Effects of Premature Ventricular Complex Burden on Left Ventricular Global Longitudinal Strain in Patients without Structural Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
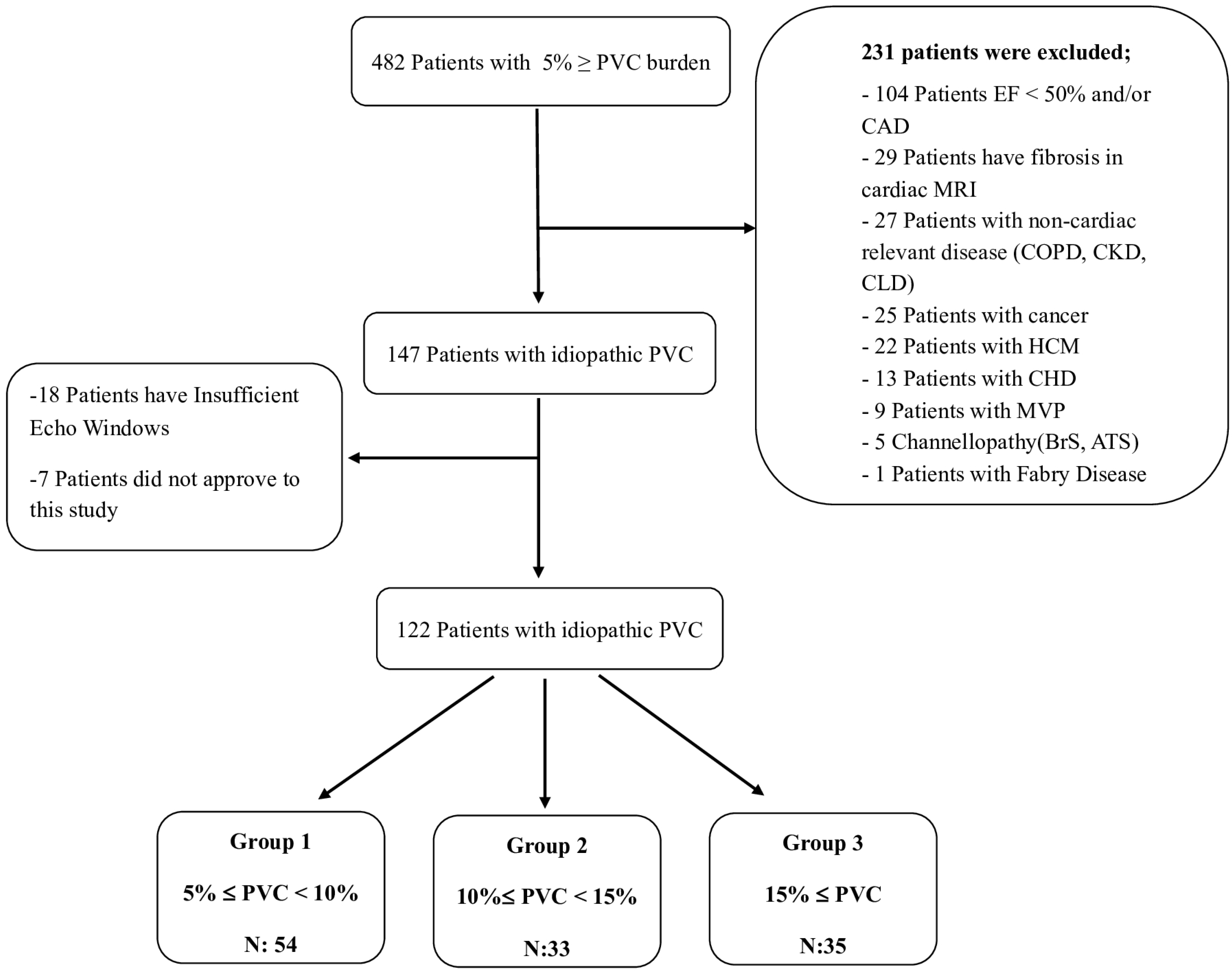
2.1. Study Population
2.2. Electrocardiography (ECG) and Rhythm Holter Analysis
2.3. Transthoracic Echocardiographic (TTE) Evaluation
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographical Features and Laboratory Parameters:
3.2. Correlation and Multiple Linear Regression Analysis
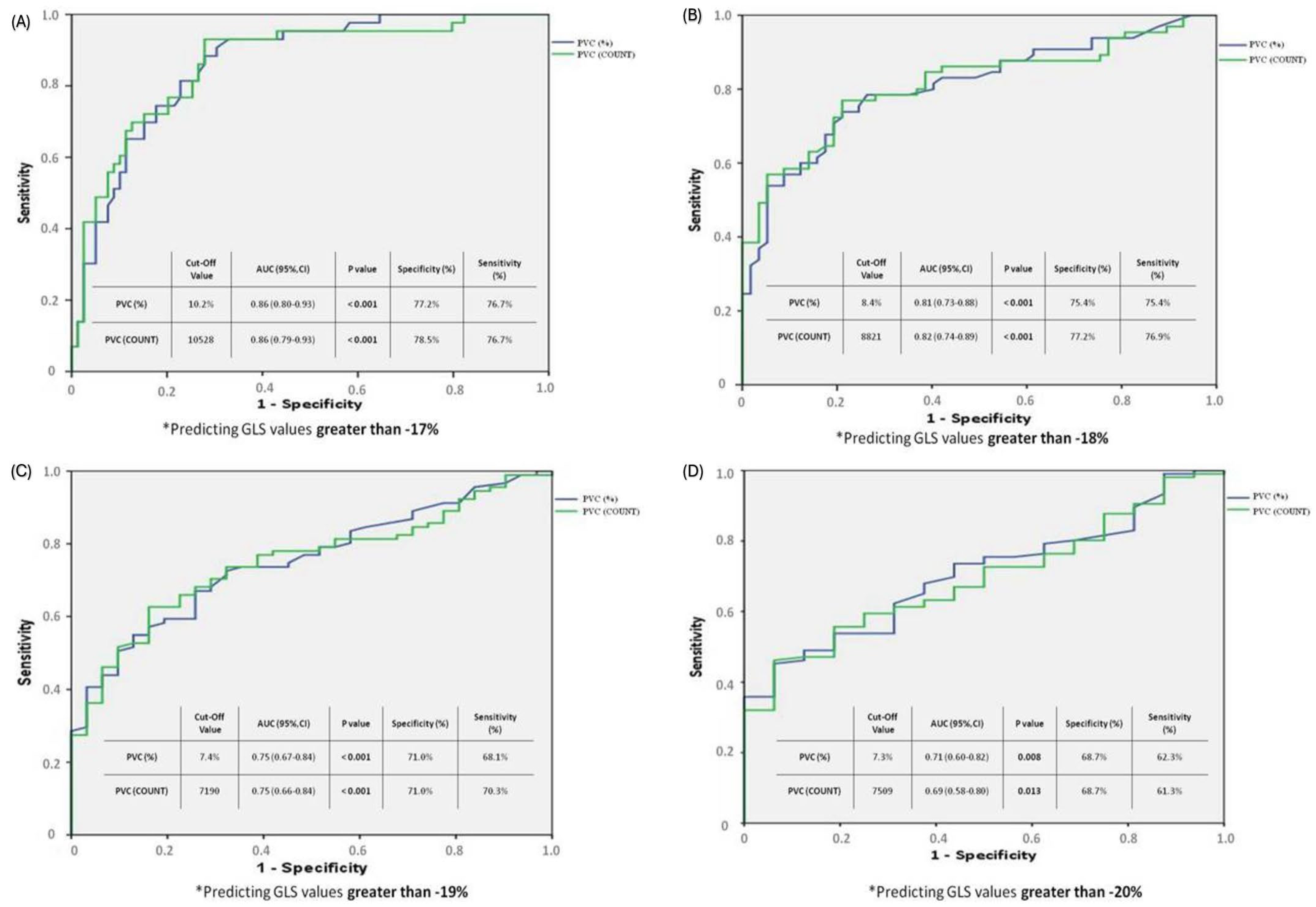
3.3. ROC Analysis
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, H.L.; Whitlock, J.A.; Sprague, M.K.; Kennedy, L.J.; Buckingham, T.A.; Goldberg, R.J. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N. Engl. J. Med. 1985, 312, 193–197. [Google Scholar] [CrossRef]
- Chugh, S.S.; Shen, W.K.; Luria, D.M.; Smith, H.C. First evidence of premature ventricular complex-induced cardiomyopathy: A potentially reversible cause of heart failure. J. Cardiovasc. Electrophysiol. 2000, 11, 328–329. [Google Scholar] [CrossRef]
- Redfearn, D.P.; Hill, J.D.; Keal, R.; Toff, W.D.; Stafford, P.J. Left ventricular dysfunction resulting from frequent unifocal ventricular ectopics with resolution following radiofrequency ablation. EP Europace 2003, 5, 247–250. [Google Scholar] [CrossRef]
- Agarwal, V.; Vittinghoff, E.; Whitman, I.R.; Dewland, T.A.; Dukes, J.W.; Marcus, G.M. Relation Between Ventricular Premature Complexes and Incident Heart Failure. Am. J. Cardiol. 2017, 119, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Niwano, S.; Wakisaka, Y.; Niwano, H.; Fukaya, H.; Kurokawa, S.; Kiryu, M.Y.; Hatakeyama, Y.; Izumi, T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009, 95, 1230–1237. [Google Scholar] [CrossRef]
- Del Carpio Munoz, F.; Syed, F.F.; Noheria, A.; Cha, Y.M.; Friedman, P.A.; Hammill, S.C.; Munger, T.M.; Venkatachalam, K.L.; Shen, W.K.; Packer, D.L.; et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J. Cardiovasc. Electrophysiol. 2011, 22, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Altıntaş, B.; Özkalaycı, F.; Çinier, G.; Kaya, İ.; Aktan, A.; Küp, A.; Onuk, R.; Özcan, S.; Uslu, A.; Akyüz, A.; et al. The effect of idiopathic premature ventricular complexes on left ventricular ejection fraction. Ann. Noninvasive Electrocardiol. 2020, 25, e12702. [Google Scholar] [CrossRef] [PubMed]
- Panizo, J.G.; Barra, S.; Mellor, G.; Heck, P.; Agarwal, S. Premature Ventricular Complex-induced Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2018, 7, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Luebbert, J.; Auberson, D.; Marchlinski, F. Premature Ventricular Complexes in Apparently Normal Hearts. Card. Electrophysiol. Clin. 2016, 8, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.A.J.; Henkens, M.T.H.M.; Wang, P.; Schummers, G.; Raafs, A.G.; Krapels, I.P.C.; van Empel, V.; Heymans, S.R.B.; Rocca, H.-P.B.-L.; Knackstedt, C. A global longitudinal strain cut-off value to predict adverse outcomes in individuals with a normal ejection fraction. ESC Heart Fail. 2021, 8, 4343–4345. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology(ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology(AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc. Imaging 2023, 16, 1516–1531. [Google Scholar] [CrossRef]
- Lie, Ø.H.; Saberniak, J.; Dejgaard, L.A.; Stokke, M.K.; Hegbom, F.; Anfinsen, O.-G.; Edvardsen, T.; Haugaa, K.H. Lower than expected burden of premature ventricular contractions impairs myocardial function. ESC Heart Fail. 2017, 4, 585–594. [Google Scholar] [CrossRef]
- Ling, Y.; Wan, Q.; Chen, Q.; Zhu, W. Assessment of subtle cardiac dysfunction in patients with frequent premature ventricular complexes by real-time three-dimensional speckle tracking echocardiography. Clin. Cardiol. 2017, 40, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.-E.; Park, H.-C.; Park, J.-S.; Nagamoto, Y.; Choi, J.-I.; Lim, H.-E.; Park, S.-W.; Kim, Y.-H. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace 2013, 15, 735–741. [Google Scholar] [CrossRef]
- Baman, T.S.; Lange, D.C.; Ilg, K.J.; Gupta, S.K.; Liu, T.-Y.; Alguire, C.; Armstrong, W.; Good, E.; Chugh, A.; Jongnarangsin, K.; et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010, 7, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.; Chua, T.P.; Ward, D. ‘Frequent’ ventricular bigeminy—A reversible cause of dilated cardiomyopathy. How frequent is ’frequent’? Eur. J. Heart Fail. 2006, 8, 869–873. [Google Scholar] [CrossRef]
- Sheel, B.K.; Badiuzzaman, M.; Haque, T.; Rahman, H.; Biswas, A.K.; Khan, S.R. Association between Myocardial Performance Index. (Tei-Index) and Severity of Coronary Artery Disease in Patients with Non-ST Segment Elevation Acute Coronary Syndrome. Bangladesh Heart J. 2022, 37, 16–26. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Biscottini, E.; Lauciello, R.; Pantano, P.; Gentile, F.; Nishimura, R.A.; et al. Improvement of myocardial performance (Tei) index closely reflects intrinsic improvement of cardiac function: Assessment in revascularized hibernating myocardium. Echocardiography 2012, 29, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Abaci, O.; Kocas, C.; Oktay, V.; Arslan, S.; Turkmen, Y.; Bostan, C.; Coskun, U.; Yildiz, A.; Ersanli, M. Relationship between myocardial performance index and severity of coronary artery disease in patients with non-ST-segment elevation acute coronary syndrome. Cardiovasc. J. Afr. 2017, 28, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Kim, H.M.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Alguire, C.; Armstrong, W.; Crawford, T.; Jongnarangsin, K.; Oral, H.; et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm. 2012, 9, 92–95. [Google Scholar] [CrossRef] [PubMed]
| Group 1 N: 54 | Group 2 N: 33 | Group 3 N: 35 | p Value | |
|---|---|---|---|---|
| Age (years) | 46.83 ± 12.6 | 46.94 ± 8.8 | 42.83 ± 14.8 | 0.280 |
| Male, n (%) | 20 (37.0%) | 16 (48.5%) | 17 (48.6%) | 0.445 |
| Hypertension, n (%) | 23 (42.6%) | 10 (30.3%) | 11 (31.4%) | 0.407 |
| Diabetes Mellitus, n (%) | 12 (22.2%) | 5 (15.2%) | 4 (11.4%) | 0.392 |
| Hyperlipidemia, n (%) | 13 (24.1%) | 4 (12.1%) | 8 (22.9%) | 0.374 |
| Previous CVA/TIA, n (%) | 2 (3.7%) | 0 | 1 (2.9%) | 0.548 |
| Current smoking, n (%) | 28 (51.9%) | 23 (69.7%) | 20 (57.1%) | 0.259 |
| Sudden death in family, n (%) | 10 (18.5%) | 5 (15.2%) | 12 (34.3%) | 0.114 |
| Drug Usage, n (%) | ||||
| B-Blockers | 35 (64.8%) | 23 (69.7%) | 30 (85.7%) | 0.093 |
| CCB | 9 (16.7%) | 5 (15.2%) | 11 (31.4%) | 0.163 |
| Class Ic AAD | 9 (16.7%) | 5 (15.2%) | 12 (34.3%) | 0.084 |
| Class III AAD | 4 (7.4%) | 2 (6.1%) | 4 (11.4%) | 0.694 |
| BMI | 25.4 ± 2.7 | 26.6 ± 3.5 | 24.7 ± 2.7 | 0.029 |
| Symptoms | ||||
| Asymptomatic, n (%) | 4 (7.4%) | 2 (6.3%) | 2 (5.7%) | 0.181 |
| Palpitations, n (%) | 39 (72.2%) | 26 (81.3%) | 20 (57.1%) | |
| Atypical symptoms, n (%) | 11 (20.4%) | 4 (12.5%) | 13 (37.1%) | |
| Symptoms duration, month | 15.57 ± 8.2 | 14.94 ± 6.8 | 18.54 ± 9.7 | 0.123 |
| CAG, n (%) | 12 (22.2%) | 7 (21.2%) | 13 (37.1%) | 0.220 |
| MPS, n (%) | 12 (22.2%) | 8 (24.2%) | 14 (40.0%) | 0.162 |
| Group 1 N: 54 | Group 2 N: 33 | Group 3 N: 35 | p Value | |
|---|---|---|---|---|
| PVC Count/day | 5890 ± 1064 | 10778 ± 2069 | 23689 ± 6452 | <0.001 |
| PVC Burden (%) * | 6.2% (5.1–8.9) | 11.3% (10–14.8) | 22.5% (16.1–39.8) | <0.001 |
| ECG-PVC, n (%) | 12 (22.2%) | 14 (42.4%) | 18 (51.4%) | 0.013 |
| QTc (msc) | 419.7 ±40.1 | 425.1 ± 32.8 | 422.4 ± 30.3 | 0.789 |
| BBB, n (%) | 10(18.5%) | 10(30.3%) | 10(28,6%) | 0.377 |
| Min. HR/min | 51.3 ± 14.6 | 49.5 ± 5.9 | 48.9 ± 6.6 | 0.490 |
| Max. HR/min | 128.7 ± 21.3 | 130.6 ± 15.1 | 132.2 ± 18.1 | 0.781 |
| Average HR/min | 74.6 ± 8.2 | 75.8 ± 9.5 | 76.3 ± 7.2 | 0.598 |
| Polymorphic PVC, n (%) | 5 (23.8%) | 2 (20.0%) | 1 (7.1%) | 0.440 |
| NSVT, n (%) | 8(14.8%) | 13(39.4%) | 15(42.9%) | 0.006 |
| LA, (mm) | 36.6 ± 4.6 | 37.4 ± 5.8 | 38.9 ± 4.8 | 0.098 |
| LVd, (mm) | 48.0 ± 3.3 | 50.5 ± 4.9 | 49.9 ± 7.7 | 0.066 |
| RVd, (mm) | 22.3 ± 1.9 | 23.5 ± 1.9 | 22.6 ± 2.1 | 0.044 |
| IVS, (mm) | 9.6 ± 1.2 | 9.7 ± 1.4 | 9.7 ± 1.1 | 0.874 |
| TAPSE, (cm) | 2.3 ± 0.3 | 2.2 ± 0.3 | 2.2 ± 0.2 | 0.737 |
| sPAP (mmHg) | 23.9 ± 4.4 | 23.8 ± 4.3 | 24.6 ± 4.3 | 0.625 |
| MPI Index | 0.50 ± 0.06 | 0.48 ± 0.08 | 0.47 ± 0.06 | 0.143 |
| LV-GLS % | −18.9 ± 1.4 | −17.5 ± 2.1 | −16.3 ± 1.3 | <0.001 |
| N | Correlation Coefficient (r) | p Value | |
|---|---|---|---|
| PVC (%) and LV-GLS | 122 | 0.536 * | <0.001 |
| PVC counts and LV-GLS | 122 | 0.555 * | <0.001 |
| PVC (%) and MPI | 122 | −0.219 ** | 0.015 |
| PVC counts and MPI | 122 | −0.220 ** | 0.015 |
| Symptom duration and LV-GLS | 122 | 0.195 ** | 0.032 |
| B | Std. ErrorB | Beta | t | Sig. (p) | Lower Bound (Min) | Upper Bound (Max) | VIF | |
|---|---|---|---|---|---|---|---|---|
| Predictors | ||||||||
| PVC (%) | 0.121 | 0.018 | 0.525 | 6.540 | <0.001 | 0.084 | 0.157 | 1.194 |
| Hb | −0.050 | 0.103 | −0.038 | −0.486 | 0.628 | −0.253 | 0.153 | 1.149 |
| ECG QTc | 0.005 | 0.004 | 0.091 | 1.204 | 0.231 | −0.003 | 0.013 | 1.052 |
| LA | 0.058 | 0.043 | 0.152 | 1.357 | 0.177 | −0.027 | 0.143 | 2.314 |
| LVd | 0.012 | 0.036 | 0.033 | 0.737 | 0.737 | −0.060 | 0.084 | 1.744 |
| MPI | −0.539 | 2.273 | −0.019 | −0.237 | 0.813 | −5.044 | 3.965 | 1.137 |
| BMI | 0.011 | 0.062 | 0.016 | 0.171 | 0.864 | −0.113 | 0.134 | 1.651 |
| AGE | 0.021 | 0.015 | 0.133 | 1.371 | 0.173 | −0.009 | 0.051 | 1.747 |
| TAPSE | −0.511 | 0.546 | −0.073 | −0.936 | 0.351 | −1.592 | 0.571 | 1.122 |
| RVd | 0.067 | 0.081 | 0.070 | 0.835 | 0.406 | −0.092 | 0.227 | 1.289 |
| R2 = 0.400 Adjusted R2 = 0.346 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukru, A.; Ozan, A.H.; Furkan, D.M.; Emin, G.M.; Seyma, A.; Veysel, O.; Mustafa, Y.; Isıl, U. Effects of Premature Ventricular Complex Burden on Left Ventricular Global Longitudinal Strain in Patients without Structural Heart Disease. J. Clin. Med. 2024, 13, 1796. https://doi.org/10.3390/jcm13061796
Sukru A, Ozan AH, Furkan DM, Emin GM, Seyma A, Veysel O, Mustafa Y, Isıl U. Effects of Premature Ventricular Complex Burden on Left Ventricular Global Longitudinal Strain in Patients without Structural Heart Disease. Journal of Clinical Medicine. 2024; 13(6):1796. https://doi.org/10.3390/jcm13061796
Chicago/Turabian StyleSukru, Arslan, Arabaci H. Ozan, Deniz M. Furkan, Gokce M. Emin, Arslan Seyma, Oktay Veysel, Yıldız Mustafa, and Uzunhasan Isıl. 2024. "Effects of Premature Ventricular Complex Burden on Left Ventricular Global Longitudinal Strain in Patients without Structural Heart Disease" Journal of Clinical Medicine 13, no. 6: 1796. https://doi.org/10.3390/jcm13061796
APA StyleSukru, A., Ozan, A. H., Furkan, D. M., Emin, G. M., Seyma, A., Veysel, O., Mustafa, Y., & Isıl, U. (2024). Effects of Premature Ventricular Complex Burden on Left Ventricular Global Longitudinal Strain in Patients without Structural Heart Disease. Journal of Clinical Medicine, 13(6), 1796. https://doi.org/10.3390/jcm13061796







