Cardiac Wolframinopathies: A Case Report of Myocarditis and a Literature Review of Cardiac Involvement in Wolfram Syndrome 1
Abstract
1. Introduction
2. Methods
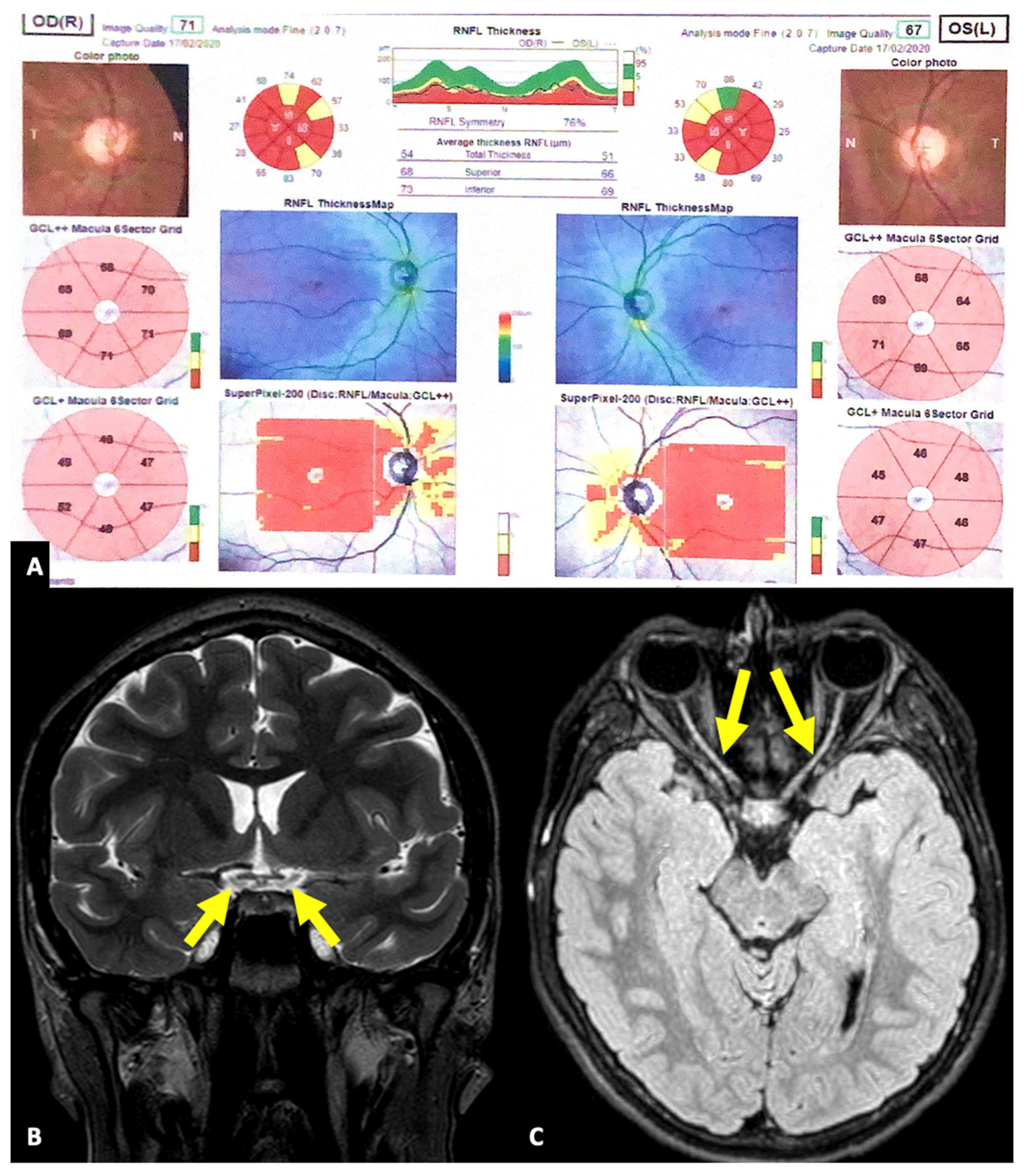
3. Results
4. Discussion
4.1. Wolfram Syndrome
4.2. Cardiovascular Involvement
4.3. Metabolic Alterations
4.4. Inflammation
4.5. Therapeutic Opportunities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peretto, G.; De Luca, G.; Villatore, A.; Di Resta, C.; Sala, S.; Palmisano, A.; Vignale, D.; Campochiaro, C.; Lazzeroni, D.; De Gaspari, M.; et al. Multimodal Detection and Targeting of Biopsy-Proven Myocardial Inflammation in Genetic Cardiomyopathies: A Pilot Report. JACC Basic. Transl. Sci. 2023, 8, 755–765. [Google Scholar] [CrossRef]
- Barrett, T.; Tranebjærg, L.; Gupta, R.; McCarthy, L.; Rendtorff, N.D.; Williams, D.; Wright, B.; Dias, R. WFS1 Spectrum Disorder. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK4144/ (accessed on 25 January 2024).
- Frontino, G.; Raouf, T.; Canarutto, D.; Tirelli, E.; Di Tonno, R.; Rigamonti, A.; Cascavilla, M.L.; Baldoli, C.; Scotti, R.; Leocani, L.; et al. Case Report: Off-Label Liraglutide Use in Children with Wolfram Syndrome Type 1: Extensive Characterization of Four Patients. Front. Pediatr. 2021, 9, 755365. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648, 2648a–2648d. [Google Scholar] [CrossRef]
- Rigoli, L.; Bramanti, P.; Di Bella, C.; De Luca, F. Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatr. Res. 2018, 83, 921–929. [Google Scholar] [CrossRef]
- Chaussenot, A.; Rouzier, C.; Quere, M.; Plutino, M.; Ait-El-Mkadem, S.; Bannwarth, S.; Barth, M.; Dollfus, H.; Charles, P.; Nicolino, M.; et al. Mutation update and uncommon phenotypes in a French cohort of 96 patients with WFS1-related disorders. Clin. Genet. 2015, 87, 430–439. [Google Scholar] [CrossRef]
- Yamada, T.; Ishihara, H.; Tamura, A.; Takahashi, R.; Yamaguchi, S.; Takei, D.; Tokita, A.; Satake, C.; Tashiro, F.; Katagiri, H.; et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum. Mol. Genet. 2006, 15, 1600–1609. [Google Scholar] [CrossRef]
- Bekir, N.A.; Güngör, K.; Güran, S. A DIDMOAD syndrome family with juvenile glaucoma and myopia findings. Acta Ophthalmol. Scand. 2000, 78, 480–482. [Google Scholar] [CrossRef]
- Salzano, G.; Rigoli, L.; Valenzise, M.; Chimenz, R.; Passanisi, S.; Lombardo, F. Clinical Peculiarities in a Cohort of Patients with Wolfram Syndrome 1. Int. J. Environ. Res. Public Health 2022, 19, 520. [Google Scholar] [CrossRef]
- Png, D.; Yeoh, E.; Tan, C.; Lim, S.C. A Pair of Siblings With Wolfram Syndrome: A Review of the Literature and Treatment Options. J. Investig. Med. High. Impact Case Rep. 2023, 11, 23247096221150631. [Google Scholar] [CrossRef] [PubMed]
- Medlej, R.; Wasson, J.; Baz, P.; Azar, S.; Salti, I.; Loiselet, J.; Permutt, A.; Halaby, G. Diabetes mellitus and optic atrophy: A study of Wolfram syndrome in the Lebanese population. J. Clin. Endocrinol. Metab. 2004, 89, 1656–1661. [Google Scholar] [CrossRef]
- Kinsley, B.T.; Swift, M.; Dumont, R.H.; Swift, R.G. Morbidity and mortality in the Wolfram syndrome. Diabetes Care 1995, 18, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Aloi, C.; Salina, A.; Pasquali, L.; Lugani, F.; Perri, K.; Russo, C.; Tallone, R.; Ghiggeri, G.M.; Lorini, R.; d’Annunzio, G. Wolfram syndrome: New mutations, different phenotype. PLoS ONE 2012, 7, e29150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, Y.; Xu, K.; Chang, H.; Zhang, X.; Li, Y. Comprehensive Genetic Analysis Unraveled the Missing Heritability in a Chinese Cohort with Wolfram Syndrome 1: Clinical and Genetic Findings. Investig. Ophthalmol. Vis. Sci. 2022, 63, 9. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, H.A.; Demir, K.; Hazan, F.; Yıldız, M.; Elmas, Ö.N.; Özkan, B. Association of Wolfram syndrome with Fallot tetralogy in a girl. Arch. Argent. Pediatr. 2016, 114, e163-6, (In English and Spanish). [Google Scholar] [CrossRef]
- Esteban Bueno, G.; Gómez Trujillo, F.M. Manifestaciones clínicas y retraso diagnóstico en el síndrome de Wolfram [Clinical manifestations and diagnostic delay in Wolfram’s syndrome]. Rev. Clin. Esp. 2006, 206, 332–335. (In Spanish) [Google Scholar] [CrossRef]
- Ganie, M.A.; Laway, B.A.; Nisar, S.; Wani, M.M.; Khurana, M.L.; Ahmad, F.; Ahmed, S.; Gupta, P.; Ali, I.; Shabir, I.; et al. Presentation and clinical course of Wolfram (DIDMOAD) syndrome from North India. Diabet. Med. 2011, 28, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Raza, S.I.; Anwar, M.Z.; Bharadwaj, T.; Liaqat, K.; Khokhar, M.A.S.; Everard, J.L.; Nasir, A.; University of Washington Center for Mendelian Genomics; Nickerson, D.A.; et al. Wolfram-like syndrome with bicuspid aortic valve due to a homozygous missense variant in CDK13. J. Hum. Genet. 2021, 66, 1009–1018. [Google Scholar] [CrossRef]
- Siani, A.; Proaspatu, M.; Lanzo, G.; Irico, P.; Rodolfi, S.; Carriero, S.; Guglielmo, M. Petrified heart in a patient with Wolfram Syndrome. J. Public Health Res. 2022, 11, 22799036221107063. [Google Scholar] [CrossRef]
- Eimre, M.; Paju, K.; Peet, N.; Kadaja, L.; Tarrend, M.; Kasvandik, S.; Seppet, J.; Ivask, M.; Orlova, E.; Kõks, S. Increased Mitochondrial Protein Levels and Bioenergetics in the Musculus Rectus Femoris of Wfs1-Deficient Mice. Oxid. Med. Cell Longev. 2018, 2018, 3175313. [Google Scholar] [CrossRef]
- Porosk, R.; Terasmaa, A.; Mahlapuu, R.; Soomets, U.; Kilk, K. Metabolomics of the Wolfram Syndrome 1 Gene (Wfs1) Deficient Mice. OMICS 2017, 21, 721–732. [Google Scholar] [CrossRef]
- Porosk, R.; Kilk, K.; Mahlapuu, R.; Terasmaa, A.; Soomets, U. Glutathione system in Wolfram syndrome 1-deficient mice. Mol. Med. Rep. 2017, 16, 7092–7097. [Google Scholar] [CrossRef]
- Tepp, K.; Aid-Vanakova, J.; Puurand, M.; Timohhina, N.; Reinsalu, L.; Tein, K.; Plaas, M.; Shevchuk, I.; Terasmaa, A.; Kaambre, T. Wolframin deficiency is accompanied with metabolic inflexibility in rat striated muscles. Biochem. Biophys. Rep. 2022, 30, 101250. [Google Scholar] [CrossRef]
- Kõks, S.; Soomets, U.; Paya-Cano, J.L.; Fernandes, C.; Luuk, H.; Plaas, M.; Terasmaa, A.; Tillmann, V.; Noormets, K.; Vasar, E.; et al. Wfs1 gene deletion causes growth retardation in mice and interferes with the growth hormone pathway. Physiol. Genom. 2009, 37, 249–259. [Google Scholar] [CrossRef]
- Plaas, M.; Seppa, K.; Reimets, R.; Jagomäe, T.; Toots, M.; Koppel, T.; Vallisoo, T.; Nigul, M.; Heinla, I.; Meier, R.; et al. Wfs1- deficient rats develop primary symptoms of Wolfram syndrome: Insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci. Rep. 2017, 7, 10220. [Google Scholar] [CrossRef]
- Cagalinec, M.; Zahradníková, A.; Zahradníková, A., Jr.; Kováčová, D.; Paulis, L.; Kureková, S.; Hot’ka, M.; Pavelková, J.; Plaas, M.; Novotová, M.; et al. Calcium Signaling and Contractility in Cardiac Myocyte of Wolframin Deficient Rats. Front. Physiol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Kureková, S.; Plaas, M.; Cagalinec, M. Lack of functional wolframin causes drop in plasmalemmal sodium-calcium exchanger type 1 expression at early stage in rat model of Wolfram syndrome. Gen. Physiol. Biophys. 2020, 39, 499–503. [Google Scholar] [CrossRef]
- Rusciano, M.R.; Sommariva, E.; Douin-Echinard, V.; Ciccarelli, M.; Poggio, P.; Maione, A.S. CaMKII Activity in the Inflammatory Response of Cardiac Diseases. Int. J. Mol. Sci. 2019, 20, 4374. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sommariva, E.; Di Resta, C.; Rabino, M.; Villatore, A.; Lazzeroni, D.; Sala, S.; Pompilio, G.; Cooper, L.T. Myocardial Inflammation as a Manifestation of Genetic Cardiomyopathies: From Bedside to the Bench. Biomolecules 2023, 13, 646. [Google Scholar] [CrossRef]
- Abreu, D.; Stone, S.I.; Pearson, T.S.; Bucelli, R.C.; Simpson, A.N.; Hurst, S.; Brown, C.M.; Kries, K.; Onwumere, C.; Gu, H.; et al. A phase Ib/IIa clinical trial of dantrolene sodium in patients with Wolfram syndrome. JCI Insight 2021, 6, e145188. [Google Scholar] [CrossRef] [PubMed]
- Panfili, E.; Mondanelli, G.; Orabona, C.; Belladonna, M.L.; Gargaro, M.; Fallarino, F.; Orecchini, E.; Prontera, P.; Proietti, E.; Frontino, G.; et al. Novel mutations in the WFS1 gene are associated with Wolfram syndrome and systemic inflammation. Hum. Mol. Genet. 2021, 30, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Wang, S.; Zhu, H.; Ye, P.; Xie, A.; Shen, B.; Liu, C.; Guo, C.; Fu, Q.; et al. The Treg/Th17 imbalance in patients with idiopathic dilated cardiomyopathy. Scand. J. Immunol. 2010, 71, 298–303. [Google Scholar] [CrossRef]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef]
- Yan, L.; Hu, F.; Yan, X.; Wei, Y.; Ma, W.; Wang, Y.; Lu, S.; Wang, Z. Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J. Mol. Med. 2016, 94, 1063–1079. [Google Scholar] [CrossRef]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Tresoldi, M.; Dagna, L. Myocarditis: An Interleukin-1-Mediated Disease? Front. Immunol. 2018, 9, 1335. [Google Scholar] [CrossRef]
- Abreu, D.; Urano, F. Current Landscape of Treatments for Wolfram Syndrome. Trends Pharmacol. Sci. 2019, 40, 711–714. [Google Scholar] [CrossRef]
- Gorgogietas, V.; Rajaei, B.; Heeyoung, C.; Santacreu, B.J.; Marín-Cañas, S.; Salpea, P.; Sawatani, T.; Musuaya, A.; Arroyo, M.N.; Moreno-Castro, C.; et al. GLP-1R agonists demonstrate potential to treat Wolfram syndrome in human preclinical models. Diabetologia 2023, 66, 1306–1321. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Yariwake, V.Y.; Alves, R.W.; de Araujo, D.R.; Andrade-Oliveira, V. Crosstalk between incretin hormones, Th17 and Treg cells in inflammatory diseases. Peptides 2022, 155, 170834. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Zempo, H.; Ogawa, M.; Watanabe, R.; Suzuki, J.; Akazawa, H.; Komuro, I.; Isobe, M. A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS ONE 2015, 10, e0119360. [Google Scholar] [CrossRef]
- Evans, M.; Hicks, D.; Patel, D.; Patel, V.; McEwan, P.; Dashora, U. Optimising the Benefits of SGLT2 Inhibitors for Type 1 Diabetes. Diabetes Ther. 2020, 11, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Talha, K.M.; Anker, S.D.; Butler, J. SGLT-2 Inhibitors in Heart Failure: A Review of Current Evidence. Int. J. Heart Fail. 2023, 5, 82–90. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 inhibitors: Role in protective reprogramming of cardiac nutrient transport and metabolism. Nat. Rev. Cardiol. 2023, 20, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Kanekura, K.; Hara, T.; Mahadevan, J.; Spears, L.D.; Oslowski, C.M.; Martinez, R.; Yamazaki-Inoue, M.; Toyoda, M.; Neilson, A.; et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, E5292–E5301. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, C.; Ishigaki, S.; Oslowski, C.M.; Fonseca, S.G.; Kato, T.; Urano, F. Valproate, a mood stabilizer, induces WFS1 expression and modulates its interaction with ER stress protein GRP94. PLoS ONE 2009, 4, e4134. [Google Scholar] [CrossRef]
- Shang, L.; Hua, H.; Foo, K.; Martinez, H.; Watanabe, K.; Zimmer, M.; Kahler, D.J.; Freeby, M.; Chung, W.; LeDuc, C.; et al. β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes 2014, 63, 923–933. [Google Scholar] [CrossRef]
- Urano, F. Wolfram syndrome iPS cells: The first human cell model of endoplasmic reticulum disease. Diabetes 2014, 63, 844–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindahl, M.; Danilova, T.; Palm, E.; Lindholm, P.; Võikar, V.; Hakonen, E.; Ustinov, J.; Andressoo, J.O.; Harvey, B.K.; Otonkoski, T.; et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014, 7, 366–375. [Google Scholar] [CrossRef]


| Study | Number of Patients (% of Cohort) | CV (No. of Patients) | References |
|---|---|---|---|
| Bekir et al. | 2 (20%) | VSD (2) | [8] |
| Salzano et al. | 2 (14.3%) | VSD (1) and ASD (1) | [9] |
| Png et al. | 1 (50%) | ASD (1) | [10] |
| Medlej et al. | 6 (16.1%) | VSD (1) and PVS (5), plus cardiac autonomic dysfunction | [11] |
| Kinsley et al. | 3 (4.4%) | ToF (2) and PVS (1), plus sinus tachycardia, SVA, and VA | [12] |
| Aloi et al. | 1 (11.1%) | ToF (1) | [13] |
| Zhang et al. | 1 (4.2%) | ToF (1) | [14] |
| Korkmaz et al. | 1 (100%) | ToF (1) | [15] |
| Ganie et al. | 1 (14%) | Cyanotic CHD (1) | [17] |
| Siani et al. | 1 (100%) | Sepsis-induced myocardial calcification (1) | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villatore, A.; Frontino, G.; Cascavilla, M.L.; Vignale, D.; Lazzeroni, D.; Peretto, G. Cardiac Wolframinopathies: A Case Report of Myocarditis and a Literature Review of Cardiac Involvement in Wolfram Syndrome 1. J. Clin. Med. 2024, 13, 1803. https://doi.org/10.3390/jcm13061803
Villatore A, Frontino G, Cascavilla ML, Vignale D, Lazzeroni D, Peretto G. Cardiac Wolframinopathies: A Case Report of Myocarditis and a Literature Review of Cardiac Involvement in Wolfram Syndrome 1. Journal of Clinical Medicine. 2024; 13(6):1803. https://doi.org/10.3390/jcm13061803
Chicago/Turabian StyleVillatore, Andrea, Giulio Frontino, Maria Lucia Cascavilla, Davide Vignale, Davide Lazzeroni, and Giovanni Peretto. 2024. "Cardiac Wolframinopathies: A Case Report of Myocarditis and a Literature Review of Cardiac Involvement in Wolfram Syndrome 1" Journal of Clinical Medicine 13, no. 6: 1803. https://doi.org/10.3390/jcm13061803
APA StyleVillatore, A., Frontino, G., Cascavilla, M. L., Vignale, D., Lazzeroni, D., & Peretto, G. (2024). Cardiac Wolframinopathies: A Case Report of Myocarditis and a Literature Review of Cardiac Involvement in Wolfram Syndrome 1. Journal of Clinical Medicine, 13(6), 1803. https://doi.org/10.3390/jcm13061803










