Comparing the Efficacy of Carboplatin plus 5-Fluorouracil, Cisplatin plus 5-Fluorouracil, and Best Supportive Care for Advanced Esophageal Squamous Cell Carcinoma: A Propensity Score Analysis from a Tertiary Hospital in Southern Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Procedures
2.3. Measurement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Treatment Information
3.3. Effectiveness
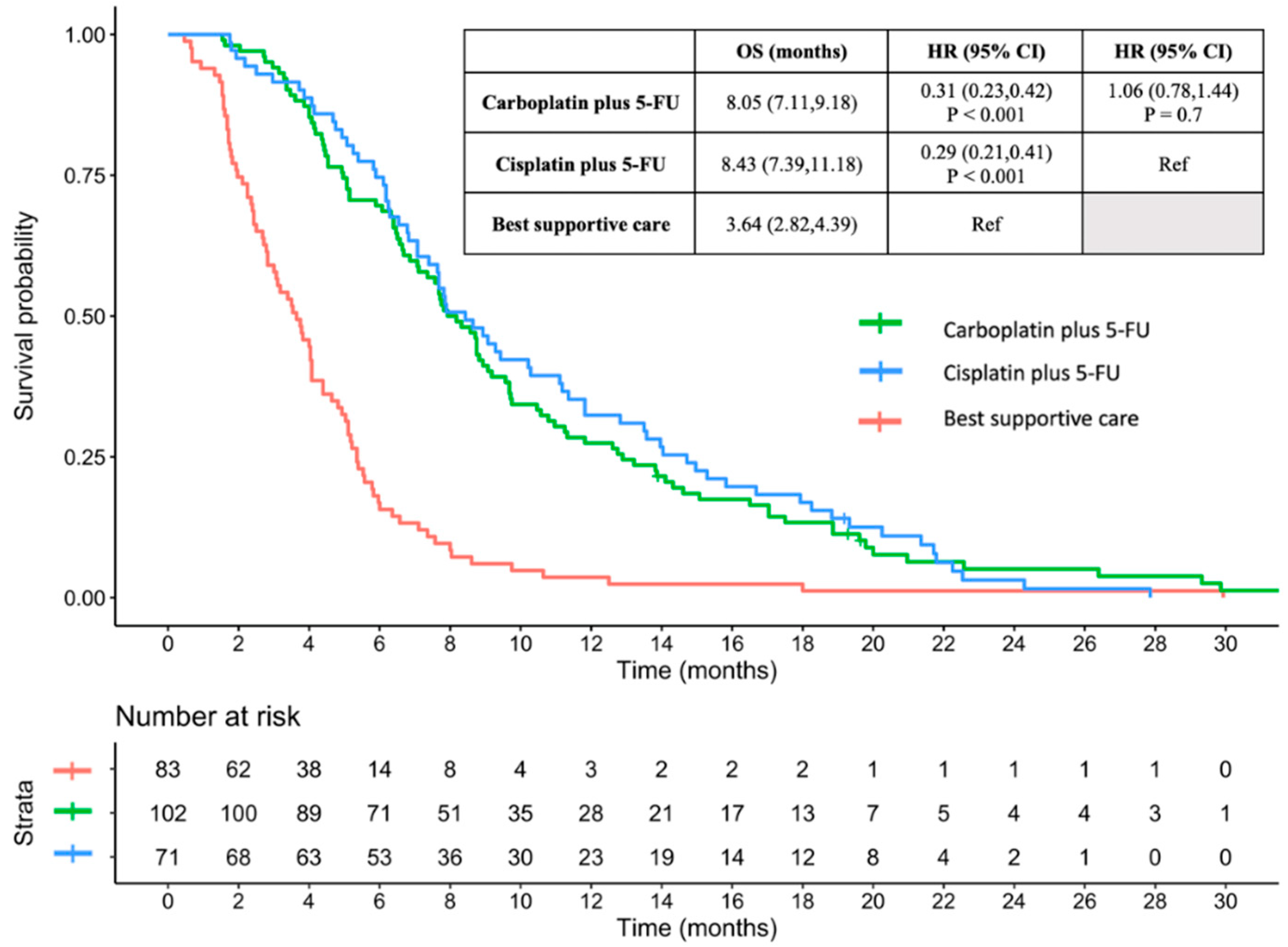
3.3.1. OS between Carboplatin plus 5-FU, Cisplatin plus 5-FU, and BSC
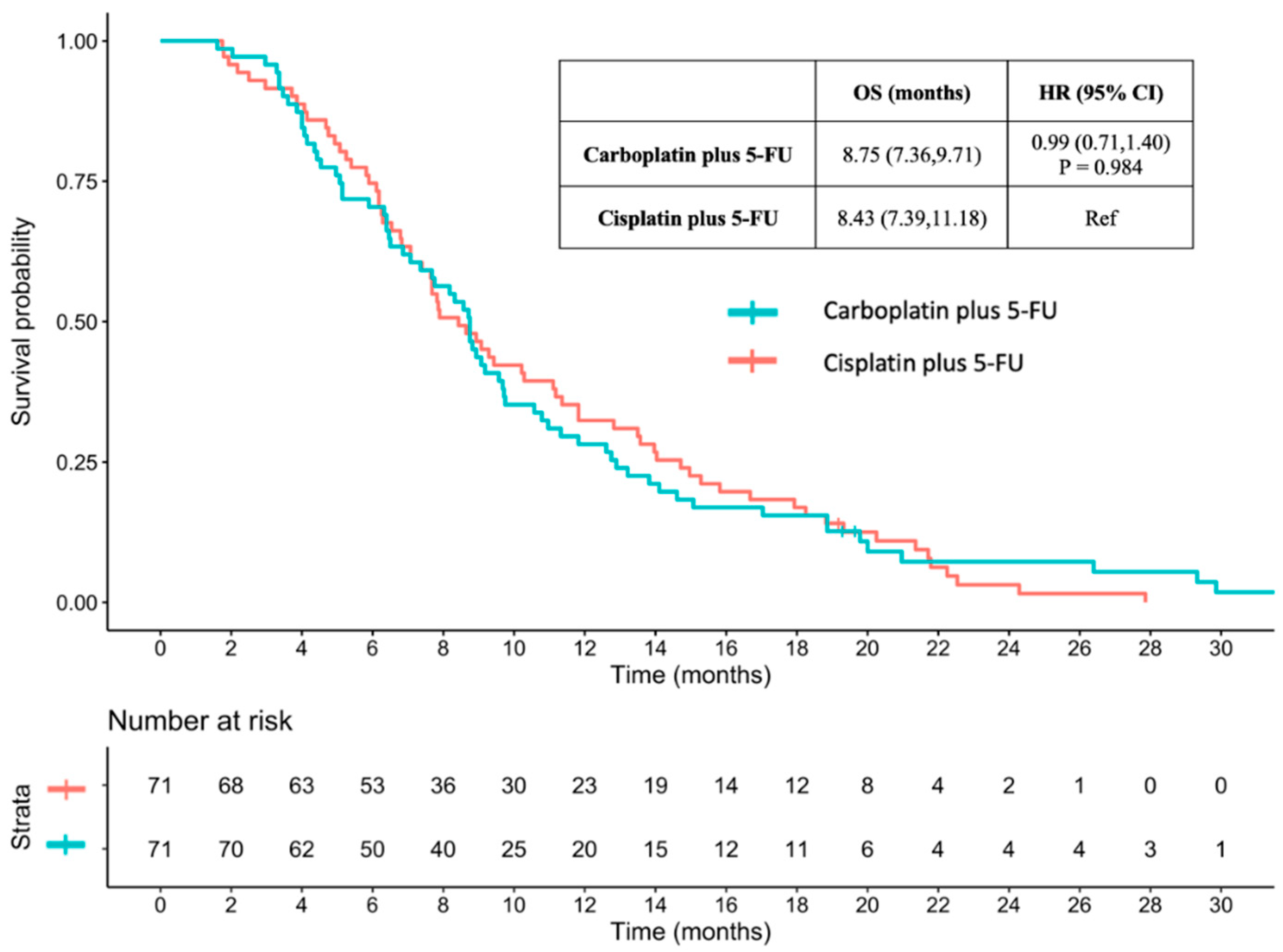
3.3.2. OS between Carboplatin plus 5-FU and Cisplatin plus 5-FU with Propensity Score Matching
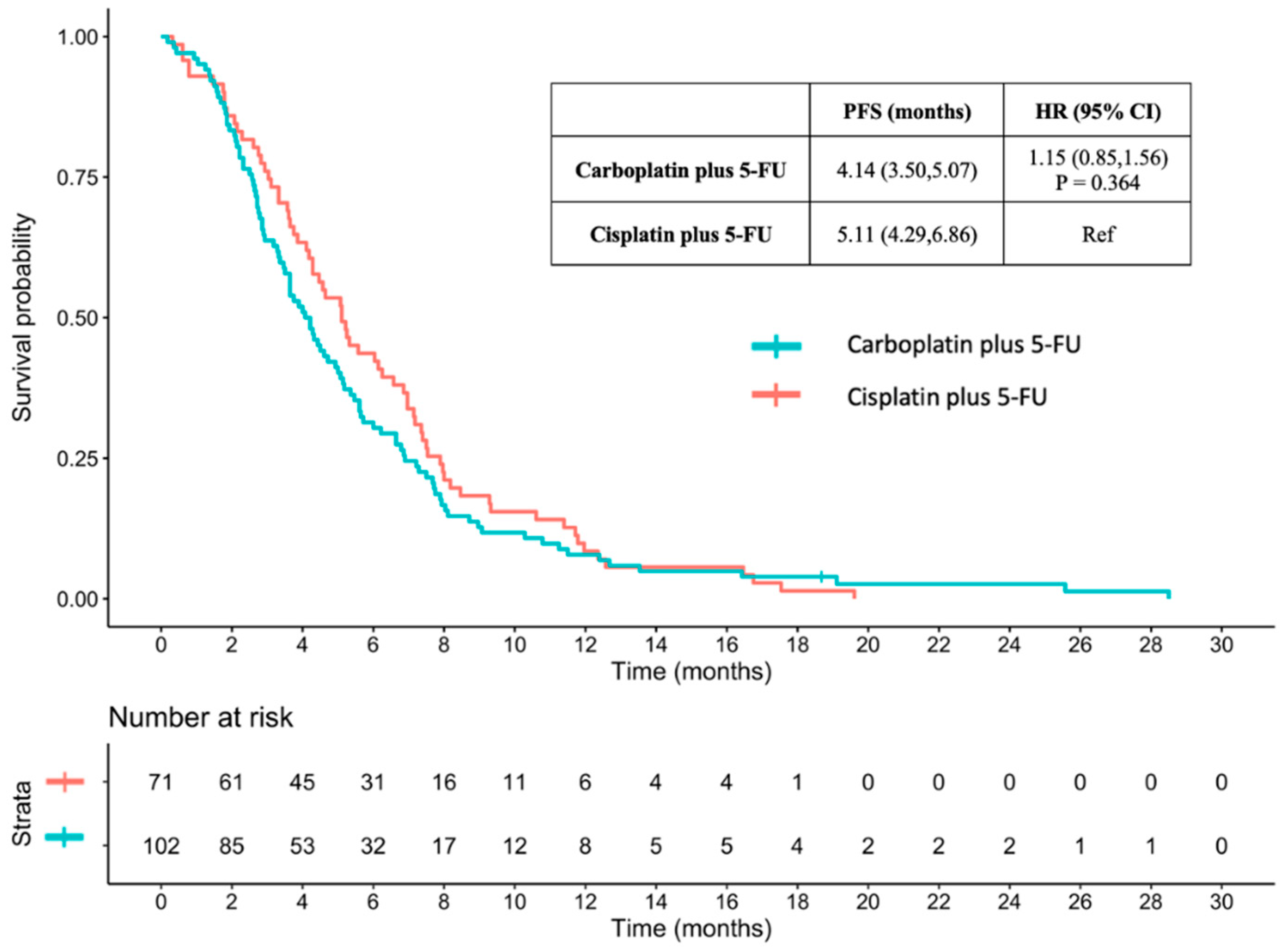
3.3.3. PFS between Carboplatin plus 5-FU and Cisplatin plus 5-FU
3.4. Prognostic Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global Incidence of Oesophageal Cancer by Histological Subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal Cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- DaSilva, L.L.; Aguiar, P.N., Jr.; de Lima Lopes, G. Immunotherapy for Advanced Esophageal Squamous Cell Carcinoma-Renewed Enthusiasm and a Lingering Challenge. JAMA Oncol. 2021, 7, 1613–1614. [Google Scholar] [CrossRef]
- van Rossum, P.S.N.; Mohammad, N.H.; Vleggaar, F.P.; van Hillegersberg, R. Treatment for Unresectable or Metastatic Oesophageal Cancer: Current Evidence and Trends. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Mukkamalla, S.K.R.; Recio-Boiles, A.; Babiker, H.M. Esophageal Cancer; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Shah, M.A.; Schwartz, G.K. Treatment of Metastatic Esophagus and Gastric Cancer. Semin. Oncol. 2004, 31, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.Y. Systemic Therapy for Esophageal Cancer: Chemotherapy. Chin. Clin. Oncol. 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Grünberger, B.; Raderer, M.; Schmidinger, M.; Hejna, M. Palliative Chemotherapy for Recurrent and Metastatic Esophageal Cancer. Anticancer Res. 2007, 27, 2705–2714. [Google Scholar] [PubMed]
- Harada, K.; Rogers, J.E.; Iwatsuki, M.; Yamashita, K.; Baba, H.; Ajani, J.A. Recent Advances in Treating Oesophageal Cancer. F1000Research 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal Cancer. Nat. Rev. Dis. Primers 2017, 3, 17049. [Google Scholar] [CrossRef]
- Valkema, M.J.; Mostert, B.; Lagarde, S.M.; Wijnhoven, B.P.L.; van Lanschot, J.J.B. The Effectivity of Targeted Therapy and Immunotherapy in Patients with Advanced Metastatic and Non-Metastatic Cancer of the Esophagus and Esophago-Gastric Junction. Updates Surg. 2023, 75, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Harada, K.; Yamamoto, S.; Kato, K. Pembrolizumab: First Anti-PD-1/L1-Based Regimen for First-Line Treatment of Advanced Esophageal Cancer in Japan. Expert Opin. Biol. Ther. 2022, 22, 1333–1338. [Google Scholar] [CrossRef]
- Puhr, H.C.; Prager, G.W.; Ilhan-Mutlu, A. How We Treat Esophageal Squamous Cell Carcinoma. ESMO Open 2023, 8, 100789. [Google Scholar] [CrossRef] [PubMed]
- Bleiberg, H.; Conroy, T.; Paillot, B.; Lacave, A.J.; Blijham, G.; Jacob, J.H.; Bedenne, L.; Namer, M.; De Besi, P.; Gay, F.; et al. Randomised Phase II Study of Cisplatin and 5-Fluorouracil (5-FU) versus Cisplatin Alone in Advanced Squamous Cell Oesophageal Cancer. Eur. J. Cancer 1997, 33, 1216–1220. [Google Scholar] [CrossRef]
- Ajani, J.A.; Barthel, J.S.; Bentrem, D.J.; D’Amico, T.A.; Das, P.; Denlinger, C.S.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; Hayman, J.A.; et al. Esophageal and Esophagogastric Junction Cancers. J. Natl. Compr. Canc. Netw. 2011, 9, 830–887. [Google Scholar] [CrossRef]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A Review of Toxicities and Therapeutic Applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R.; et al. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef]
- Queisser, W.; Preusser, P.; Mross, K.B.; Fritze, D.; Rieche, K.; Beyer, J.H.; Achterrath, W.; Edler, L. Phase II Evaluation of Carboplatin in Advanced Esophageal Carcinoma. A Trial of the Phase I/II Study Group of the Association for Medical Oncology of the German Cancer Society. Onkologie 1990, 13, 190–193. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Shields, A.; Zalupski, M.; Heilbrun, L.K.; Jain, V.; Terry, D.; Ferris, A.; Philip, P.A. A Phase II Study of Carboplatin and Paclitaxel in Esophageal Cancer. Ann. Oncol. 2004, 15, 960–965. [Google Scholar] [CrossRef]
- Prithviraj, G.K.; Baksh, K.; Fulp, W.; Meredith, K.; Hoffe, S.; Shridhar, R.; Almhanna, K. Carboplatin and Paclitaxel as First-Line Treatment of Unresectable or Metastatic Esophageal or Gastric Cancer: Chemotherapy in Advanced Esophageal Cancer. Dis. Esophagus 2015, 28, 782–787. [Google Scholar] [CrossRef]
- de Man, F.M.; van Eerden, R.A.G.; Oomen-de Hoop, E.; Veraart, J.N.; van Doorn, N.; van Doorn, L.; van der Gaast, A.; Mathijssen, R.H.J. Efficacy and Toxicity of Weekly Carboplatin and Paclitaxel as Induction or Palliative Treatment in Advanced Esophageal Cancer Patients. Cancers 2019, 11, 826. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant Chemoradiotherapy plus Surgery versus Surgery Alone for Oesophageal or Junctional Cancer (CROSS): Long-Term Results of a Randomised Controlled Trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Athauda, A.; Watkins, D.; Mohammed, K.; Chau, I.; Starling, N.; Rao, S.; Tait, D.; Aitken, K.; Cunningham, D. Cisplatin Substitution with Carboplatin during Radical Chemoradiotherapy for Oesophagogastric Carcinoma: Outcomes from a Tertiary Centre. Anticancer Res. 2018, 38, 5943–5949. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Electronic address: [email protected] Oesophageal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Li, E.; Xu, N.; Shi, D.; Qian, J. Efficacy and Safety of Chemotherapy Regimens for First-Line Treatment of Advanced Esophageal Squamous Cell Carcinoma in Asia: A Systematic Review. Expert Rev. Anticancer Ther. 2022, 22, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Homs, M.Y.V.; vd Gaast, A.; Siersema, P.D.; Steyerberg, E.W.; Kuipers, E.J. Chemotherapy for Metastatic Carcinoma of the Esophagus and Gastro-Esophageal Junction. Cochrane Database Syst. Rev. 2006, 4, CD004063. [Google Scholar] [CrossRef]
- Levard, H.; Pouliquen, X.; Hay, J.M.; Fingerhut, A.; Langlois-Zantain, O.; Huguier, M.; Lozach, P.; Testart, J. 5-Fluorouracil and Cisplatin as Palliative Treatment of Advanced Oesophageal Squamous Cell Carcinoma. A Multicentre Randomised Controlled Trial. The French Associations for Surgical Research: A Multicentre Randomised Controlled Trial. Eur. J. Surg. 1998, 164, 849–857. [Google Scholar] [CrossRef]
- Hayashi, K.; Ando, N.; Watanabe, H.; Ide, H.; Nagai, K.; Aoyama, N.; Takiyama, W.; Ishida, K.; Isono, K.; Makuuchi, H.; et al. Phase II Evaluation of Protracted Infusion of Cisplatin and 5-Fluorouracil in Advanced Squamous Cell Carcinoma of the Esophagus: A Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn. J. Clin. Oncol. 2001, 31, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Schuster, T.; Porschen, R.; Al-Batran, S.-E.; Hofheinz, R.; Thuss-Patience, P.; Moehler, M.; Grabowski, P.; Arnold, D.; Greten, T.; et al. Cetuximab plus Cisplatin-5-Fluorouracil versus Cisplatin-5-Fluorouracil Alone in First-Line Metastatic Squamous Cell Carcinoma of the Esophagus: A Randomized Phase II Study of the Arbeitsgemeinschaft Internistische Onkologie. Ann. Oncol. 2009, 20, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, V.T.; Steyerberg, E.W.; van der Gaast, A.; Mathijssen, R.H.; Bruno, M.J.; Peppelenbosch, M.P.; Kuipers, E.J.; Spaander, M.C. Palliative Chemotherapy and Targeted Therapies for Esophageal and Gastroesophageal Junction Cancer. Cochrane Database Syst. Rev. 2017, 11, CD004063. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Galais, M.-P.; Raoul, J.-L.; Bouché, O.; Gourgou-Bourgade, S.; Douillard, J.-Y.; Etienne, P.-L.; Boige, V.; Martel-Lafay, I.; Michel, P.; et al. Definitive Chemoradiotherapy with FOLFOX versus Fluorouracil and Cisplatin in Patients with Oesophageal Cancer (PRODIGE5/ACCORD17): Final Results of a Randomised, Phase 2/3 Trial. Lancet Oncol. 2014, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B.; et al. Pembrolizumab plus Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, S.; Kato, K.; Shoji, H.; Okita, N.; Takashima, A.; Honma, Y.; Iwasa, S.; Hamaguchi, T.; Yamada, Y.; Shimada, Y.; et al. A Retrospective Analysis of 5-Fluorouracil plus Cisplatin as First-Line Chemotherapy in the Recent Treatment Strategy for Patients with Metastatic or Recurrent Esophageal Squamous Cell Carcinoma. Int. J. Clin. Oncol. 2018, 23, 466–472. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Yuan, L.; Xu, S.; Yao, Z.; Qiao, L.; Li, K. Paclitaxel plus Cisplatin vs. 5-Fluorouracil plus Cisplatin as First-Line Treatment for Patients with Advanced Squamous Cell Esophageal Cancer. Am. J. Cancer Res. 2016, 6, 2345–2350. [Google Scholar]
- Singel, K.L.; Segal, B.H. Neutrophils in the Tumor Microenvironment: Trying to Heal the Wound That Cannot Heal. Immunol. Rev. 2016, 273, 329–343. [Google Scholar] [CrossRef]
- Qiu, M.-Z.; Xu, R.-H.; Ruan, D.-Y.; Li, Z.-H.; Luo, H.-Y.; Teng, K.-Y.; Wang, Z.-Q.; Li, Y.-H.; Jiang, W.-Q. Incidence of Anemia, Leukocytosis, and Thrombocytosis in Patients with Solid Tumors in China. Tumour Biol. 2010, 31, 633–641. [Google Scholar] [CrossRef]
- Schernberg, A.; Moureau-Zabotto, L.; Rivin Del Campo, E.; Escande, A.; Ducreux, M.; Nguyen, F.; Goere, D.; Chargari, C.; Deutsch, E. Leukocytosis and Neutrophilia Predict Outcome in Locally Advanced Esophageal Cancer Treated with Definitive Chemoradiation. Oncotarget 2017, 8, 11579–11588. [Google Scholar] [CrossRef]
- Almasaudi, A.S.; Dolan, R.D.; Edwards, C.A.; McMillan, D.C. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers 2020, 12, 1986. [Google Scholar] [CrossRef]
- Sohda, M.; Sakai, M.; Yamaguchi, A.; Watanabe, T.; Nakazawa, N.; Ubukata, Y.; Kuriyam, K.; Sano, A.; Yokobori, T.; Ogawa, H.; et al. Pre-Treatment CRP and Albumin Determines Prognosis for Unresectable Advanced Oesophageal Cancer. Vivo 2022, 36, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, T.; Shimokawa, M.; Matsumoto, T.; Boku, S.; Yasuda, T.; Shibata, N.; Kurioka, Y.; Takatani, M.; Nobuhisa, T.; Namikawa, T.; et al. Inflammatory Prognostic Factors in Advanced or Recurrent Esophageal Squamous Cell Carcinoma Treated with Nivolumab. Cancer Immunol. Immunother. 2023, 72, 427–435. [Google Scholar] [CrossRef]
- Mohri, J.; Katada, C.; Ueda, M.; Sugawara, M.; Yamashita, K.; Moriya, H.; Komori, S.; Hayakawa, K.; Koizumi, W.; Atsuda, K. Predisposing Factors for Chemotherapy-Induced Nephrotoxicity in Patients with Advanced Esophageal Cancer Who Received Combination Chemotherapy with Docetaxel, Cisplatin, and 5-Fluorouracil. J. Transl. Int. Med. 2018, 6, 32–37. [Google Scholar] [CrossRef]
- Luo, P.; Wei, X.; Liu, C.; Chen, X.; Yang, Y.; Zhang, R.; Kang, X.; Qin, J.; Qi, X.; Li, Y. The Risk and Prognostic Factors for Liver Metastases in Esophageal Cancer Patients: A Large-Cohort Based Study. Thorac. Cancer 2022, 13, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Chen, Y.; Liu, Q.; Deng, J.; Zhao, K. The Effect of Tumor Locations of Esophageal Cancer on the Metastasis to Liver or Lung. J. Thorac. Dis. 2019, 11, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
| Carboplatin Plus 5-FU (n = 102) | Cisplatin Plus 5-FU (n = 71) | Best Supportive Care (n = 83) | |
|---|---|---|---|
| Median Age, years (IQR) *,** | 63.0 (58.2, 68.6) | 54.6 (49.9, 59.9) | 62.1 (54.2, 71) |
| Age ≥ 65 years, n (%) *,** | 40 (39.2) | 5 (7.0) | 37 (44.6) |
| Sex, n (%) | |||
| Male | 96 (94.1) | 67 (94.4) | 76 (91.6) |
| Female | 6 (5.9) | 4 (5.6) | 7 (8.4) |
| ECOG PS, n (%) *,** | |||
| 0 | 2 (2) | 0 (0) | 0 (0) |
| 1 | 70 (68.6) | 61 (85.9) | 11 (13.3) |
| 2 | 29 (28.4) | 10 (14.1) | 48 (57.8) |
| 3 | 1 (1) | 0 (0) | 22 (26.5) |
| 4 | 0 (0) | 0 (0) | 2 (2.4) |
| BMI, n (%) ** | |||
| <18.5 kg/m2 | 59 (57.8) | 40 (56.3) | 63 (75.9) |
| 18.5–22.9 kg/m2 | 33 (32.4) | 23 (32.4) | 19 (22.9) |
| 23.0–24.9 kg/m2 | 5 (4.9) | 5 (7.0) | 1 (1.2) |
| ≥25 kg/m2 | 5 (4.9) | 3 (4.2) | 0 (0) |
| Smoking, n (%) ** | |||
| Current or former | 92 (90.2) | 63 (88.7) | 60 (72.3) |
| Never | 10 (9.8) | 8 (11.3) | 23 (27.7) |
| Alcohol drinking, n (%) ** | |||
| Current or former | 88 (86.3) | 62 (87.3) | 55 (66.3) |
| Never | 14 (13.7) | 9 (12.7) | 28 (33.7) |
| Comorbidities, n (%) *,** | 59 (57.8) | 26 (36.6) | 38 (45.8) |
| Hypertension *,** | 35 (34.3) | 11 (15.5) | 26 (31.3) |
| Diabetes mellitus | 7 (6.9) | 4 (5.6) | 9 (10.8) |
| Ischemic heart disease | 3 (2.9) | 1 (1.4) | 5 (6) |
| Cerebrovascular disease | 8 (7.8) | 2 (2.8) | 6 (7.2) |
| COPD | 5 (4.9) | 2 (2.8) | 2 (2.4) |
| Cirrhosis | 6 (5.9) | 4 (5.6) | 1 (1.2) |
| Chronic kidney disease *,** | 78 (76.5) | 43 (60.6) | 75 (90.4) |
| History of previous cancer, n (%) | 11 (10.8) | 2 (2.8) | 5 (6) |
| Concurrent second primary cancer, n (%) ** | 3 (2.9) | 7 (9.9) | 0 (0) |
| Tumor Location, n (%) | |||
| Cervical | 7 (6.9) | 10 (14.1) | 2 (2.4) |
| Upper thoracic | 23 (22.5) | 10 (14.1) | 16 (19.3) |
| Middle thoracic | 53 (52) | 28 (39.4) | 42 (50.6) |
| Lower thoracic | 17 (16.7) | 21 (29.6) | 20 (24.1) |
| Esophagogastric junction | 2 (2) | 2 (2.8) | 3 (3.6) |
| T stage, n (%) | |||
| T1 | 0 (0) | 0 (0) | 1 (1.2) |
| T2 | 5 (4.9) | 4 (5.6) | 6 (7.2) |
| T3 | 48 (47.1) | 25 (35.2) | 37 (44.6) |
| T4 | 49 (48) | 42 (59.2) | 39 (47) |
| N stage, n (%) | |||
| N0 | 5 (4.9) | 2 (2.8) | 5 (6) |
| N1 | 56 (54.9) | 42 (59.2) | 45 (54.2) |
| N2 | 22 (21.6) | 17 (23.9) | 21 (25.3) |
| N3 | 19 (18.6) | 10 (14.1) | 12 (14.5) |
| M stage, n (%) ** | |||
| M0 | 38 (37.3) | 30 (42.3) | 17 (20.5) |
| M1 | 64 (62.7) | 41 (57.7) | 66 (79.5) |
| Tumor differentiation, n (%) * | |||
| Well differentiated SCC | 23 (22.5) | 8 (11.3) | 15 (18.1) |
| Moderately differentiated SCC | 44 (43.1) | 43 (60.6) | 41 (49.4) |
| Poorly differentiated SCC | 25 (24.5) | 18 (25.4) | 19 (22.9) |
| Missing | 10 (9.8) | 2 (2.8) | 8 (9.6) |
| Number of organ metastasis, n (%) ** | |||
| 1 | 40 (39.2) | 27 (38.0) | 41 (50.0) |
| 2 | 18 (17.6) | 12 (16.9) | 18 (22.0) |
| ≥3 | 6 (5.9) | 1 (1.4) | 6 (7.5) |
| Organ metastasis, n (%) | |||
| Lung | 24 (23.5) | 12 (16.9) | 16 (19.3) |
| Lymph node ** | 28 (27.5) | 25 (35.2) | 41 (49.4) |
| Liver | 20 (19.6) | 13 (18.3) | 21 (25.3) |
| Bone | 10 (9.8) | 4 (5.6) | 12 (14.5) |
| Adrenal gland | 2 (2.0) | 1 (1.4) | 1 (1.2) |
| Peritoneum | 1 (1.0) | 0 (0) | 1 (1.2) |
| Pleura ** | 3 (2.9) | 0 (0) | 6 (7.2) |
| Others | 4 (3.9) | 0 (0) | 0 (0) |
| Laboratory | 8905 (7090, 11,527) | 9530 (7650, 12,185) | 10,020 (7700, 13,185) |
| White blood cell count, per µL (IQR) | 10.8 | 11.7 | 10.6 |
| Hemoglobin, g/dL (IQR) *,** | (9.8, 11.9) | (10.6, 12.9) | (9.3, 11.8) |
| Platelet count, per µL (IQR) | 351,670 (124,937.5) | 379,169 (147,677.9) | 361,791.4 (133,515.2) |
| Creatinine, mg/dL (IQR) | 0.8 (0.7, 0.9) | 0.7 (0.6, 0.9) | 0.8 (0.6, 1) |
| CrCl, mL/min (IQR) *,** | 50.4 (45.2, 58.3) | 57.5 (50.3, 65.3) | 45.2 (39.5, 53.1) |
| CrCl < 60 mL/min, n (%) *,** | 78 (76.5) | 43 (60.6) | 75 (90.4) |
| CrCl ≥ 60 mL/min, n (%) *,** | 24 (23.5) | 28 (39.4) | 8 (9.6) |
| Albumin, g/dL (SD) ** | 3.5 (0.5) | 3.7 (0.6) | 3.2 (0.5) |
| Previous treatment, n (%) | |||
| Esophagectomy | 2 (2.0) | 2 (2.8) | 6 (7.2) |
| Definitive CCRT | 2 (2.0) | 0 (0) | 0 (0) |
| Palliative RT *,** | 27 (26.5) | 5 (7.0) | 38 (45.8) |
| Carboplatin Plus 5-FU (n = 102) | Cisplatin Plus 5-FU (n = 71) | |
|---|---|---|
| Treatment | ||
| Number of chemotherapy cycles (IQR) | 3 (2, 4) | 4 (2, 4) |
| Dose of carboplatin, AUC (IQR) | 5 (4, 5) | - |
| Dose of cisplatin, mg/m2 | - | 80 (80, 80) |
| Dose of 5-FU, mg/m2/cycle | 4000 (3200, 4000) | 4000 (4000, 4000) |
| Any dose reduction, n (%) | 55 (53.9) | 21 (29.5) |
| One level | 52 (51.0) | 17 (23.9) |
| Two level | 3 (2.9) | 4 (5.6) |
| Concurrent RT, n (%) * | 47 (46.1) | 51 (71.8) |
| Dose of RT, Gray (IQR) | 5040 (5000, 5040) | 5040 (5000, 5040) |
| Discontinuation, n (%) | ||
| Complete treatment | 20 (19.6) | 24 (33.8) |
| Progressive disease | 35 (34.3) | 19 (26.8) |
| Death | 31 (30.4) | 16 (22.5) |
| Worsening ECOG PS | 10 (9.8) | 8 (11.3) |
| Patient preference | 5 (4.9) | 2 (2.8) |
| Refer | 0 (0) | 2 (2.8) |
| Loss of follow-up | 1 (1.0) | 0 (0) |
| Subsequent therapy, n (%) | 27 (26.5) | 19 (26.8) |
| Second line treatment | 27 (26.5) | 19 (26.8) |
| Third line treatment | 2 (2.0) | 4 (5.6) |
| Fourth line treatment | 1 (1.0) | 0 (0) |
| Fifth line treatment | 1 (1.0) | 0 (0) |
| Carboplatin Plus 5-FU (n = 102) | Cisplatin Plus 5-FU (n = 71) | p-Value | |
|---|---|---|---|
| Evaluable, n (%) | 74 (72.5) | 55 (77.5) | |
| Complete response, n (%) | 0 (0) | 0 (0) | |
| Partial response, n (%) | 31 (30.4) | 26 (36.6) | |
| Stable disease, n (%) | 21 (20.6) | 15 (21.1) | |
| Progressive disease, n (%) | 22 (21.6) | 14 (19.7) | |
| Missing, n (%) | 28 (27.5) | 16 (22.5) | |
| ORR in the entire population, n (%) | 31 (30.4) | 26 (36.6) | 0.436 |
| ORR in available data, n (%) | 31 (41.9) | 26 (47.3) | 0.531 |
| Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Receiving chemotherapy | 0.31 * | 0.23, 0.40 | 0.46 * | 0.33, 0.64 |
| Age ≥ 65 years | 1.41 * | 1.08, 1.84 | 1.16 | 0.85, 1.58 |
| Sex–Male | 1.23 | 0.74, 2.04 | ||
| ECOG PS ≥ 2 | 2.06 * | 1.60, 2.66 | 1.43 * | 1.06, 1.94 |
| BMI < 18.5 kg/m2 | 1.27 | 0.98, 1.64 | ||
| Smoking | 0.87 | 0.62, 1.22 | ||
| Alcohol drinking | 0.79 | 0.58, 1.07 | ||
| History of previous cancer | 0.89 | 0.55, 1.45 | ||
| Concurrent two primary cancer | 1.51 | 0.80, 2.85 | ||
| Tumor Location | ||||
| Cervical | Ref | |||
| Upper thoracic | 1.25 | 0.74, 2.14 | ||
| Middle thoracic | 1.18 | 0.73, 1.92 | ||
| Lower thoracic | 1.27 | 0.75, 2.13 | ||
| Esophagogastric junction | 1.17 | 0.49, 2.80 | ||
| T stage | ||||
| T1 | Ref | |||
| T2 | 0.12 * | 0.02, 0.92 | 0.69 | 0.08, 5.57 |
| T3 | 0.11 * | 0.01, 0.81 | 0.44 | 0.06, 3.35 |
| T4 | 0.10 * | 0.01, 0.71 | 0.44 | 0.06, 3.40 |
| N stage | ||||
| N0 | Ref | |||
| N1 | 1.03 | 0.56, 1.91 | ||
| N2 | 1.08 | 0.56, 2.06 | ||
| N3 | 0.99 | 0.51, 1.95 | ||
| M stage–M1 | 1.56 * | 1.19, 2.03 | 1.21 | 0.86, 1.70 |
| Tumor differentiation | ||||
| Well differentiated SCC | Ref | |||
| Moderately differentiated SCC | 1.19 | 0.85, 1.68 | ||
| Poorly differentiated SCC | 1.08 | 0.73, 1.60 | ||
| Number of organ metastasis ≥ 2 | 1.01 | 0.79, 1.30 | ||
| Liver metastasis | 1.57 * | 1.16, 2.13 | 1.43 * | 1.01, 2.03 |
| White blood cell count > 10,000/uL | 1.78 * | 1.38, 2.30 | 1.86 * | 1.38, 2.50 |
| Hemoglobin < 10 g/dL | 1.58 * | 1.19, 2.09 | 1.06 | 0.77, 1.45 |
| Platelet | ||||
| <150,000/uL | 0.99 | 0.51, 1.96 | ||
| >450,000/uL | 1.09 | 0.82, 1.46 | ||
| CrCl < 60 mL/min | 1.50 * | 1.12, 2.02 | 1.47 * | 1.05, 2.05 |
| Albumin < 3.5 g/dL | 2.13 * | 1.65, 2.76 | 1.48 * | 1.09, 2.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonglhow, J.; Wetwittayakhlang, P.; Sunpaweravong, P.; Sathitruangsak, C.; Dechaphunkul, A. Comparing the Efficacy of Carboplatin plus 5-Fluorouracil, Cisplatin plus 5-Fluorouracil, and Best Supportive Care for Advanced Esophageal Squamous Cell Carcinoma: A Propensity Score Analysis from a Tertiary Hospital in Southern Thailand. J. Clin. Med. 2024, 13, 1735. https://doi.org/10.3390/jcm13061735
Wonglhow J, Wetwittayakhlang P, Sunpaweravong P, Sathitruangsak C, Dechaphunkul A. Comparing the Efficacy of Carboplatin plus 5-Fluorouracil, Cisplatin plus 5-Fluorouracil, and Best Supportive Care for Advanced Esophageal Squamous Cell Carcinoma: A Propensity Score Analysis from a Tertiary Hospital in Southern Thailand. Journal of Clinical Medicine. 2024; 13(6):1735. https://doi.org/10.3390/jcm13061735
Chicago/Turabian StyleWonglhow, Jirapat, Panu Wetwittayakhlang, Patrapim Sunpaweravong, Chirawadee Sathitruangsak, and Arunee Dechaphunkul. 2024. "Comparing the Efficacy of Carboplatin plus 5-Fluorouracil, Cisplatin plus 5-Fluorouracil, and Best Supportive Care for Advanced Esophageal Squamous Cell Carcinoma: A Propensity Score Analysis from a Tertiary Hospital in Southern Thailand" Journal of Clinical Medicine 13, no. 6: 1735. https://doi.org/10.3390/jcm13061735





