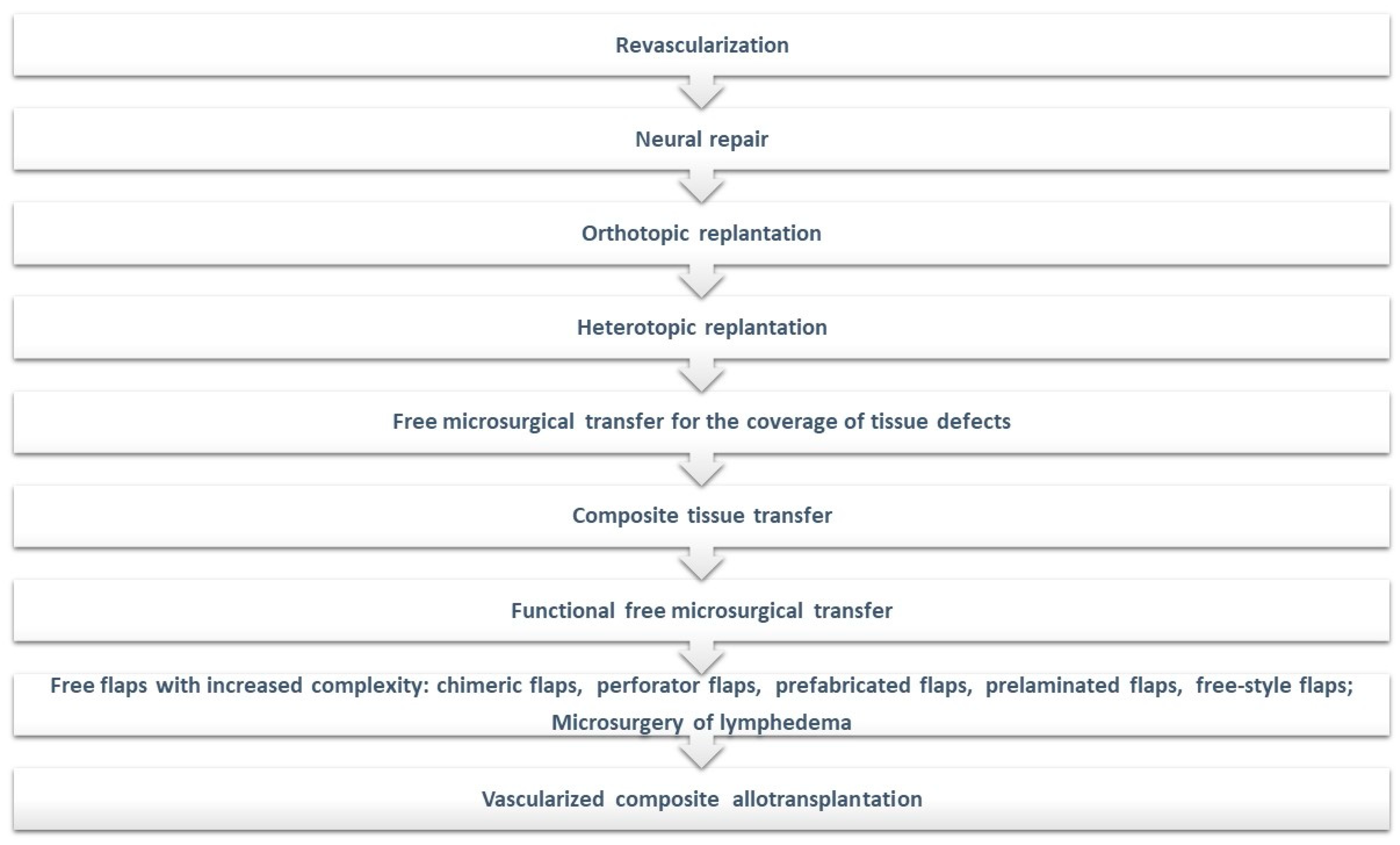
2. Overview of Reconstructive Paradigms
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouhadana, G.; Aljerian, A.; Thibaudeau, S. The Reconstruction of Plastic Surgery: A Historical Perspective on the Etymology of Plastic and Reconstructive Surgery. Plast. Surg. 2023, 31, 366–370. [Google Scholar] [CrossRef]
- Fang, F.; Chung, K.C. An evolutionary perspective on the history of flap reconstruction in the upper extremity. Hand Clin. 2014, 30, 109–122. [Google Scholar] [CrossRef]
- Chambers, J.A.; Ray, P.D. Achieving growth and excellence in medicine: The case history of armed conflict and modern reconstructive surgery. Ann. Plast. Surg. 2009, 63, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Nealeigh, M.; Oh, J.S.; Rothberg, P.; Elster, E.A.; Rich, N.M. Combat casualty care and lessons learned from the past 100 years of war. Curr. Probl. Surg. 2017, 54, 315–351. [Google Scholar] [CrossRef]
- Kwasnicki, R.M.; Hughes-Hallett, A.; Marcus, H.J.; Yang, G.Z.; Darzi, A.; Hettiaratchy, S. Fifty Years of Innovation in Plastic Surgery. Arch. Plast. Surg. 2016, 43, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; O’Connor, M.J.; Attalla, P.; Ewing, E.; Lee, C.J.; Greene, A.; Lee, C.N.; Lifchez, S.; Sacks, J.M.; Gosman, A. Game Changers: Plastic and Reconstructive Surgery Innovations of the Last 100 Years. Plast. Reconstr. Surg. Glob. Open. 2023, 11, e5209. [Google Scholar] [CrossRef] [PubMed]
- Al Deek, N.F.; Wei, F.C. It is the time to say good bye to the reconstructive ladder/lift and its variants. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.C.; Swanson, J.A.; Schmitz, D.; Sullivan, D.; Rohrich, R.J. Introducing evidence-based medicine to plastic and reconstructive surgery. Plast. Reconstr. Surg. 2009, 123, 1385–1389. [Google Scholar] [CrossRef]
- Kuhn, T. The Structure of Scientific Revolutions; The University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Kuhn, T.S. Structura Revoluțiilor Științifice; Editura Humanitas: București, Romania, 2008. [Google Scholar]
- Mathes, S.; Nahai, F. Clinical Application for Muscle and Musculocutaneous Flaps; Mosby: St. Louis, MI, USA, 1982. [Google Scholar]
- Tintle, S.M.; Levin, L.S. The reconstructive microsurgery ladder in orthopaedics. Injury 2013, 44, 376–385. [Google Scholar] [CrossRef]
- De Francesco, F.; Zingaretti, N.; Parodi, P.C.; Riccio, M. The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine. Cells 2023, 12, 2567. [Google Scholar] [CrossRef]
- Wolf, J.M.; Athwal, G.S.; Shin, A.Y.; Dennison, D.G. Acute trauma to the upper extremity: What to do and when to do it. J. Bone Joint. Surg. Am. 2009, 91, 1240–1252. [Google Scholar] [PubMed]
- Simman, R. Wound closure and the reconstructive ladder in plastic surgery. J. Am. Col. Certif. Wound Spec. 2009, 1, 6–11. [Google Scholar] [CrossRef]
- Gosman, A.A. Basics of Flaps. In Essentials of Plastic Surgery; Janis, J.E., Ed.; Quality Medical Publishing Inc.: St. Louis, MI, USA, 2007; pp. 20–38. [Google Scholar]
- Vamadeva, S.V.; Curnier, A. The role of plastic surgery in reconstruction after oncological surgery. Br. J. Hosp. Med. 2016, 77, 328–333. [Google Scholar] [CrossRef]
- Hansen, S.L.; Young, D.M.; Lang, P.; Sbitany, H. Flap classification and applications. In Plastic Surgery, 3rd ed.; Neligan, P.C., Ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Janis, J.E.; Kwon, R.K.; Attinger, C.E. The new reconstructive ladder: Modifications to the traditional model. Plast. Reconstr. Surg. 2011, 127 (Suppl. 1), 205S–212S. [Google Scholar] [CrossRef]
- Nordback, P.H.; Hakkarainen, M.; Mattila, S. A treatment algorithm for reconstruction of soft tissue defects in the hand: A narrative review. Ann. Transl. Med. 2023, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, L.J.; Krieger, L.M. From the reconstructive ladder to the reconstructive elevator. Plast. Reconstr. Surg. 1994, 93, 1503–1504. [Google Scholar] [CrossRef] [PubMed]
- Zeiderman, M.R.; Pu, L.L.Q. Contemporary reconstruction after complex facial trauma. Burns Trauma. 2020, 8, tkaa003. [Google Scholar] [CrossRef]
- Mathes, S.J.; Nahai, F. Reconstructive Surgery: Principles, Anatomy & Technique; Churchill Livingstone St. Louis Quality Medical: New York, NY, USA, 1997; Volume 2. [Google Scholar]
- Zenn, M.; Jones, G. Reconstructive Surgery: Anatomy, Technique, and Clinical Application; Quality Medical Publishing Inc.: St Louis, MI, USA, 2012. [Google Scholar]
- Hallock, G.G. The Reconstructive Toolbox. Arch. Plast. Surg. 2023, 50, 331–334. [Google Scholar] [CrossRef]
- Erba, P.; Ogawa, R.; Vyas, R.; Orgill, D.P. The reconstructive matrix: A new paradigm in reconstructive plastic surgery. Plast. Reconstr. Surg. 2010, 126, 492–498. [Google Scholar] [CrossRef]
- Wong, C.; Niranjan, N. Reconstructive Stages as an Alternative to the Reconstructive Ladder. Plast. Reconstr. Surg. 2008, 121, 362e–363e. [Google Scholar] [CrossRef]
- Zhang, L.; Weng, T.; Wu, P.; Li, Q.; Han, C.; Wang, X. The Combined Use of Negative-Pressure Wound Therapy and Dermal Substitutes for Tissue Repair and Regeneration. Biomed. Res. Int. 2020, 2020, 8824737. [Google Scholar] [CrossRef]
- Katari, R.; Peloso, A.; Orlando, G. Tissue engineering and regenerative medicine: Semantic considerations for an evolving paradigm. Front. Bioeng. Biotechnol. 2015, 2, 57. [Google Scholar] [CrossRef]
- Himdani, S.; Jessop, Z.M.; Al-Sabah, A.; Combellack, E.; Ibrahim, A.; Doak, S.H.; Hart, A.M.; Archer, C.W.; Thornton, C.A.; Whitaker, I.S. Tissue-Engineered Solutions in Plastic and Reconstructive Surgery: Principles and Practice. Front. Surg. 2017, 4, 4. [Google Scholar] [CrossRef]
- Salibian, A.A.; Widgerow, A.D.; Abrouk, M.; Evans, G.R. Stem cells in plastic surgery: A review of current clinical and translational applications. Arch. Plast. Surg. 2013, 40, 666–675. [Google Scholar] [CrossRef]
- Rusu, M.C.; Vrapciu, A.D.; Hostiuc, S.; Hariga, C.S. Brown adipocytes, cardiac protection and a common adipo- and myogenic stem precursor in aged human hearts. Med. Hypotheses 2015, 85, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Bahadoram, M.; Helalinasab, A.; Namehgoshay-Fard, N.; Akade, E.; Moghaddar, R. Platelet-Rich Plasma Applications in Plastic Surgery. World J. Plast. Surg. 2023, 12, 100–102. [Google Scholar] [CrossRef]
- Teodoreanu, R.N.; Popescu, S.A.; Lascăr, I.; Vulturescu, V.; Grigore, A. Therapeutic protocol using growth factors in electrocution wounds--case reports and review of the literature. Rom. J. Morphol. Embryol. 2014, 55, 473–482. [Google Scholar] [PubMed]
- Behr, B.; Ko, S.H.; Wong, V.W.; Gurtner, G.C.; Longaker, M.T. Stem cells. Plast. Reconstr. Surg. 2010, 126, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in Adipose-Derived Stem Cells Isolation, Characterization, and Application in Regenerative Tissue Engineering. Stem. Cells Int. 2016, 2016, 3206807. [Google Scholar] [CrossRef]
- Gir, P.; Oni, G.; Brown, S.A.; Mojallal, A.; Rohrich, R.J. Human adipose stem cells: Current clinical applications. Plast. Reconstr. Surg. 2012, 129, 1277–1290. [Google Scholar] [CrossRef]
- Fang, J.; Chen, F.; Liu, D.; Gu, F.; Wang, Y. Adipose tissue-derived stem cells in breast reconstruction: A brief review on biology and translation. Stem. Cell Res. Ther. 2021, 12, 8. [Google Scholar] [CrossRef]
- Lin, J.Y.; Wang, C.; Pu, L.L. Can we standardize the techniques for fat grafting? Clin. Plast. Surg. 2015, 42, 199–208. [Google Scholar] [CrossRef]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008, 32, 48–55; discussion 56–57. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef]
- Li, M.; Chen, C. The Efficacy of Cell-Assisted Lipotransfer Versus Conventional Lipotransfer in Breast Augmentation: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2021, 45, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Sharma, S.; Ganta, C.; Ranjan, R.; Jha, S.K. Nanofat: A therapeutic paradigm in regenerative medicine. World J. Stem. Cells 2021, 13, 1733–1746. [Google Scholar] [CrossRef]
- Segreto, F.; Marangi, G.F.; Nobile, C.; Alessandri-Bonetti, M.; Gregorj, C.; Cerbone, V.; Gratteri, M.; Caldaria, E.; Tirindelli, M.C.; Persichetti, P. Use of platelet-rich plasma and modified nanofat grafting in infected ulcers: Technical refinements to improve regenerative and antimicrobial potential. Arch. Plast. Surg. 2020, 47, 217–222. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.E.; Di Vito, C.; Ughi, N.; Tabano, S.; Miozzo, M.; Tremolada, C.; Bolla, G.; Crotti, C.; Ingegnoli, F.; et al. Mesenchymal stem cells: Potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis 2015, 11, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.W.; Mao, J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006, 420, 339–361. [Google Scholar] [CrossRef]
- Chen, J.S.; Wong, V.W.; Gurtner, G.C. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front. Immunol. 2012, 3, 192. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem. Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, I.; Elvira, G.; Zapata, A.G.; Lamana, M.L.; Ramírez, M.; Castro, J.G.; Arranz, M.G.; Vicente, A.; Bueren, J.; García-Olmo, D. Mesenchymal stem cells: Biological properties and clinical applications. Expert Opin. Biol. Ther. 2010, 10, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Meirelles Lda, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Gómez-García, R.; Granados-Montiel, J.; Berebichez-Fastlicht, E.; Olivos-Meza, A.; Granados, J.; Velasquillo, C.; Ibarra, C. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells-Their Current Uses and Potential Applications. Stem. Cells Int. 2017, 2017, 2638305. [Google Scholar] [CrossRef] [PubMed]
- Conci, C.; Bennati, L.; Bregoli, C.; Buccino, F.; Danielli, F.; Gallan, M.; Gjini, E.; Raimondi, M.T. Tissue engineering and regenerative medicine strategies for the female breast. J. Tissue Eng. Regen. Med. 2020, 14, 369–387. [Google Scholar] [CrossRef]
- Colazo, J.M.; Evans, B.C.; Farinas, A.F.; Al-Kassis, S.; Duvall, C.L.; Thayer, W.P. Applied Bioengineering in Tissue Reconstruction, Replacement, and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 259–290. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent advances in decellularized biomaterials for wound healing. Mater. Today Bio. 2023, 19, 100589. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Ren, Y.; Lu, D.; Li, T.; Li, W.; Wang, J.; Ma, H.; Zhao, J. Tissue Engineering Strategies for Peripheral Nerve Regeneration. Front. Neurol. 2021, 12, 768267. [Google Scholar] [CrossRef]
- Brown, P.T.; Handorf, A.M.; Jeon, W.B.; Li, W.J. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr. Pharm. Des. 2013, 19, 3429–3445. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Cool, S. Concepts of scaffold-based tissue engineering--the rationale to use solid free-form fabrication techniques. J. Cell Mol. Med. 2007, 11, 654–669. [Google Scholar] [CrossRef]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin Substitutes and Bioscaffolds: Temporary and Permanent Coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Nyame, T.T.; Chiang, H.A.; Leavitt, T.; Ozambela, M.; Orgill, D.P. Tissue-Engineered Skin Substitutes. Plast. Reconstr. Surg. 2015, 136, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Walgenbach, K.J.; Voigt, M.; Riabikhin, A.W.; Andree, C.; Schaefer, D.J.; Galla, T.J.; Björn, G. Tissue engineering in plastic reconstructive surgery. Anat. Rec. 2001, 263, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem. Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Qin, C.; Wu, C. 3D printing of cell-delivery scaffolds for tissue regeneration. Regen. Biomater. 2023, 10, rbad032. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell-scaffold interactions in tissue engineering for oral and craniofacial reconstruction. Bioact. Mater. 2022, 23, 16–44. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; He, C.; Gao, C.; Zhu, P.; Lu, G.; Li, H. 3D-Printed Degradable Anti-Tumor Scaffolds for Controllable Drug Delivery. Int. J. Bioprint. 2021, 7, 418. [Google Scholar] [CrossRef]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Xu, M.; Geng, Z.; Ji, P.; Liu, Y. Hydrogel systems for targeted cancer therapy. Front. Bioeng. Biotechnol. 2023, 11, 1140436. [Google Scholar] [CrossRef]
- Johnson, P.J.; Wood, M.D.; Moore, A.M.; Mackinnon, S.E. Tissue engineered constructs for peripheral nerve surgery. Eur. Surg. 2013, 45, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Vancea, C.V.; Grosu-Bularda, A.; Cretu, A.; Hodea, F.V.; Al-Falah, K.; Stoian, A.; Chiotoroiu, A.L.; Mihai, C.; Hariga, C.S.; Lascăr, I.; et al. Therapeutic strategies for nerve injuries: Current findings and future perspectives. Are textile technologies a potential solution? Ind. Textila 2022, 73, 704–712. [Google Scholar] [CrossRef]
- Supra, R.; Agrawal, D.K. Peripheral Nerve Regeneration: Opportunities and Challenges. J. Spine Res. Surg. 2023, 5, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zegrea, I.; Chivu, L.I.; Albu, M.G.; Zamfirescu, D.; Chivu, R.D.; Ion, D.A.; Lascăr, I. A Romanian therapeutic approach to peripheral nerve injury. Rom. J. Morphol. Embryol. 2012, 53, 357–361. [Google Scholar]
- Zhang, M.; Xing, J.; Zhong, Y.; Zhang, T.; Liu, X.; Xing, D. Advanced function, design and application of skin substitutes for skin regeneration. Mater. Today Bio. 2024, 24, 100918. [Google Scholar] [CrossRef]
- Vecin, N.M.; Kirsner, R.S. Skin substitutes as treatment for chronic wounds: Current and future directions. Front. Med. 2023, 10, 1154567. [Google Scholar] [CrossRef]
- Bay, C.; Chizmar, Z.; Reece, E.M.; Yu, J.Z.; Winocour, J.; Vorstenbosch, J.; Winocour, S. Comparison of Skin Substitutes for Acute and Chronic Wound Management. Semin. Plast. Surg. 2021, 35, 171–180. [Google Scholar] [CrossRef]
- Varkey, M.; Ding, J.; Tredget, E.E. Advances in Skin Substitutes-Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J. Funct. Biomater. 2015, 6, 547–563. [Google Scholar] [CrossRef]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatolog. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Zaver, V.; Kankanalu, P. Negative Pressure Wound Therapy. In StatPearls [Internet]; Updated 4 September 2023; StatPearls Publishing: Treasure Island, FL, USA, January 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576388/ (accessed on 15 January 2024).
- Pappalardo, V.; Frattini, F.; Ardita, V.; Rausei, S. Negative Pressure Therapy (NPWT) for Management of Surgical Wounds: Effects on Wound Healing and Analysis of Devices Evolution. Surg. Technol. Int. 2019, 34, 56–67. [Google Scholar]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef]
- Avino, A.; Cozma, C.; Balcangiu-Stroescu, A.; Tanasescu, M.; Balan, D.G.; Timofte, D.; Stoicescu, S.M.; Hariga, C.; Ionescu, D. Our Experience in Skin Grafting and Silver Dressing for Venous Leg Ulcers. Rev. Chim. 2019, 70, 742–744. [Google Scholar] [CrossRef]
- Hasan, M.Y.; Teo, R.; Nather, A. Negative-pressure wound therapy for management of diabetic foot wounds: A review of the mechanism of action, clinical applications, and recent developments. Diabet Foot Ankle 2015, 6, 27618. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Yu, X.T.; Liao, X.C.; Liu, M.Z.; Fu, Z.H.; Min, D.H.; Guo, G.H. Negative-pressure wound therapy in skin grafts: A systematic review and meta-analysis of randomized controlled trials. Burns 2021, 47, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.; Skladman, R.; Varagur, K.; Tenenbaum, E.; Sacks, J.L.; Martin, C.; Gordon, T.; Murphy, J.; Moritz, W.R.; Sacks, J.M. From Augmented to Virtual Reality in Plastic Surgery: Blazing the Trail to a New Frontier. J. Reconstr. Microsurg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.B.; Fleischmann, D. Computed Tomography Angiography of the Upper Extremities. Radiol. Clin. N. Am. 2016, 54, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Pomahac, B.; Wake, N.; Schultz, K.; Prior, R.F.; Kumamaru, K.; Steigner, M.L.; Mitsouras, D.; Signorelli, J.; Bueno, E.M.; et al. CT angiography for surgical planning in face transplantation candidates. AJNR Am. J. Neuroradiol. 2013, 34, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Kiruluta, A.J.M.; González, R.G. Magnetic resonance angiography: Physical principles and applications. Handb. Clin. Neurol. 2016, 135, 137–149. [Google Scholar] [CrossRef]
- Vasile, J.V.; Levine, J.L. Magnetic resonance angiography in perforator flap breast reconstruction. Gland Surg. 2016, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.; Cornejo-Aguilar, J.A.; Vargas, M.; Helguero, C.G.; Milanezi de Andrade, R.; Torres-Montoya, S.; Asensio-Salazar, J.; Rivero Calle, A.; Martínez Santos, J.; Damon, A.; et al. Anatomical Engineering and 3D Printing for Surgery and Medical Devices: International Review and Future Exponential Innovations. Biomed. Res. Int. 2022, 2022, 6797745. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Dedhia, R.; Cervenka, B.; Tollefson, T.T. 3D Printing: Current use in facial plastic and reconstructive surgery. Curr. Opin. Otolaryngol. Head. Neck. Surg. 2017, 25, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann. Plast. Surg. 2016, 77, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Harrison, P.; Cheng, A.; Bray, B.; Bell, R.B. Fibular Reconstruction of the Maxilla and Mandible with Immediate Implant-Supported Prosthetic Rehabilitation: Jaw in a Day. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Qaisi, M.; Kolodney, H.; Swedenburg, G.; Chandran, R.; Caloss, R. Fibula Jaw in a Day: State of the Art in Maxillofacial Reconstruction. J. Oral Maxillofac. Surg. 2016, 74, 1284.E1–1284.E15. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.F.; Ge, Y.J.; Lv, X.M.; Ding, M.K.; Shan, X.F.; Cai, Z.G. One-stage jaw reconstruction and prosthetic rehabilitation with an iliac flap: A case report and literature review. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 3. [Google Scholar] [CrossRef]
- Williams, F.C. History of the Jaw in a Day. Oral Maxillofac. Surg. Cases. 2023, 9, 100306. [Google Scholar] [CrossRef]
- Antúnez-Conde, R.; Salmerón, J.I.; Díez-Montiel, A.; Agea, M.; Gascón, D.; Sada, Á.; Navarro Cuéllar, I.; Tousidonis, M.; Ochandiano, S.; Arenas, G.; et al. Mandibular Reconstruction With Fibula Flap and Dental Implants Through Virtual Surgical Planning and Three Different Techniques: Double-Barrel Flap, Implant Dynamic Navigation and CAD/CAM Mesh With Iliac Crest Graft. Front. Oncol. 2021, 11, 719712. [Google Scholar] [CrossRef]
- Kwon, J.G.; Hong, D.W.; Choi, J.W. Clinical Applications of Augmented Reality Technology in Microsurgical Planning of Head and Neck Reconstruction. J. Craniofac. Surg. 2022, 33, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Pribaz, J.J.; Fine, N.A. Prefabricated and prelaminated flaps for head and neck reconstruction. Clin. Plast. Surg. 2001, 28, 261–272. [Google Scholar] [CrossRef]
- Levin, L.S. The reconstructive ladder. An orthoplastic approach. Orthop. Clin. N. Am. 1993, 24, 393–409. [Google Scholar] [CrossRef]
- Levin, L.S. From replantation to transplantation: The evolution of orthoplastic extremity reconstruction. J. Orthop. Res. 2023, 41, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I. The angiosomes of the body and their supply to perforator flaps. Clin. Plast. Surg. 2003, 30, 331–342. [Google Scholar] [CrossRef]
- Taylor, G.I.; Corlett, R.J.; Dhar, S.C.; Ashton, M.W. The anatomical (angiosome) and clinical territories of cutaneous perforating arteries: Development of the concept and designing safe flaps. Plast. Reconstr. Surg. 2011, 127, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The perforasome theory: Vascular anatomy and clinical implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I.; Corlett, R.J.; Ashton, M.W. The Functional Angiosome: Clinical Implications of the Anatomical Concept. Plast. Reconstr. Surg. 2017, 140, 721–733. [Google Scholar] [CrossRef]
- Gunnarsson, G.L.; Jackson, I.T.; Thomsen, J.B. Freestyle facial perforator flaps—A safe reconstructive option for moderate-sized facial defects. Eur. J. Plast. Surg. 2014, 37, 315–318. [Google Scholar] [CrossRef]
- Mohan, A.T.; Saint-Cyr, M. Recent Advances in Microsurgery: An Update in the Past 4 Years. Clin. Plast. Surg. 2020, 47, 663–677. [Google Scholar] [CrossRef]
- Badash, I.; Gould, D.J.; Patel, K.M. Supermicrosurgery: History, Applications, Training and the Future. Front. Surg. 2018, 5, 23. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Kageyama, T.; Sakai, H.; Fuse, Y.; Tsuihiji, K.; Tsukuura, R. Supermicrosurgery for oncologic reconstructions. Glob. Health Med. 2020, 2, 18–23. [Google Scholar] [CrossRef]
- Will, P.A.; Wan, Z.; Seide, S.E.; Berner, J.E.; Kneser, U.; Gazyakan, E.; Hirche, C. Supermicrosurgical treatment for lymphedema: A systematic review and network meta-analysis protocol. Syst. Rev. 2022, 11, 18. [Google Scholar] [CrossRef]
- van Mulken, T.J.M.; Wolfs, J.A.G.N.; Qiu, S.S.; Scharmga, A.M.J.; Schols, R.M.; Spiekerman van Weezelenburg, M.A.; Cau, R.; van der Hulst, R.R.W.J.; MicroSurgical Robot Research Group. One-Year Outcomes of the First Human Trial on Robot-Assisted Lymphaticovenous Anastomosis for Breast Cancer-Related Lymphedema. Plast. Reconstr. Surg. 2022, 149, 151–161. [Google Scholar] [CrossRef]
- Dubernard, J.M.; Owen, E.; Herzberg, G.; Martin, X.; Guigal, V.; Dawahra, M.; Pasticier, G.; Mongin-Long, D.; Kopp, C.; Ostapetz, A.; et al. Première transplantation de main chez l’homme. Résultats précoces [The first transplantation of a hand in humans. Early results]. Chirurgie 1999, 124, 358–365; discussion 365–367. [Google Scholar] [CrossRef]
- Shores, J.T.; Malek, V.; Lee, W.P.A.; Brandacher, G. Outcomes after hand and upper extremity transplantation. J. Mater. Sci. Mater. Med. 2017, 28, 72. [Google Scholar] [CrossRef]
- Kaufman, C.L.; Bhutiani, N.; Ramirez, A.; Tien, H.Y.; Palazzo, M.D.; Galvis, E.; Farner, S.; Ozyurekoglu, T.; Jones, C.M. Current Status of Vascularized Composite Allotransplantation. Am. Surg. 2019, 85, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, L.; Knoedler, S.; Panayi, A.C.; Lee, C.A.A.; Sadigh, S.; Huelsboemer, L.; Stoegner, V.A.; Schroeter, A.; Kern, B.; Mookerjee, V.; et al. Cellular activation pathways and interaction networks in vascularized composite allotransplantation. Front. Immunol. 2023, 14, 1179355. [Google Scholar] [CrossRef] [PubMed]
- Carty, M.J.; Pribaz, J.J.; Talbot, S.G.; Caterson, E.J.; Pomahac, B. The advent of the restorative plastic surgeon. Plast. Reconstr. Surg. 2014, 133, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Siso, J.R.; Bueno, E.M.; Sisk, G.C.; Marty, F.M.; Pomahac, B.; Tullius, S.G. Vascularized composite tissue allotransplantation--state of the art. Clin. Transplant. 2013, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Pribaz, J.J.; Caterson, E.J. Evolution and limitations of conventional autologous reconstruction of the head and neck. J. Craniofac. Surg. 2013, 24, 99–107. [Google Scholar] [CrossRef]
- Cho, K.H.; Papay, F.A.; Yanof, J.; West, K.; Bassiri Gharb, B.; Rampazzo, A.; Gastman, B.; Schwarz, G.S. Mixed Reality and 3D Printed Models for Planning and Execution of Face Transplantation. Ann. Surg. 2021, 274, e1238–e1246. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, L.; Knoedler, S.; Allam, O.; Remy, K.; Miragall, M.; Safi, A.F.; Alfertshofer, M.; Pomahac, B.; Kauke-Navarro, M. Application possibilities of artificial intelligence in facial vascularized composite allotransplantation-a narrative review. Front. Surg. 2023, 10, 1266399. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, J.J.; Den Hondt, M.; Delaere, P. Prefabrication and prelamination strategies for the reconstruction of complex defects of trachea and larynx. J. Reconstr. Microsurg. 2014, 30, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Den Hondt, M.; Vanaudenaerde, B.M.; Maughan, E.F.; Butler, C.R.; Crowley, C.; Verbeken, E.K.; Verleden, S.E.; Vranckx, J.J. An optimized non-destructive protocol for testing mechanical properties in decellularized rabbit trachea. Acta Biomater. 2017, 60, 291–301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu-Bularda, A.; Hodea, F.-V.; Cretu, A.; Lita, F.-F.; Bordeanu-Diaconescu, E.-M.; Vancea, C.-V.; Lascar, I.; Popescu, S.A. Reconstructive Paradigms: A Problem-Solving Approach in Complex Tissue Defects. J. Clin. Med. 2024, 13, 1728. https://doi.org/10.3390/jcm13061728
Grosu-Bularda A, Hodea F-V, Cretu A, Lita F-F, Bordeanu-Diaconescu E-M, Vancea C-V, Lascar I, Popescu SA. Reconstructive Paradigms: A Problem-Solving Approach in Complex Tissue Defects. Journal of Clinical Medicine. 2024; 13(6):1728. https://doi.org/10.3390/jcm13061728
Chicago/Turabian StyleGrosu-Bularda, Andreea, Florin-Vlad Hodea, Andrei Cretu, Flavia-Francesca Lita, Eliza-Maria Bordeanu-Diaconescu, Cristian-Vladimir Vancea, Ioan Lascar, and Serban Arghir Popescu. 2024. "Reconstructive Paradigms: A Problem-Solving Approach in Complex Tissue Defects" Journal of Clinical Medicine 13, no. 6: 1728. https://doi.org/10.3390/jcm13061728
APA StyleGrosu-Bularda, A., Hodea, F.-V., Cretu, A., Lita, F.-F., Bordeanu-Diaconescu, E.-M., Vancea, C.-V., Lascar, I., & Popescu, S. A. (2024). Reconstructive Paradigms: A Problem-Solving Approach in Complex Tissue Defects. Journal of Clinical Medicine, 13(6), 1728. https://doi.org/10.3390/jcm13061728






