Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies
Abstract
:1. Introduction
2. Scope and Methodology
3. Hypertensive Disorders of Pregnancy and PE
4. Evidence for the Increased Risk of PE in Women with PCOS and the Inclusion of PCOS in Clinical Practice Guidelines
4.1. Summary of Systematic Reviews
4.2. Risk of PE according to the United States National Inpatient Database
4.3. PCOS and Clinical Practice Guidelines
5. Evidence for the Role of Nutritional Factors in the Pathophysiology of PE
5.1. Role of a Healthy Lifestyle and Diet in the Pathogenesis of PE
5.2. Future Research into the Role of Lifestyle Modification in Reducing the Risk of PE in Women with PCOS
6. Mechanisms of Action of Nutritional Factors in the Pathophysiology of PE
6.1. Altered Placental Physiology and the Development of PE

6.2. Maternal Nutrition, Epigenetics, and Metabolism
6.3. Insulin Resistance
6.3.1. Insulin Resistance in PCOS
6.3.2. Physiological and Pathological Effects of Insulin and IR
| Author (Reference) | Experimental Tissue | Treatment | Main Findings |
|---|---|---|---|
| Hyperinsulinemia | |||
| Nestler [117] | Cytotrophoblasts from human term placenta | Insulin | Inhibition of aromatase via insulin receptor |
| Vega [28] | Cultured primary first-trimester trophoblasts | Insulin | ↑ DNA damage, ↑ apoptosis, ↓ cell survival |
| O’Tierney-Ginn [118] | Women in early pregnancy | IV GTT | Total insulin secretory response related to placental size and volume |
| Lassance [119] | Placental villous tissue from TOP | Insulin | Altered transcriptome signature, 30-fold-↓ insulin sensitivity in obese women |
| Inflammation | |||
| Cotechini [120] | Pregnant rats | Low-dose LPS | Inflammation associated with deficient trophoblast invasion and SA remodeling |
| Liu [121] | Retrospective case–control study | Endometrial Biopsy | ↑ endometrial macrophages, dendritic cells, and T cells, correlated with QUICKI (IR) |
| Wilson [122] | Primate model of PCOS | Testosterone | Syncytiotrophoblast inflammation |
| Matteo [123] | Experimental clinical study | Endometrial Biopsy | Abnormal lymphocyte subsets, impaired cytokines (IL-15/18, CL10) |
| Hyperandrogenemia | |||
| Gopalakrishnan [124] | Pregnant rats | Testosterone | ↓ SA remodeling, ↑ placental hypoxia |
| Sathishkumar [125] | Rat fetus | Testosterone SCI | ↓ placental amino acid transport, ↓ placental size and weight |
| Frolova [126] | In vitro stromal cells In vivo mouse model | DHEA DHEA | Inhibition of decidualization, reduced decidualization |
| Parsons [127] | Trophoblast cell lines | Testosterone | ↑ mitochondrial ROS, ↑ placental oxidative stress |
| Pan [128] | In vitro cell line | Testosterone | ↓ trophoblast cell invasion |
6.3.3. Pathological Effects of IR in the Placenta
6.3.4. Maternal Vascular Endothelial Dysfunction and IR
6.3.5. Effect of the Oral Contraceptive Pill on IR and Inflammation
6.4. Chronic Systemic Inflammation
6.4.1. Impact of Maternal Inflammation on Low-Grade Placental Inflammation
6.4.2. Interaction of IR, Inflammation, and Hyperandrogenism in the Regulation of Blood Pressure in PCOS and PE
6.4.3. Impact of Maternal Diet on Placental Inflammation
6.5. Hyperandrogenism
6.5.1. Role of Maternal Hyperandrogenemia in the Pathophysiology of PE
6.5.2. Synergistic Effects of IR and Chronic Inflammation on Hyperandrogenemia
6.6. Role of the Gastrointestinal Microbiome in PCOS and PE
6.6.1. Summary of the Role of the Microbiome in Women with PCOS
6.6.2. Emerging Role of the Microbiome in the Pathophysiology of PE
7. Identification, Assessment, and Management of Women with PCOS in Pregnancy
7.1. Identification of Women with PCOS and Increased Risk of PE in Early Pregnancy
7.2. Early-Pregnancy Screening and Treatment of High-Risk Women with PCOS
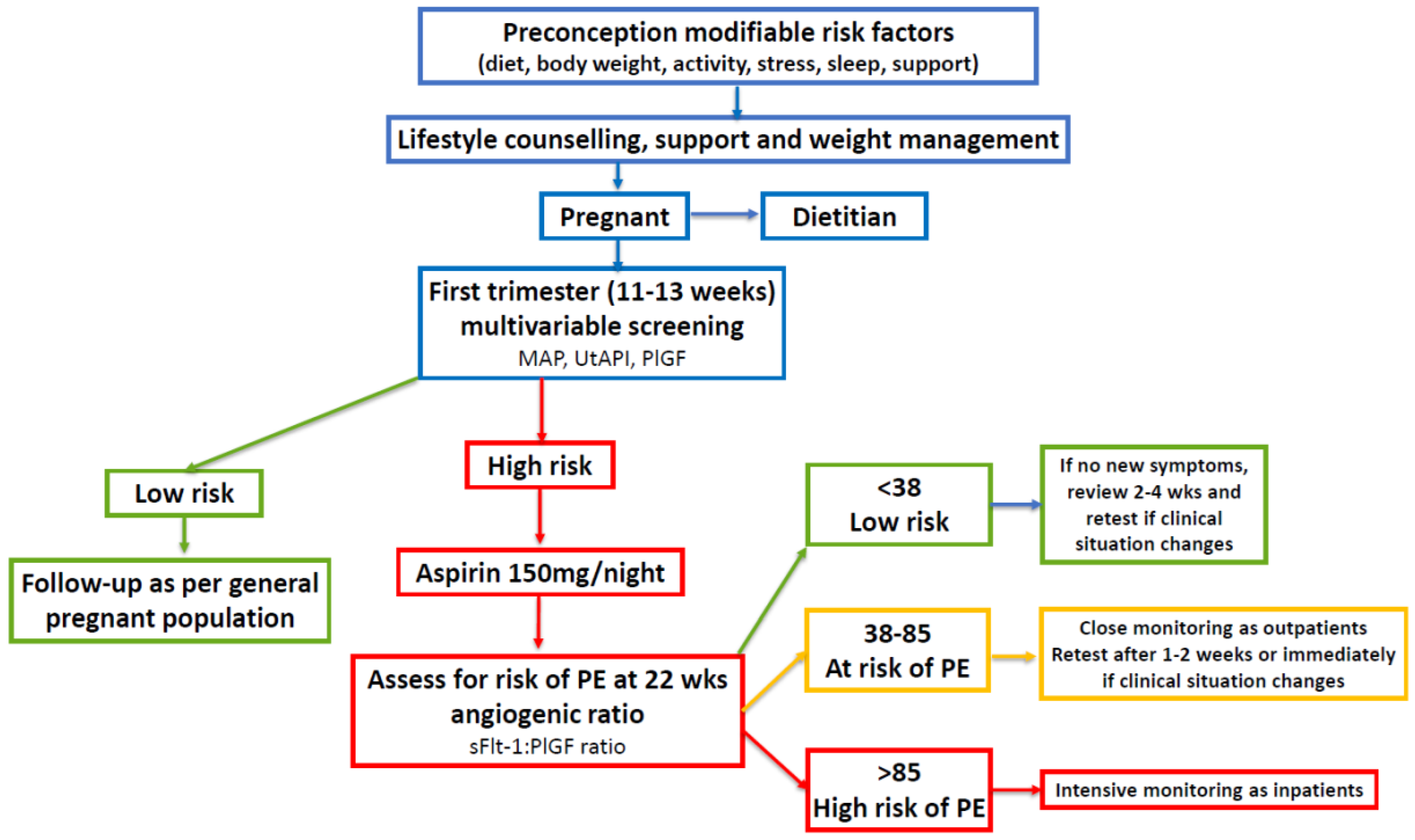
7.3. Future Investigation of Periconception Low-Dose Aspirin
7.4. Future Investigation into the Timing of Cessation of Low-Dose Aspirin
8. Strengths and Limitations of the Current Review
8.1. Strengths
8.2. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, J.; O’brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Health 2022, 19, 1336. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Abbott, D.H.; Chazenbalk, G.D.; Scholar, G. An Evolutionary Model for the Ancient Origins of Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 6120. [Google Scholar] [CrossRef] [PubMed]
- Parker, J. Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome. Life 2023, 13, 1056. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Padmanabhan, V.; Chazenbalk, G.D.; Abbott, D.H. Polycystic ovary syndrome as a plausible evolutionary outcome of metabolic adaptation. Reprod. Biol. Endocrinol. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rather, H.A.; Neelam; Kumar, R.; Basheer, M.; Reshi, M.S. A review on critical appraisal and pathogenesis of polycystic ovarian syndrome. Endocr. Metab. Sci. 2024, 14, 100162. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Shaw, L.M.A.; Elton, S. Polycystic ovary syndrome: A transgenerational evolutionary adaptation. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 144–148. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2021, 106, E1071–E1083. [Google Scholar] [CrossRef]
- Zore, T.; Joshi, N.V.; Lizneva, D.; Azziz, R. Polycystic Ovarian Syndrome: Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 271–281. [Google Scholar] [CrossRef]
- Reyes-Muñoz, E.; Castellanos-Barroso, G.; Ramírez-Eugenio, B.Y.; Ortega-González, C.; Parra, A.; Castillo-Mora, A.; De La Jara-Díaz, J.F. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil. Steril. 2012, 97, 1467–1471. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Avery, J.C.; Moore, V.M.; Davies, M.J.; Azziz, R.; Stener-Victorin, E.; Moran, L.J.; Robertson, S.A.; Stepto, N.K.; Norman, R.J.; et al. Complex diseases and co-morbidities: Polycystic ovary syndrome and type 2 diabetes mellitus. Endocr. Connect. 2019, 8, R71–R75. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yao, X.Y.; Shi, R.X.; Liu, S.F.; Wang, X.Y. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: An update meta-analysis. Reprod. Health 2018, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; De Wilde, M.A.; Falbo, A.; Koster, M.P.H.; La Sala, G.B.; Fauser, B.C.J.M. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Misso, M.; Costello, M.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.; Andersen, M.; Azziz, R.; et al. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2023; RCOG: London, UK, 2023. [Google Scholar]
- Du, Y.; Li, F.; Li, S.; Ding, L.; Liu, M. Causal relationship between polycystic ovary syndrome and chronic kidney disease: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1120119. [Google Scholar] [CrossRef]
- Boomsma, C.M.; Eijkemans, M.J.C.; Hughes, E.G.; Visser, G.H.A.; Fauser, B.C.J.M.; Macklon, N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 2006, 12, 673–683. [Google Scholar] [CrossRef]
- Kjerulff, L.E.; Sanchez-Ramos, L.; Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 558.e1–558.e6. [Google Scholar] [CrossRef]
- Qin, J.Z.; Pang, L.H.; Li, M.J.; Fan, X.J.; Huang, R.D.; Chen, H.Y. Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2013, 11, 56. [Google Scholar] [CrossRef]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4863. [Google Scholar] [CrossRef]
- Bahri Khomami, M.; Joham, A.E.; Boyle, J.A.; Piltonen, T.; Silagy, M.; Arora, C.; Misso, M.L.; Teede, H.J.; Moran, L.J. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—A systematic review, meta-analysis, and meta-regression. Obes. Rev. 2019, 20, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Khan, M.Z.; Gowda, S.; Faza, N.N.; Honigberg, M.C.; Vaught, A.; Guan, C.; Minhas, A.S.; Michos, E.D. Trends, Predictors, and Outcomes of Cardiovascular Complications Associated With Polycystic Ovary Syndrome During Delivery Hospitalizations: A National Inpatient Sample Analysis (2002–2019). J. Am. Heart Assoc. 2022, 11, e025839. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc. R. Soc. B Biol. Sci. 2023, 290, 20230191. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Elawad, T.; Scott, G.; Bone, J.N.; Elwell, H.; Lopez, C.E.; Filippi, V.; Green, M.; Khalil, A.; Kinshella, M.L.W.; Mistry, H.D.; et al. Risk factors for pre-eclampsia in clinical practice guidelines: Comparison with the evidence. BJOG Int. J. Obstet. Gynaecol. 2022, 131, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes—A molecular link for altered immunoregulation and inflammation mediated vascular dysfunction in preeclampsia. Int. J. Mol. Sci. 2020, 21, 1406. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Mauro, M.; Williams, Z. Direct toxicity of insulin on the human placenta and protection by metformin. Fertil. Steril. 2019, 111, 489–496.e5. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, B.; Qiao, S.; Feng, X.; Li, Y.; Zhang, S.; Lin, Y.; Hou, L. Elevated maternal androgen is associated with dysfunctional placenta and lipid disorder in newborns of mothers with polycystic ovary syndrome. Fertil. Steril. 2020, 113, 1275–1285.e2. [Google Scholar] [CrossRef]
- Myers, J.E. What are the metabolic precursors which increase the risk of pre-eclampsia and how could these be investigated further. Placenta 2017, 60, 110–114. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Baker, P.N.; Tong, C. Revisiting preeclampsia: A metabolic disorder of the placenta. FEBS J. 2022, 289, 336–354. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynecol. Obstet. 2019, 145, 1. [Google Scholar] [CrossRef]
- Verlohren, S.; Dröge, L.A. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S1048–S1058. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef]
- Davis, E.F.; Lazdam, M.; Lewandowski, A.J.; Worton, S.A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A.R.; McCormick, K.; Sargent, I.; et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012, 129, e1552–e1561. [Google Scholar] [CrossRef]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: A review. J. Am. Coll. Cardiol. 2014, 63, 1815–1822. [Google Scholar] [CrossRef]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Pan, H.; Xian, P.; Yang, D.; Zhang, C.; Tang, H.; He, X.; Lin, H.; Wen, X.; Ma, H.; Lai, M. Polycystic ovary syndrome is an independent risk factor for hypertensive disorders of pregnancy: A systematic review, meta-analysis, and meta-regression. Endocrine 2021, 74, 518–529. [Google Scholar] [CrossRef]
- Riestenberg, C.; Jagasia, A.; Markovic, D.; Buyalos, R.P.; Azziz, R. Health Care-Related Economic Burden of Polycystic Ovary Syndrome in the United States: Pregnancy-Related and Long-Term Health Consequences. J. Clin. Endocrinol. Metab. 2022, 107, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Tay, C.T.; Teede, H. Technical Report for the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome; Monash University: Clayton, Australia, 2023. [Google Scholar]
- Verlohren, S.; Brennecke, S.P.; Galindo, A.; Karumanchi, S.A.; Mirkovic, L.B.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022, 27, 42–50. [Google Scholar] [CrossRef]
- Wright, D.; Wright, A.; Nicolaides, K.H. The competing risk approach for prediction of preeclampsia. Am. J. Obstet. Gynecol. 2020, 223, 12–23.e7. [Google Scholar] [CrossRef]
- Serrano, B.; Bonacina, E.; Rodo, C.; Garcia-Manau, P.; Sanchez-Duran, M.Á.; Pancorbo, M.; Forcada, C.; Murcia, M.T.; Perestelo, A.; Armengol-Alsina, M.; et al. First-trimester screening for pre-eclampsia and small for gestational age: A comparison of the gaussian and Fetal Medicine Foundation algorithms. Int. J. Gynecol. Obstet. 2023, 160, 150–160. [Google Scholar] [CrossRef]
- ACOG. Low-Dose Aspirin Use during Pregnancy. Am. Coll. Obstet. Gynecol. 2018, 132, E44–E52. [Google Scholar] [CrossRef]
- Kane, S.C.; Da Silva Costa, F. Risk factors for pre-eclampsia: Received wisdom versus reality. BJOG Int. J. Obstet. Gynaecol. 2022, 131, 63. [Google Scholar] [CrossRef]
- Bahri Khomami, M.; Teede, H.J.; Joham, A.E.; Moran, L.J.; Piltonen, T.T.; Boyle, J.A. Clinical management of pregnancy in women with polycystic ovary syndrome: An expert opinion. Clin. Endocrinol. 2022, 97, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Howeler, J.F.; Hewson, A. Dietary Fibre and Toxaemia of Pregnancy. Med. J. Aust. 1957, 1, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Hipsley, E. Dietary Fibre and Pregnancy Toxaemia. Br. Med. J. 1953, 2, 420. [Google Scholar] [CrossRef] [PubMed]
- Harding, V.J.; Van Wyck, H.B. Diet in the Treatment of Pre-Eclampsia. J. Obstet. Gynaecol. 1926, 33, 17–32. [Google Scholar] [CrossRef]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef] [PubMed]
- Traore, S.S.; Bo, Y.; Amoah, A.N.; Khatun, P.; Kou, G.; Hu, Y.; Lyu, Q. A meta-analysis of maternal dietary patterns and preeclampsia. Clin. Nutr. Open Sci. 2021, 40, 15–29. [Google Scholar] [CrossRef]
- Kinshella, M.L.W.; Pickerill, K.; Bone, J.N.; Prasad, S.; Campbell, O.; Vidler, M.; Craik, R.; Volvert, M.L.; Mistry, H.D.; Tsigas, E.; et al. An evidence review and nutritional conceptual framework for pre-eclampsia prevention. Br. J. Nutr. 2023, 130, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Stephanou, A.; Rayman, M.P. Dietary factors that affect the risk of pre-eclampsia. BMJ Nutr. Prev. Health 2022, 5, 118–133. [Google Scholar] [CrossRef]
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 2019, 22, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.O.; Patriota, E.S.O.; Gonçalves, V.S.S.; Pizato, N. Maternal Consumption of Ultra-Processed Foods-Rich Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3242. [Google Scholar] [CrossRef]
- Pant, A.; Gribbin, S.; Machado, P.; Hodge, A.; Moran, L.; Marschner, S.; Zaman, S. Association of ultra-processed foods with cardiovascular disease and hypertension in australian women. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655-2388. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and future cardiovascular health. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Tuncalp, Ö.; Rogers, L.M.; Lawrie, T.A.; Barreix, M.; Peña-Rosas, J.P.; Bucagu, M.; Neilson, J.; Oladapo, O.T. WHO recommendations on antenatal nutrition: An update on multiple micronutrient supplements. BMJ Glob. Health 2022, 5, 3–6. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Bahri Khomami, M.; Moran, L.J.; Kenny, L.; Grieger, J.A.; Myers, J.; Poston, L.; McCowan, L.; Walker, J.; Dekker, G.; Norman, R.; et al. Lifestyle and pregnancy complications in polycystic ovary syndrome: The SCOPE cohort study. Clin. Endocrinol. 2019, 90, 814–821. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Bianco-Miotto, T.; Jankovic-Karasoulos, T.; Moran, L.J.; Wilson, R.L.; Leemaqz, S.Y.; Poston, L.; Mccowan, L.; Kenny, L.C.; et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Hum. Reprod. 2018, 33, 1063–1070. [Google Scholar] [CrossRef]
- Sampathkumar, S.; Parkhi, D.; Ghebremichael-Weldeselassie, Y.; Sukumar, N.; Saravanan, P. Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—A systematic review and meta-analysis of 257,876 pregnancies. Nutr. Diabetes 2023, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Mey, J.T.; Axelrod, C.L.; Paul, D.; Gordesky, L.; Russell, K.; Barkoukis, H.; O’Tierney-Ginn, P.; Fielding, R.A.; Kirwan, J.P.; et al. Rationale and study design for lifestyle intervention in preparation for pregnancy (LIPP): A randomized controlled trial. Contemp. Clin. Trials 2020, 94, 106024. [Google Scholar] [CrossRef]
- Eriksson, G.; Li, C.; Risal, S.; Pui, H.P.; Torstensson, S.; Linden, A.H.; Petropoulos, S.; Deng, Q.; Stener-Victorin, E. Mapping Endometrial Cell-type-specific Disease Signatures and Endometrial Organoids In Polycystic Ovary Syndrome. J. Endocr. Soc. 2023, 7 (Suppl. 1), bvad114-1587. [Google Scholar] [CrossRef]
- Parker, J.; Hawrelak, J.; Gersh, F.L. Nutritional role of polyphenols as a component of a wholefood diet in the management of polycystic ovary syndrome. J. Australas. Coll. Nutr. Environ. Med. 2021, 40, 6–12. [Google Scholar]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2022, 226, S907–S927. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Sharkey, D.J.; Green, E.S.; Chan, H.Y.; Matias, R.A.; Moldenhauer, L.M.; Robertson, S.A. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun. Biol. 2021, 4, 572. [Google Scholar] [CrossRef]
- Robertson, S.A.; Prins, J.R.; Sharkey, D.J.; Moldenhauer, L.M. Seminal Fluid and the Generation of Regulatory T Cells for Embryo Implantation. Am. J. Reprod. Immunol. 2013, 69, 315–330. [Google Scholar] [CrossRef]
- Ward, K.; Taylor, R.N. Genetic Factors in the Etiology of Preeclampsia/Eclampsia. In Chesley’s Hypertensive Disorders in Pregnancy, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 57–80. [Google Scholar] [CrossRef]
- Christians, J.K.; Leavey, K.; Cox, B.J. Associations between imprinted gene expression in the placenta, human fetal growth and preeclampsia. Biol. Lett. 2017, 13, 20170643. [Google Scholar] [CrossRef]
- Ashraf, U.M.; Hall, D.L.; Rawls, A.Z.; Alexander, B.T. Epigenetic processes during preeclampsia and effects on fetal development and chronic health. Clin. Sci. 2021, 135, 2307–2327. [Google Scholar] [CrossRef]
- Neubrand, L.; Pothmann, H.; Besenfelder, U.; Havlicek, V.; Gabler, C.; Dolezal, M.; Aurich, C.; Drillich, M.; Wagener, K. In vivo dynamics of pro-inflammatory factors, mucins, and polymorph nuclear neutrophils in the bovine oviduct during the follicular and luteal phase. Sci. Rep. 2023, 13, 22353. [Google Scholar] [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Turco, M.Y. Review: Histotrophic nutrition and the placental-endometrial dialogue during human early pregnancy. Placenta 2020, 102, 21–26. [Google Scholar] [CrossRef]
- O’Brien, K.; Wang, Y. The Placenta: A Maternofetal Interface. Annu. Rev. Nutr. 2023, 43, 301–325. [Google Scholar] [CrossRef]
- Conrad, K.P.; Rabaglino, M.B.; Post Uiterweer, E.D. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta 2017, 60, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Rabaglino, M.B.; Conrad, K.P. Evidence for shared molecular pathways of dysregulated decidualization in preeclampsia and endometrial disorders revealed by microarray data integration. FASEB J. 2019, 33, 11682–11695. [Google Scholar] [CrossRef] [PubMed]
- Spracklen, C.N.; Smith, C.J.; Saftlas, A.F.; Robinson, J.G.; Ryckman, K.K. Maternal hyperlipidemia and the risk of preeclampsia: A meta-analysis. Am. J. Epidemiol. 2014, 180, 346–358. [Google Scholar] [CrossRef]
- Tippetts, T.S.; Sieber, M.H.; Solmonson, A. Beyond energy and growth: The role of metabolism in developmental signaling, cell behavior and diapause. Development 2023, 150, dev201610. [Google Scholar] [CrossRef] [PubMed]
- Trigg, N.A.; Skerrett-Byrne, D.A.; Xavier, M.J.; Zhou, W.; Anderson, A.L.; Stanger, S.J.; Katen, A.L.; De Iuliis, G.N.; Dun, M.D.; Roman, S.D.; et al. Acrylamide modulates the mouse epididymal proteome to drive alterations in the sperm small non-coding RNA profile and dysregulate embryo development. Cell Rep. 2021, 37, 109787. [Google Scholar] [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 2018, 9, 421448. [Google Scholar] [CrossRef]
- Foidart, J.M.; Hustin, J.; Dubois, M.; Schaaps, J.P. The human placenta becomes haemochorial at the 13th week of pregnancy. Int. J. Dev. Biol. 1992, 36, 451–453. [Google Scholar]
- Kingdom, J.C.P.; Drewlo, S. Is heparin a placental anticoagulant in high-risk pregnancies? Blood 2011, 118, 4780–4788. [Google Scholar] [CrossRef]
- Kamrani, A.; Alipourfard, I.; Ahmadi-Khiavi, H.; Yousefi, M.; Rostamzadeh, D.; Izadi, M.; Ahmadi, M. The role of epigenetic changes in preeclampsia. BioFactors 2019, 45, 712–724. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Méhats, C.; Miralles, F.; Vaiman, D. The role of epigenetics in placental development and the etiology of preeclampsia. Int. J. Mol. Sci. 2019, 20, 2837. [Google Scholar] [CrossRef]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The impact of hypoxia in early pregnancy on placental cells. Int. J. Mol. Sci. 2021, 22, 9675. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Cedrón, M.; Moreno Palomares, R.; Ramírez de Molina, A. Metabolo-epigenetic interplay provides targeted nutritional interventions in chronic diseases and ageing. Front. Oncol. 2023, 13, 1169168. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 2020, 21, 782. [Google Scholar] [CrossRef] [PubMed]
- Doshani, A.; Konje, J.C. Placental dysfunction in obese women and antenatal surveillance. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 91, 102407. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Young, S.L.; Grattan, D.R.; Jasoni, C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014, 90, 117259. [Google Scholar] [CrossRef]
- Tiffon, C. The impact of nutrition and environmental epigenetics on human health and disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef] [PubMed]
- Barrón-Cabrera, E.; Ramos-Lopez, O.; González-Becerra, K.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Epigenetic Modifications as Outcomes of Exercise Interventions Related to Specific Metabolic Alterations: A Systematic Review. Lifestyle Genom. 2019, 12, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenet. 2012, 4, 4. [Google Scholar] [CrossRef]
- Abraham, E.; Rousseaux, S.; Agier, L.; Giorgis-Allemand, L.; Tost, J.; Galineau, J.; Hulin, A.; Siroux, V.; Vaiman, D.; Charles, M.A.; et al. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ. Int. 2018, 118, 334–347. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; Vandevoort, C.A.; et al. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tang, Y.; Xiong, Y.; Feng, L.; Li, X. Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J. 2019, 33, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Tassinari, R.; Maranghi, F.; Mantovani, A. Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. J. Appl. Toxicol. 2015, 35, 1278–1291. [Google Scholar] [CrossRef]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern.-Fetal Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef]
- Indrio, F.; Martini, S.; Francavilla, R.; Corvaglia, L.; Cristofori, F.; Mastrolia, S.A.; Neu, J.; Rautava, S.; Spena, G.R.; Raimondi, F.; et al. Epigenetic matters: The link between early nutrition, microbiome, and long-term health development. Front. Pediatr. 2017, 5, 178. [Google Scholar] [CrossRef]
- Kinshella, M.L.W.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.A.; von Dadelszen, P.; Moore, S.E.; Elango, R.; Poston, L.; Mistry, H.D.; et al. Maternal nutritional risk factors for pre-eclampsia incidence: Findings from a narrative scoping review. Reprod. Health 2022, 19, 188. [Google Scholar] [CrossRef]
- Abbott, D.H.; Dumesic, D.A.; Franks, S. Developmental origin of polycystic ovary syndrome—A hypothesis. J. Endocrinol. 2002, 174, 1–5. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Gersh, F.L. Developmental origins and transgenerational inheritance of polycystic ovary syndrome. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 922–926. [Google Scholar] [CrossRef]
- Sharma, S.; Mahajan, N. Polycystic ovarian syndrome and menopause in forty plus women. J. Midlife. Health 2021, 12, 3–7. [Google Scholar] [CrossRef]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Abhari, F.R.; Ghanbari Andarieh, M.; Farokhfar, A.; Ahmady, S. Estimating rate of insulin resistance in patients with preeclampsia using Homa-IR index and comparison with nonpreeclampsia pregnant women. Biomed Res. Int. 2014, 2014, 140851. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Iantorno, M.; Quon, M. An Integrated View of Insulin Resistance and Endothelial Dysfunction. Endocrinol. Metab. Clin. N. Am. 2008, 37, 685. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Leask, M.P.; Estiverne, C.; Choi, H.K.; Merriman, T.R.; Mount, D.B. Genetic and Physiological Effects of Insulin on Human Urate Homeostasis. Front. Physiol. 2021, 12, 713710. [Google Scholar] [CrossRef]
- DeFronzo, R.A. The effect of insulin on renal sodium metabolism. Diabetologia 1981, 21, 165–171. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Nestler, J.E. Regulation of the aromatase activity of human placental cytotrophoblasts by insulin, insulin-like growth factor-I, and -II. J. Steroid Biochem. Mol. Biol. 1993, 44, 449–457. [Google Scholar] [CrossRef] [PubMed]
- O’Tierney-Ginn, P.; Presley, L.; Myers, S.; Catalano, P. Placental growth response to maternal insulin in early pregnancy. J. Clin. Endocrinol. Metab. 2015, 100, 159–165. [Google Scholar] [CrossRef]
- Lassance, L.; Haghiac, M.; Leahy, P.; Basu, S.; Minium, J.; Zhou, J.; Reider, M.; Catalano, P.M.; Hauguel-De Mouzon, S. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am. J. Obstet. Gynecol. 2015, 212, 647.e1–647.e11. [Google Scholar] [CrossRef] [PubMed]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hong, L.; Mo, M.; Xiao, S.; Chen, C.; Li, Y.; Lian, R.; Wang, X.; Cai, S.; Diao, L.; et al. Evaluation of endometrial immune status of polycystic ovary syndrome. J. Reprod. Immunol. 2021, 144, 103282. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Lo, J.O.; Romero Jimenez, G.; Lindner, J.R.; Slayden, O.D.; Roberts, V.H.J. Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques. Molecules 2023, 28, 2894. [Google Scholar] [CrossRef]
- Matteo, M.; Serviddio, G.; Massenzio, F.; Scillitani, G.; Castellana, L.; Picca, G.; Sanguedolce, F.; Cignarelli, M.; Altomare, E.; Bufo, P.; et al. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 2222–2227.e3. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Mishra, J.S.; Chinnathambi, V.; Vincent, K.L.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Hankins, G.D.; Sathishkumar, K. Elevated Testosterone Reduces Uterine Blood Flow, Spiral Artery Elongation, and Placental Oxygenation in Pregnant Rats. Hypertension 2016, 67, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Balakrishnan, M.; Chinnathambi, V.; Chauhan, M.; Hankins, G.D.V.; Yallampalli, C. Fetal sex-related dysregulation in testosterone production and their receptor expression in the human placenta with preeclampsia. J. Perinatol. 2012, 32, 328–335. [Google Scholar] [CrossRef]
- Frolova, A.I.; O’Neill, K.; Moley, K.H. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol. Endocrinol. 2011, 25, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.M.; Rajendran, R.R.; Whitcomb, L.A.; Bouma, G.J.; Chicco, A.J. Characterization of trophoblast mitochondrial function and responses to testosterone treatment in ACH-3P cells. Placenta 2023, 137, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; He, G.; Chen, M.; Bao, C.; Chen, Y.; Liu, G.; Zhou, M.; Li, S.; Xu, W.; Liu, X. Abnormal CYP11A1 gene expression induces excessive autophagy, contributing to the pathogenesis of preeclampsia. Oncotarget 2017, 8, 89824–89836. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Ma, Q.; Fan, J.; Wang, J.; Yang, S.; Cong, Q.; Wang, R.; Lv, Q.; Liu, R.; Ning, G. High levels of chorionic gonadotrophin attenuate insulin sensitivity and promote inflammation in adipocytes. J. Mol. Endocrinol. 2015, 54, 161–170. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30, S112–S119. [Google Scholar] [CrossRef]
- Sonagra, A.D. Normal Pregnancy- A State of Insulin Resistance. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zuo, Y.; Gao, J.; Liu, Y.; Zheng, W. The risk factors of gestational diabetes mellitus in patients with polycystic ovary syndrome: What should we care. Medcine 2021, 100, E26521. [Google Scholar] [CrossRef]
- Yan, Q.; Qiu, D.; Liu, X.; Xing, Q.; Liu, R.; Hu, Y. The incidence of gestational diabetes mellitus among women with polycystic ovary syndrome: A meta-analysis of longitudinal studies. BMC Pregnancy Childbirth 2022, 22, 370. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.M.B.; Tsai, J.H.; Moley, K.H. Obesity and PCOS: The effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod. Sci. 2015, 22, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Scalisi, N.M.; Milluzzo, A.; Ettore, G.; Vigneri, R.; Sciacca, L. Maternal Diabetes Impairs Insulin and IGF-1 Receptor Expression and Signaling in Human Placenta. Front. Endocrinol. 2021, 12, 621680. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; DeMouzon, S.H.; O’Tierney-Ginn, P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 2016, 103, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Haghiac, M.; Minium, J.; Glazebrook, P.; Ranasinghe, G.C.; Hoppel, C.; De-Mouzon, S.H.; Catalano, P.; O’Tierney-Ginn, P. Effect of maternal obesity on placental lipid metabolism. Endocrinology 2017, 158, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Leoni, M.; Padilla, N.; Fabbri, A.; Della-Morte, D.; Ricordi, C.I.M. Mechanisms of Insulin Resistance during Pregnancy. Evolving Concepts in Insulin Resistance. In Evolving Concepts in Insulin Resistance; Infante, M., Ed.; Intech Open: London, UK, 2022; Available online: https://www.intechopen.com/chapters/83989 (accessed on 29 November 2023).
- Gou, R.; Zhang, X. Glycolysis: A fork in the path of normal and pathological pregnancy. FASEB J. 2023, 37, e23263. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, G.; Steinberg, H.O.; Hempfling, A.; Cronin, J.; Hook, G.; Shepard, M.K.; Baron, A.D. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001, 103, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.A.; Teede, H.; Sari, C.I.; Jona, E.; Shorakae, S.; Woodington, K.; Hemmes, R.; Eikelis, N.; Straznicky, N.E.; De Courten, B.; et al. Sympathetic activation and endothelial dysfunction in polycystic ovary syndrome are not explained by either obesity or insulin resistance. Clin. Endocrinol. 2015, 83, 812–819. [Google Scholar] [CrossRef]
- Oncul, M.; Albayrak, M.; Sozer, V.; Karakus, B.; Gelisgen, R.; Karatas, S.; Simsek, G.; Uzun, H. Polycystic ovary syndrome and endothelial dysfunction: A potential role for soluble lectin-like oxidized low density lipoprotein receptor-1. Reprod. Biol. 2020, 20, 396–401. [Google Scholar] [CrossRef]
- Chen, L.H.; Lin, C.P.; Wu, H.M.; Chu, P.H. Endothelial dysfunction in subfertile women with polycystic ovary syndrome. Reprod. Biomed. Online 2023, 46, 391–398. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G.; Kandarakis, S.A.; Chrousos, G.P. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol. Metab. 2007, 18, 280–285. [Google Scholar] [CrossRef]
- Avagliano, L.; Bulfamante, G.; Pietro; Morabito, A.; Marconi, A.M. Abnormal spiral artery remodelling in the decidual segment during pregnancy: From histology to clinical correlation. J. Clin. Pathol. 2011, 64, 1064–1068. [Google Scholar] [CrossRef]
- Wang, Q.; Würtz, P.; Auro, K.; Morin-Papunen, L.; Kangas, A.J.; Soininen, P.; Tiainen, M.; Tynkkynen, T.; Joensuu, A.; Havulinna, A.S.; et al. Effects of hormonal contraception on systemic metabolism: Cross-sectional and longitudinal evidence. Int. J. Epidemiol. 2016, 45, 1445–1457. [Google Scholar] [CrossRef]
- Teede, H.J.; Meyer, C.; Hutchison, S.K.; Zoungas, S.; McGrath, B.P.; Moran, L.J. Endothelial function and insulin resistance in polycystic ovary syndrome: The effects of medical therapy. Fertil. Steril. 2010, 93, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Bordin, L.; Donà, G.; Sabbadin, C.; Bakdounes, L.; Ragazzi, E.; Giorgino, F.L.; Fiore, C. Polycystic ovary syndrome: Implications of measurement of plasma aldosterone, renin activity and progesterone. Steroids 2012, 77, 655–658. [Google Scholar] [CrossRef]
- Zamolodchikova, T.S.; Tolpygo, S.M.; Kotov, A.V. Insulin in the regulation of the renin-angiotensin system: A new perspective on the mechanism of insulin resistance and diabetic complications. Front. Endocrinol. 2024, 15, 1293221. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.G.; Stanhewicz, A.E.; Nielsen, K.E.; Wong, B.J. Microvascular endothelial function following cessation of long-term oral contraceptive pill use: A case report. Exp. Physiol. 2023, 108, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.; Mokadem, I.; Smeets, N.J.; Spaanderman, M.E.; Roeleveld, N.; Lupattelli, A.; Van Gelder, M.M. Associations of periconceptional oral contraceptive use with pregnancy complications and adverse birth outcomes. Int. J. Epidemiol. 2023, 52, 1388–1399. [Google Scholar] [CrossRef]
- Hauguel-de Mouzon, S.; Guerre-Millo, M. The Placenta Cytokine Network and Inflammatory Signals. Placenta 2006, 27, 794–798. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, F.; Liu, F.; Zhao, J.; Huang, X. Metaflammation in glucolipid metabolic disorders: Pathogenesis and treatment. Biomed. Pharmacother. 2023, 161, 114545. [Google Scholar] [CrossRef]
- Wolf, M.; Sandler, L.; Hsu, K.; Vossen-Smirnakis, K.; Ecker, J.L.; Thadhani, R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 2003, 26, 819–824. [Google Scholar] [CrossRef]
- Du, M.; Basu, A.; Fu, D.; Wu, M.; Centola, M.; Jenkins, A.J.; Hanssen, K.F.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum inflammatory markers and preeclampsia in type 1 diabetes: A prospective study. Diabetes Care 2013, 36, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Parchim, N.F.; Wang, W.; Iriyama, T.; Ashimi, O.A.; Siddiqui, A.H.; Blackwell, S.; Sibai, B.; Kellems, R.E.; Xia, Y. Neurokinin 3 receptor and phosphocholine transferase: Missing factors for pathogenesis of C-reactive protein in preeclampsia. Hypertension 2015, 65, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Herrock, O.; Deer, E.; LaMarca, B. Setting a stage: Inflammation during preeclampsia and postpartum. Front. Physiol. 2023, 14, 1130116. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Ali, N. Mechanisms involved in regulation of Systemic Blood Pressure. Arch. Clin. Hypertens. 2017, 3, 016–020. [Google Scholar] [CrossRef]
- Triebel, H.; Castrop, H. The renin angiotensin aldosterone system. Pflugers Arch. Eur. J. Physiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Drury, E.R.; Wu, J.; Gigliotti, J.C.; Le, T.H. Sex differences in blood pressure regulation and hypertension: Renal, hemodynamic, and hormonal mechanisms. Physiol. Rev. 2024, 104, 199–251. [Google Scholar] [CrossRef]
- Troisi, R.; Braekke, K.; Harsem, N.K.; Hyer, M.; Hoover, R.N.; Staff, A.C. Blood pressure augmentation and maternal circulating concentrations of angiogenic factors at delivery in preeclamptic and uncomplicated pregnancies. Am. J. Obstet. Gynecol. 2008, 199, 653.e1–653.e10. [Google Scholar] [CrossRef]
- Makris, A.; Yeung, K.R.; Lim, S.M.; Sunderland, N.; Heffernan, S.; Thompson, J.F.; Iliopoulos, J.; Killingsworth, M.C.; Yong, J.; Xu, B.; et al. Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension 2016, 67, 1263–1272. [Google Scholar] [CrossRef]
- Armanini, D.; Andrisani, A.; Ambrosini, G.; Donà, G.; Bordin, L.; Sabbadin, C. Hypertension in pregnancy: Role of body mass index, insulin resistance, aldosterone, and calcium homeostasis. J. Clin. Hypertens. 2019, 21, 624–626. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Van Der Graaf, A.M.; Toering, T.J.; Faas, M.M.; Titia Lely, A. From preeclampsia to renal disease: A role of angiogenic factors and the renin-angiotensin aldosterone system? Nephrol. Dial. Transplant. 2012, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tarkun, I.; Arslan, B.C.; Cantürk, Z.; Türemen, E.; Şahin, T.; Duman, C. Endothelial dysfunction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low-grade chronic inflammation. J. Clin. Endocrinol. Metab. 2004, 89, 5592–5596. [Google Scholar] [CrossRef]
- Utkan Korun, Z.E.; Gocmez, S.S.; Furat Rencber, S.; Kavram Sarıhan, K.; Eraldemir, F.C.; Sahin, D. Etanercept Ameliorates Vascular, Endocrine, and Ovarian Changes in a Rat Model of DHEA-Induced Polycystic Ovary Syndrome. Reprod. Sci. 2023, 31, 714–726. [Google Scholar] [CrossRef]
- Usselman, C.W.; Yarovinsky, T.O.; Steele, F.E.; Leone, C.A.; Taylor, H.S.; Bender, J.R.; Stachenfeld, N.S. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: Role of the endothelin B receptor. J. Physiol. 2019, 597, 2853–2865. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, K.; Jiang, Z.Y.; Takahara, N.; Ha, S.W.; Igarashi, M.; Yamauchi, T.; Feener, E.P.; Herbert, T.P.; Rhodes, C.J.; King, G.L. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo—A specific vascular action of insulin. Circulation 2000, 101, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, C.; Nambi, S.S.; Kilcoyne, C.M.; Choucair, W.K.; Katz, A.; Quon, M.J.; Panza, J.A. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 1999, 100, 820–825. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Akolekar, R.; Zaragoza, E.; Poon, L.C.; Nicolaides, K.H. First trimester urinary placental growth factor and development of pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Ambrosini, G.; Sabbadin, C.; Donà, G.; Clari, G.; Bordin, L. Microalbuminuria and hypertension in pregnancy: Role of aldosterone and inflammation. J. Clin. Hypertens. 2013, 15, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Sabbadin, C.; Andrisani, A.; Ambrosini, G.; Bordin, L.; Donà, G.; Manso, J.; Ceccato, F.; Scaroni, C.; Armanini, D. Aldosterone in Gynecology and Its Involvement on the Risk of Hypertension in Pregnancy. Front. Endocrinol. 2019, 10, 467151. [Google Scholar] [CrossRef] [PubMed]
- Gersh, F.L.; O’Keefe, J.H.; Lavie, C.J.; Henry, B.M. The Renin-Angiotensin-Aldosterone System in Postmenopausal Women: The Promise of Hormone Therapy. Mayo Clin. Proc. 2021, 96, 3130–3141. [Google Scholar] [CrossRef]
- Armanini, D.; Strasser, T.; Weber, P. Binding of agonists and antagonists to mineralocorticoid receptors in human peripheral mononuclear leucocytes. Suppl. Off. J. Int. Soc. Hypertens. 1985, 3, S157–S159. [Google Scholar]
- Calò, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22phox in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Armanini, D.; Sabbadin, C.; Donà, G.; Andrisani, A.; Ambrosini, G.; Bordin, L. Maternal and Fetal Outcomes in Preeclampsia: Interrelations Between Insulin Resistance, Aldosterone, Metabolic Syndrome, and Polycystic Ovary Syndrome. J. Clin. Hypertens. 2015, 17, 783–785. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2003, 88, 2399–2403. [Google Scholar] [CrossRef]
- Belkacemi, L.; Michael Nelson, D.; Desai, M.; Ross, M.G. Maternal undernutrition influences placental-fetal development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef]
- Francis, E.C.; Dabelea, D.; Boyle, K.E.; Jansson, T.; Perng, W. Maternal Diet Quality Is Associated with Placental Proteins in the Placental Insulin/Growth Factor, Environmental Stress, Inflammation, and MTOR Signaling Pathways: The Healthy Start ECHO Cohort. J. Nutr. 2021, 152, 816–825. [Google Scholar] [CrossRef]
- Rosario, F.J.; Powell, T.L.; Jansson, T. Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R87–R93. [Google Scholar] [CrossRef]
- Guenther, P.M.; Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Buckman, D.W.; Dodd, K.W.; Casavale, K.O.; Carroll, R.J. The healthy eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for Americans. J. Nutr. 2014, 144, 399–407. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Daan, N.M.P.; Louwers, Y.V.; Koster, M.P.H.; Eijkemans, M.J.C.; De Rijke, Y.B.; Lentjes, E.W.G.; Fauser, B.C.J.M.; Laven, J.S.E. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: Who is really at risk? Fertil. Steril. 2014, 102, 1444–1451.e3. [Google Scholar] [CrossRef]
- Jeanes, Y.M.; Reeves, S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr. Res. Rev. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Shroff, R.; Syrop, C.H.; Davis, W.; Van Voorhis, B.J.; Dokras, A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil. Steril. 2007, 88, 1389–1395. [Google Scholar] [CrossRef]
- Persson, S.; Elenis, E.; Turkmen, S.; Kramer, M.S.; Yong, E.L.; Poromaa, I.S. Higher risk of type 2 diabetes in women with hyperandrogenic polycystic ovary syndrome. Fertil. Steril. 2021, 116, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Mumm, H.; Jensen, D.M.; Sørensen, J.A.; Andersen, L.L.T.; Ravn, P.; Andersen, M.; Glintborg, D. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet. Gynecol. Scand. 2015, 94, 204–211. [Google Scholar] [CrossRef]
- de Wilde, M.A.; Lamain-de Ruiter, M.; Veltman-Verhulst, S.M.; Kwee, A.; Laven, J.S.; Lambalk, C.B.; Eijkemans, M.J.C.; Franx, A.; Fauser, B.C.J.M.; Koster, M.P.H. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil. Steril. 2017, 108, 333–340. [Google Scholar] [CrossRef]
- Naver, K.V.; Grinsted, J.; Larsen, S.O.; Hedley, P.L.; Jørgensen, F.S.; Christiansen, M.; Nilas, L. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yao, Y.; Wang, T.; Li, B.; Jiang, P.; Wang, F.; Qu, F. Preeclampsia in pregnant women with polycystic ovary syndrome: Risk factor analysis based on a retrospective cohort study. Ginekol. Pol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Palomba, S.; Russo, T.; Falbo, A.; Di Cello, A.; Amendola, G.; Mazza, R.; Tolino, A.; Zullo, F.; Tucci, L.; La Sala, G.B. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: An experimental case-control study. J. Clin. Endocrinol. Metab. 2012, 97, 2441–2449. [Google Scholar] [CrossRef]
- Palomba, S.; Russo, T.; Falbo, A.; Di Cello, A.; Tolino, A.; Tucci, L.; La Sala, G.B.; Zullo, F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum. Reprod. 2013, 28, 2838–2847. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Chiossi, G.; Tolino, A.; Tucci, L.; La Sala, G.B.; Zullo, F. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod. Biomed. Online 2014, 29, 370–381. [Google Scholar] [CrossRef]
- Kumar, S.; Gordon, G.H.; Abbott, D.H.; Mishra, J.S. Androgens in maternal vascular and placental function: Implications for Preeclampsia Pathogenesis. Reproduction 2018, 156, R155–R167. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, V.; Balakrishnan, M.; Ramadoss, J.; Yallampalli, C.; Sathishkumar, K. Testosterone alters maternal vascular adaptations: Role of the endothelial no system. Hypertension 2013, 61, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, V.; Blesson, C.S.; Vincent, K.L.; Saade, G.R.; Hankins, G.D.; Yallampalli, C.; Sathishkumar, K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 2014, 64, 405–414. [Google Scholar] [CrossRef]
- Diamond, M.P.; Grainger, D.; Diamond, M.C.; Sherwin, R.S.; Defronzo, R.A. Effects of methyltestosterone on insulin secretion and sensitivity in women. J. Clin. Endocrinol. Metab. 1998, 83, 4420–4425. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Gilling-Smith, C.; Watson, H.; Willis, D. Insulin action in the normal and polycystic ovary. Endocrinol. Metab. Clin. N. Am. 1999, 28, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)—Searching for Epigenetic Factors. Int. J. Mol. Sci. 2022, 23, 14663. [Google Scholar] [CrossRef] [PubMed]
- González, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012, 77, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Smith, Y.R.; Padmanabhan, V. A Narrative Review of Placental Contribution to Adverse Pregnancy Outcomes in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 5299–5315. [Google Scholar] [CrossRef] [PubMed]
- Kanbour, S.A.; Dobs, A.S. Hyperandrogenism in Women with Polycystic Ovarian Syndrome: Pathophysiology and Controversies. Androgens 2022, 3, 22–30. [Google Scholar] [CrossRef]
- Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022, 65, 14. [Google Scholar] [CrossRef]
- Colonetti, T.; Limas Carmo Teixeira, D.; Grande, A.J.; Rodrigues Uggioni, M.L.; Generoso, J.; Harding, S.; Rodriguez-Mateos, A.; Rech, P.; Rosa Silva, F.; Toreti, I.; et al. The role of intestinal microbiota on pre-eclampsia: Systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 291, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, M.; Kan, Z. Causal relationship between gut microbiota and polycystic ovary syndrome: A literature review and mendelian randomization study. Front. Endocrinol. 2024, 15, 1280983. [Google Scholar] [CrossRef]
- Min, Q.; Geng, H.; Gao, Q.; Xu, M. The association between gut microbiome and PCOS: Evidence from meta-analysis and two-sample mendelian randomization. Front. Microbiol. 2023, 14, 120390. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd. 2020, 80, 161–171. [Google Scholar] [CrossRef]
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2021, 5, bvaa177. [Google Scholar] [CrossRef]
- Li, J.W.; Chen, Y.Z.; Zhang, Y.; Zeng, L.H.; Li, K.W.; Xie, B.Z.; Luo, S.P.; Gao, J. Gut microbiota and risk of polycystic ovary syndrome: Insights from Mendelian randomization. Heliyon 2023, 9, e22155. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Fiori, P.L.; Capobianco, G.; Carru, C.; Chen, Z. Gut microbiota and polycystic ovary syndrome, focus on genetic associations: A bidirectional Mendelian randomization study. Front. Endocrinol. 2024, 15, 1275419. [Google Scholar] [CrossRef]
- Jin, J.; Gao, L.; Zou, X.; Zhang, Y.; Zheng, Z.; Zhang, X.; Li, J.; Tian, Z.; Wang, X.; Gu, J.; et al. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ. Res. 2022, 131, 492–506. [Google Scholar] [CrossRef]
- Yong, W.; Zhao, Y.; Jiang, X.; Li, P. Sodium butyrate alleviates pre-eclampsia in pregnant rats by improving the gut microbiota and short-chain fatty acid metabolites production. J. Appl. Microbiol. 2022, 132, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, J.; Wang, Y. The role of short-chain fatty acids produced by gut microbiota in the regulation of pre-eclampsia onset. Front. Cell. Infect. Microbiol. 2023, 13, 1177768. [Google Scholar] [CrossRef]
- Miao, T.; Yu, Y.; Sun, J.; Ma, A.; Yu, J.; Cui, M.; Yang, L.; Wang, H. Decrease in abundance of bacteria of the genus bifidobacterium in gut microbiota may be related to pre-eclampsia progression in women from east China. Food Nutr. Res. 2021, 65, 1–12. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between gut microbiota and preeclampsia-eclampsia: A two-sample Mendelian randomization study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, Q.; Pei, S.; Zhu, Z. The causal role of intestinal microbiome in development of pre-eclampsia. Funct. Integr. Genom. 2023, 23, 127. [Google Scholar] [CrossRef] [PubMed]
- Rôças, I.N.; Siqueira, J.F. Species-directed 16S rRNA gene nested PCR detection of Olsenella species in association with endodontic diseases. Lett. Appl. Microbiol. 2005, 41, 12–16. [Google Scholar] [CrossRef]
- Lv, L.J.; Li, S.H.; Li, S.C.; Zhong, Z.C.; Duan, H.L.; Tian, C.; Li, H.; He, W.; Chen, M.C.; He, T.W.; et al. Early-onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front. Cell. Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef]
- Panzer, J.J.; Romero, R.; Greenberg, J.M.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N.; Theis, K.R. Is there a placental microbiota? A critical review and re-analysis of published placental microbiota datasets. BMC Microbiol. 2023, 23, 76. [Google Scholar] [CrossRef]
- Ishimwe, J.A. Maternal microbiome in preeclampsia pathophysiology and implications on offspring health. Physiol. Rep. 2021, 9, e14875. [Google Scholar] [CrossRef]
- Piltonen, T.T.; Ruokojärvi, M.; Karro, H.; Kujanpää, L.; Morin-Papunen, L.; Tapanainen, J.S.; Stener-Victorin, E.; Sundrström-Poromaa, I.; Hirschberg, A.L.; Ravn, P.; et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS ONE 2019, 14, e226074. [Google Scholar] [CrossRef]
- Valdimarsdottir, R.; Vanky, E.; Elenis, E.; Lindström, L.; Junus, K.; Jonsson, M.; Sundström Poromaa, I.; Wikström, A.K. Polycystic ovary syndrome and risk of pre-eclampsia: A national register-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2023, 1–11. [Google Scholar] [CrossRef]
- Tan, M.Y.; Syngelaki, A.; Poon, L.C.; Rolnik, D.L.; O’Gorman, N.; Delgado, J.L.; Akolekar, R.; Konstantinidou, L.; Tsavdaridou, M.; Galeva, S.; et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2018, 52, 186–195. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 2016, 214, 103.e1–103.e12. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.J.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: Ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet. Gynecol. 2019, 53, 367–375. [Google Scholar] [CrossRef]
- Bian, X.; Biswas, A.; Huang, X.; Lee, K.J.; Li, T.K.T.; Masuyama, H.; Ohkuchi, A.; Park, J.S.; Saito, S.; Tan, K.H.; et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women with Suspected Preeclampsia. Hypertension 2019, 74, 164–172. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Jamal, A.; Milani, F.; Al-Yasin, A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012, 10, 265–270. [Google Scholar]
- Rolnik, D.L.; Syngelaki, A.; O’Gorman, N.; Wright, D.; Poon, L.C.; Nicolaides, K.H. ASPRE trial: Effects of aspirin on mean arterial blood pressure and uterine artery pulsatility index trajectories in pregnancy. Ultrasound Obstet. Gynecol. 2023, 61, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef] [PubMed]
- Panagodage, S.; Yong, H.E.J.; Da Silva Costa, F.; Borg, A.J.; Kalionis, B.; Brennecke, S.P.; Murthi, P. Low-Dose Acetylsalicylic Acid Treatment Modulates the Production of Cytokines and Improves Trophoblast Function in an in Vitro Model of Early-Onset Preeclampsia. Am. J. Pathol. 2016, 186, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Raikwar, N.S.; Santillan, M.K.; Santillan, D.A.; Thomas, C.P. Aspirin inhibits expression of sFLT1 from human cytotrophoblasts induced by hypoxia, via cyclo-oxygenase 1. Placenta 2015, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Khanabdali, R.; Shakouri-Motlagh, A.; Wilkinson, S.; Murthi, P.; Georgiou, H.M.; Brennecke, S.P.; Kalionis, B. Low-dose aspirin treatment enhances the adhesion of preeclamptic decidual mesenchymal stem/stromal cells and reduces their production of pro-inflammatory cytokines. J. Mol. Med. 2018, 96, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- McDougall, A.R.A.; Hastie, R.; Goldstein, M.; Tuttle, A.; Tong, S.; Ammerdorffer, A.; Gülmezoglu, A.M.; Vogel, J.P. Systematic evaluation of the pre-eclampsia drugs, dietary supplements and biologicals pipeline using target product profiles. BMC Med. 2022, 20, 393. [Google Scholar] [CrossRef]
- Gomes, F.; Ashorn, P.; Askari, S.; Belizan, J.M.; Boy, E.; Cormick, G.; Dickin, K.L.; Driller-Colangelo, A.R.; Fawzi, W.; Hofmeyr, G.J.; et al. Calcium supplementation for the prevention of hypertensive disorders of pregnancy: Current evidence and programmatic considerations. Ann. N. Y. Acad. Sci. 2022, 1510, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, J.N.; Babu, A.N.; Kiran, S.S.M.; Nori, L.P.; Hassan, N.; Ashames, A.; Bhandare, R.R.; Shaik, A.B. Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review. BioTech 2023, 12, 4. [Google Scholar] [CrossRef]
- Society of Obstetric Medicine Australia and New Zealand. Hypertension in Pregnancy Guideline; Society of Obstetric Medicine Australia and New Zealand: Sydney, Australia, 2023; Available online: https://www.somanz.org/hypertension-in-pregnancy-guideline-2023/ (accessed on 5 February 2024).
- Poon, L.C.; Galindo, A.; Surbek, D.; Chantraine, F.; Stepan, H.; Hyett, J.; Tan, K.H.; Verlohren, S. From first-trimester screening to risk stratification of evolving pre-eclampsia in second and third trimesters of pregnancy: Comprehensive approach. Ultrasound Obstet. Gynecol. 2020, 55, 5–12. [Google Scholar] [CrossRef]
- Chaemsaithong, P.; Gil, M.M.; Chaiyasit, N.; Cuenca-Gomez, D.; Plasencia, W.; Rolle, V.; Poon, L.C. Accuracy of placental growth factor alone or in combination with soluble fms-like tyrosine kinase-1 or maternal factors in detecting preeclampsia in asymptomatic women in the second and third trimesters: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 229, 222–247. [Google Scholar] [CrossRef]
- Tawfeek, M.; Hassan, M.; Ahmed, M.; Mohamed, N.; Ahmed, N. Low dose Aspirin with clomid in pco. Minia J. Med. Res. 2021, 32, 100–106. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Su, F.; Wang, M. Effectiveness and safety of aspirin combined with letrozole in the treatment of polycystic ovary syndrome: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4632–4641. [Google Scholar] [CrossRef]
- Aref, N.K.; Ahmed, W.A.S.; Ahmed, M.R.; Sedik, W.F. A new look at low-dose aspirin: Co-administration with tamoxifen in ovulation induction in anovulatory PCOS women. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, B.; Jiang, X.; Ma, M.; Shi, L.; Zhang, Q.; Zhou, L. Effects of combining lowdose aspirin with a Chinese patent medicine on follicular blood flow and pregnancy outcome. Mol. Med. Rep. 2014, 10, 2372–2376. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Silver, R.M.; Perkins, N.J.; Mumford, S.L.; Whitcomb, B.W.; Stanford, J.B.; Lesher, L.L.; Faraggi, D.; Wactawski-Wende, J.; Browne, R.W.; et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: Design and baseline characteristics. Paediatr. Perinat. Epidemiol. 2013, 27, 598–609. [Google Scholar] [CrossRef]
- Naimi, A.I.; Perkins, N.J.; Sjaarda, L.A.; Mumford, S.L.; Platt, R.W.; Silver, R.M.; Schisterman, E.F. The effect of preconception-initiated low-dose aspirin on human chorionic gonadotropin-detected pregnancy, pregnancy loss, and live birth: Per protocol analysis of a randomized trial. Ann. Intern. Med. 2021, 174, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.; Bonacina, E.; Garcia-Manau, P.; López, M.; Caamiña, S.; Vives, À.; Lopez-Quesada, E.; Ricart, M.; Maroto, A.; de Mingo, L.; et al. Aspirin Discontinuation at 24 to 28 Weeks’ Gestation in Pregnancies at High Risk of Preterm Preeclampsia: A Randomized Clinical Trial. JAMA 2023, 329, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, E.; Garcia-Manau, P.; López, M.; Caamiña, S.; Vives, À.; Lopez-Quesada, E.; Ricart, M.; Maroto, A.; de Mingo, L.; Pintado, E.; et al. Mid-trimester uterine artery Doppler for aspirin discontinuation in pregnancies at high risk for preterm pre-eclampsia: Post-hoc analysis of StopPRE trial. BJOG Int. J. Obstet. Gynaecol. 2023, 131, 334–342. [Google Scholar] [CrossRef]
- Hastie, R.; Tong, S.; Wikström, A.K.; Sandström, A.; Hesselman, S.; Bergman, L. Aspirin use during pregnancy and the risk of bleeding complications: A Swedish population-based cohort study. Am. J. Obstet. Gynecol. 2021, 224, 95.e1–95.e12. [Google Scholar] [CrossRef]
- Bedrick, B.S.; Eskew, A.M.; Chavarro, J.E.; Jungheim, E.S. Self-Administered Questionnaire to Screen for Polycystic Ovarian Syndrome. Women’s Health Rep. 2020, 1, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Day, F.R.; Hinds, D.A.; Tung, J.Y.; Stolk, L.; Styrkarsdottir, U.; Saxena, R.; Bjonnes, A.; Broer, L.; Dunger, D.B.; Halldorsson, B.V.; et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 2015, 6, 8464. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. Guideline for the Management of Hypertensive Disorders of Pregnancy; Society of Obstetric Medicine of Australia and New Zealand: Sydney, Australia, 2014. [Google Scholar]
- Ayala, D.E.; Ucieda, R.; Hermida, R.C. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol. Int. 2013, 30, 260–279. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Odds Ratio (95% CI) 1 | Number of Studies | Reference |
|---|---|---|---|
| Boomsma et al. (2006) | 3.47 (1.95–6.17) | 8 | [18] |
| Kjerulff et al. (2011) | 4.23 (2.77–6.46) | 12 | [19] |
| Qin et al. (2013) | 3.28 (2.06–5.22) | 15 | [20] |
| Yu et al. (2016) | 2.79 (2.29–3.38) | 25 | [21] |
| Khomami (2019) | 1.87 (1.55–2.25) | 26 | [22] |
| Pan (2021) | 2.07 (1.91–2.24) | 20 | [40] |
| Riestenberg (2022) | 2.03 (1.43–2.87) | 15 | [41] |
| Mousa (2023) | 2.28 (1.88–2.77) | 36 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, J.; O’Brien, C.L.; Yeoh, C.; Gersh, F.L.; Brennecke, S. Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies. J. Clin. Med. 2024, 13, 1774. https://doi.org/10.3390/jcm13061774
Parker J, O’Brien CL, Yeoh C, Gersh FL, Brennecke S. Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies. Journal of Clinical Medicine. 2024; 13(6):1774. https://doi.org/10.3390/jcm13061774
Chicago/Turabian StyleParker, Jim, Claire Louise O’Brien, Christabelle Yeoh, Felice L. Gersh, and Shaun Brennecke. 2024. "Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies" Journal of Clinical Medicine 13, no. 6: 1774. https://doi.org/10.3390/jcm13061774
APA StyleParker, J., O’Brien, C. L., Yeoh, C., Gersh, F. L., & Brennecke, S. (2024). Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies. Journal of Clinical Medicine, 13(6), 1774. https://doi.org/10.3390/jcm13061774








