The Possible Effect of β-Blocker Use on the Circulating MMP-2/TIMP-2 System in Patients with Chronic Kidney Disease on Conservative Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
3.1. The Characteristics of Patients with CKD Treated and Not Treated with β-Blockers
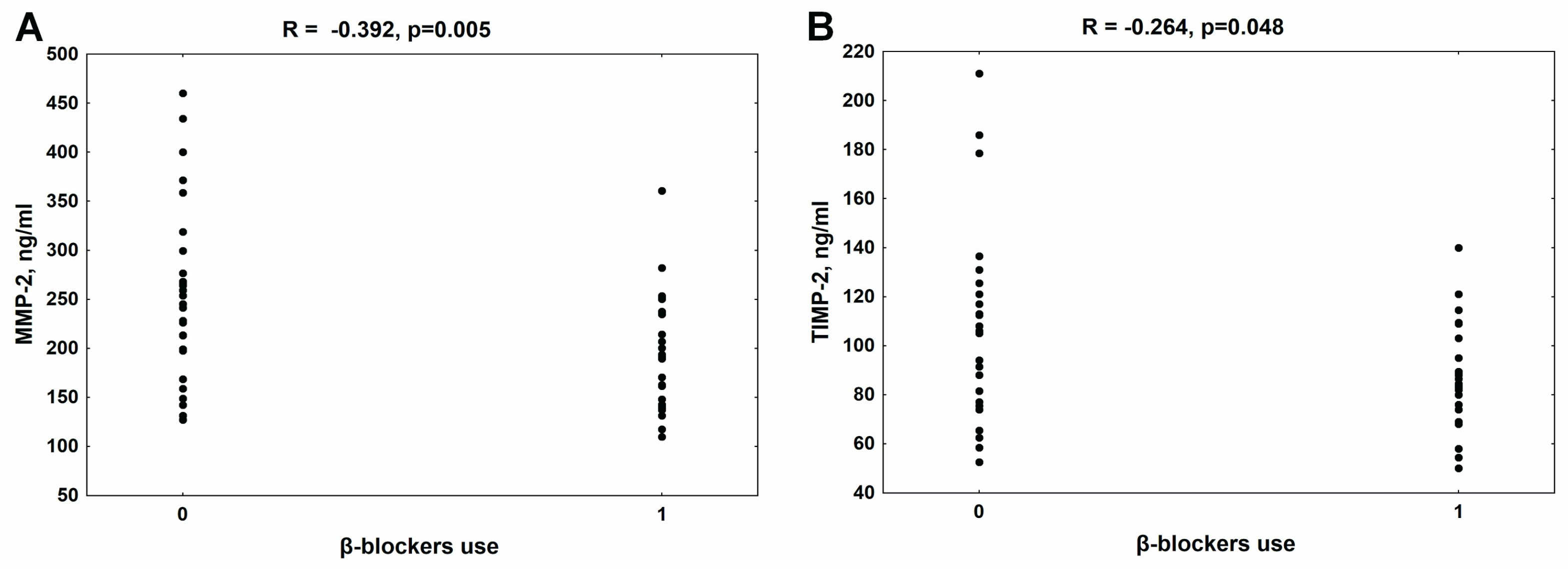
3.2. The Relationship between the Use of β-Blockers and MMP-2 and TIMP-2 Concentrations in the Whole Group of Patients with CKD
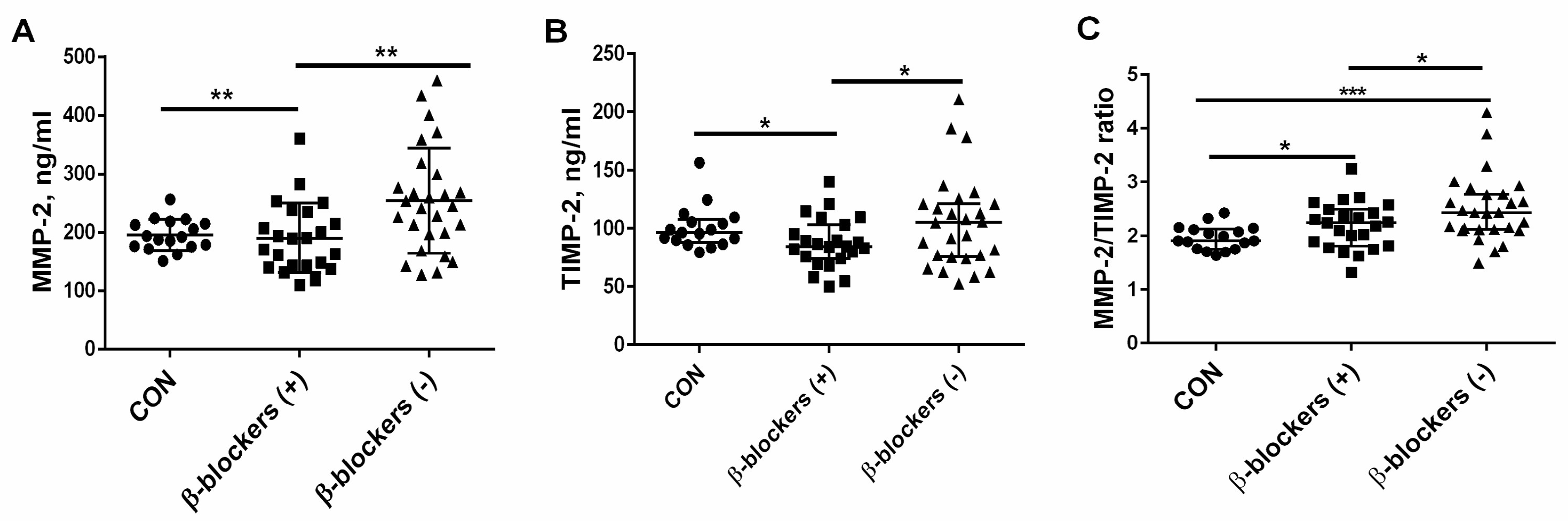
3.3. The Effect of β-Blocker Treatment on MMP-2 and TIMP-2 Levels and the MMP-2/TIMP-2 Ratio in Patients with CKD
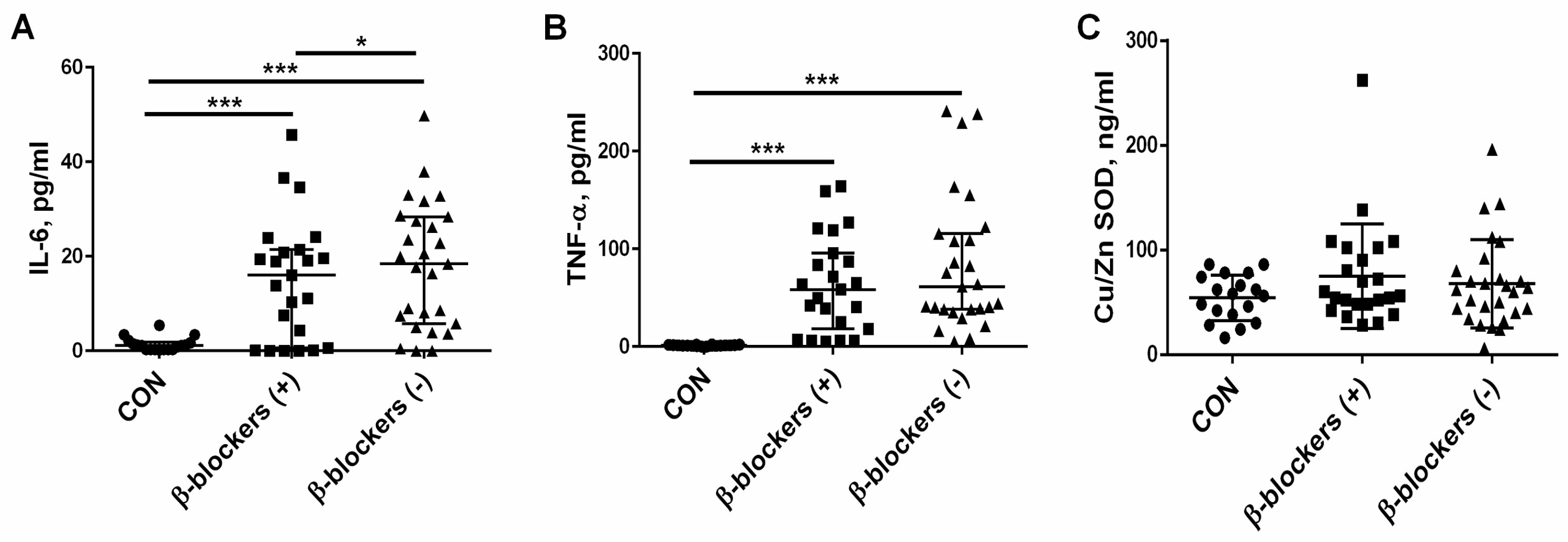
3.4. The Effect of β-Blocker Treatment on Proinflammatory Cytokines and the Marker of Oxidative Stress—Cu/Zn SOD— Levels
3.5. The Factors Affecting the MMP-2/TIMP-2 System in the β-Blockers (+) Group and β-Blockers (−) Group
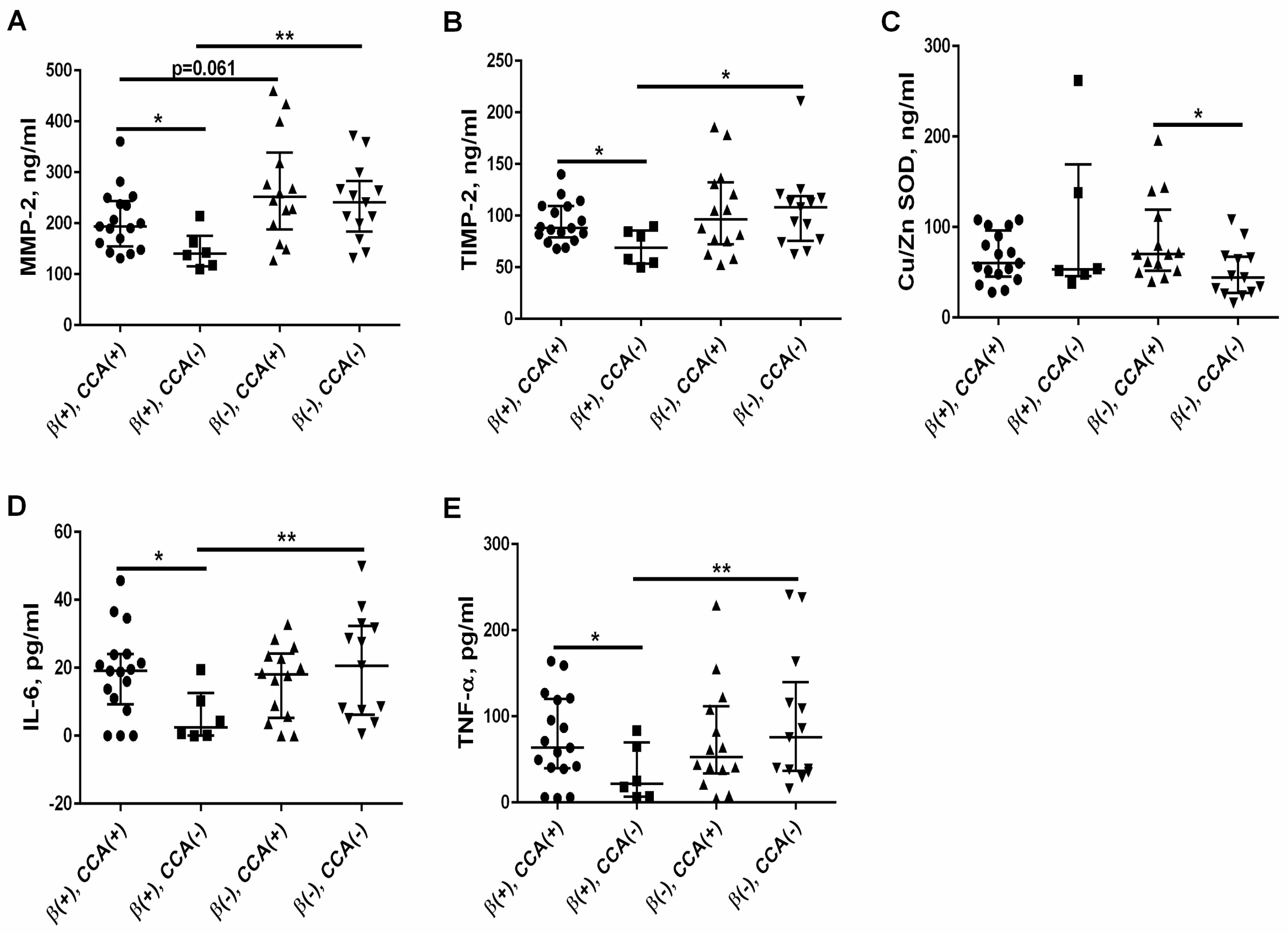
3.6. The Effect of Calcium Channel Antagonists (CCA) on the MMP-2/TIMP-2 System, Oxidative Status, and Proinflammatory Cytokines in the β-Blockers (+) Group and β-Blockers (−) Group
3.7. Variables Independently Predicting the MMP-2/TIMP-2 System in the β-Blockers Group (+) and β-Blockers (−) Group
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Nagaraju, S.P.; Bhojaraja, M.V.; Swaminathan, S.M.; Mohan, P.B. Hypertension in Chronic Kidney Disease: An Update on Diagnosis and Management. South. Med. J. 2023, 116, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management on Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Agarwal, R. Hypertension in chronic kidney disease-treatment standard 2023. Nephrol. Dial. Transplant. 2023, 38, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Converse, R.L., Jr.; Jacobsen, T.N.; Toto, R.D.; Jost, C.M.; Cosentino, F.; Fouad-Tarazi, F.; Victor, R.G. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 1992, 327, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Shina, A.D.; Agarwal, R. Clinical Pharmacology of Antihypertensive Therapy for the Treatment of Hypertension in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 757–764. [Google Scholar]
- AlHabeeb, W.; Mrabeti, S.; Abdelsalam, A.A.I. Therapeutic Properties of Highly Selective β-blockers With or Without Additional Vasodilator Properties: Focus on Bisoprolol and Nebivolol in Patients With Cardiovascular Disease. Cardiovasc. Drugs Ther. 2022, 36, 959–971. [Google Scholar] [CrossRef]
- Mookerjee, R.P.; Pavesi, M.; Thomsen, K.L.; Mehta, G.; Macnaughtan, J.; Bendtsen, F.; Coenraad, M.; Sperl, J.; Gines, P.; Moreau, R.; et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on chronic liver failure. J. Hepatol. 2016, 64, 574–582. [Google Scholar] [CrossRef]
- Le, D.E.; Pascotto, M.; Leong-Poi, H.; Sari, I.; Micari, A.; Kaul, S. Antiinflammatory and pro-angiogenic effects of beta blockers in a canine model of chronic ischemic cardiomyopathy: Comparison between carvedilol and metoprolol. Basic. Res. Cardiol. 2013, 108, 384. [Google Scholar] [CrossRef]
- Lin, T.T.; Sung, Y.L.; Syu, J.Y.; Lin, K.Y.; Hsu, H.J.; Liao, M.T.; Liu, Y.B.; Lin, S.F. Anti-Inflammatory and Antiarrhythmic Effects of Beta Blocker in a Rat Model of Rheumatoid Arthritis. J. Am. Heart Assoc. 2020, 9, e016084. [Google Scholar] [CrossRef]
- Serg, M.; Kampus, P.; Kals, J.; Zagura, M.; Zilmer, M.; Zilmer, K.; Kullisaar, T.; Eha, J. Nebivolol and metoprolol: Long-term effects on inflammation and oxidative stress in essential hypertension. Scand. J. Clin. Lab. Investig. 2012, 72, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kukin, M.L.; Kalman, J.; Charney, R.H.; Levy, D.K.; Buchholz-Varley, C.; Ocampo, O.N.; Eng, C. Prospective, randomized comparison of effect of long-term treatment with metoprolol or carvedilol on symptoms, exercise, ejection fraction, and oxidative stress in heart failure. Circulation 1999, 99, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, E.; Guimaraes, D.A.; Ceron, C.S.; Prado, C.M.; Pinheiro, L.C.; Martins-Oliveira, A.; Gerlach, R.F.; Tanus-Santos, J.E. β1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophy. Free Radic. Biol. Med. 2014, 73, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Chen, Y.H.; Leu, H.B.; Chen, Y.L.; Lin, F.Y.; Lin, S.J.; Chen, J.W. Carvedilol, a pharmacological antioxidant, inhibits neointimal matrix metalloproteinase-2 and -9 in experimental atherosclerosis. Free Radic. Biol. Med. 2007, 43, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Skrzypiec-Spring, M.; Haczkiewicz, K.; Sapa, A.; Piasecki, T.; Kwiatkowska, J.; Ceremuga, I.; Wozniak, M.; Biczysko, W.; Kobierzycki, C.; Dziegiel, P.; et al. Carvedilol Inhibits Matrix Metalloproteinase-2 Activation in Experimental Autoimmune Myocarditis: Possibilities of Cardioprotective Application. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 89–97. [Google Scholar] [CrossRef]
- Ersan, S.; Tanrısev, M.; Cavdar, Z.; Celık, A.; Unlu, M.; Kocak, A.; Kose, T. Pretreatment with nebivolol attenuates level and expression of matrix metalloproteinases in a rat model of renal ischaemia-reperfusion injury. Nephrology 2017, 22, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.; Hardy-Sosa, A.; Fernandez-Patron, C. MMP-2: Is too low as bad as too high in the cardiovascular system? Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1332–H1340. [Google Scholar] [CrossRef]
- Strongin, A.Y.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J. Biol. Chem. 1993, 268, 14033–14039. [Google Scholar] [CrossRef]
- Kandalam, V.; Basu, R.; Moore, L.; Fan, D.; Wang, X.; Jaworski, D.M.; Oudit, G.Y.; Kassiri, Z. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation 2011, 124, 2094–2105. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Peripheral blood level alterations of MMP-2 and MMP-9 in patients with chronic kidney disease on conservative treatment and on hemodialysis. Clin. Biochem. 2011, 44, 838–843. [Google Scholar] [CrossRef]
- Pawlak, K.; Pawlak, D.; Mysliwiec, M. Extrinsic coagulation pathway activation and metalloproteinase-2/TIMPs system are related to oxidative stress and atherosclerosis in hemodialysis patients. Thromb. Haemost. 2004, 92, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Pawlak, D.; Mysliwiec, M. Urokinase-type plasminogen activator and metalloproteinase-2 are independently related to the carotid atherosclerosis in haemodialysis patients. Thromb. Res. 2008, 121, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Tankiewicz, J.; Mysliwiec, M.; Pawlak, D. Systemic levels of MMP2/TIMP2 and cardiovascular risk in CAPD patients. Nephron. Clin. Pract. 2010, 115, c251–c258. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Pappas, M.K.; Abraham, T.N.; Tegegne, G.G. Hypertension in hemodialysis patients treated with atenolol or lisinopril: A randomized controlled trial. Nephrol. Dial. Transplant. 2014, 29, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Pilmore, H.L.; Ierino, F.L.; Badve, S.V.; Cass, A.; Garg, A.X.; Isbel, N.M.; Krum, H.; Pascoe, E.M.; Perkovic, V.; et al. The beta-blocker to lower cardiovascular dialysis events (BLOCADE) Feasibility study: A randomized controlled trial. Am. J. Kidney Dis. 2016, 67, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Guo, X.; Yu, Q. Efects of beta-blockers on cardiovascular events and mortality in dialysis patients: A systematic review and meta-analysis. Blood Purif. 2019, 48, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sim, J.J.; Shi, J.; Shaw, S.F.; Lee, M.-S.; Neyer, J.R.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Jacobsen, S.J. β-Blocker use and risk of mortality in heart failure patients initiating maintenance dialysis. Am. J. Kidney Dis. 2021, 77, 704–712. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, L.; Yang, L.; Lu, H.; Cao, S.; Song, H.; Fu, S. β-Blockers could improve the 28-day and 3-year survival of patients with end-stage renal disease: A retrospective cohort study. Int. Urol. Nephrol. 2023, 55, 1597–1607. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Pawlak, K.; Pawlak, D.; Mysliwiec, M. Cu/Zn superoxide dismutase plasma levels as a new useful clinical biomarker of oxidative stress in patients with end-stage renal disease. Clin. Biochem. 2005, 38, 700–705. [Google Scholar] [CrossRef]
- Washio, K.; Inagaki, M.; Tsuji, M.; Morio, Y.; Akiyama, S.; Gotoh, H.; Gotoh, T.; Gotoh, Y.; Oguchi, K. Oral vitamin C supplementation in hemodialysis patients and its effect on the plasma level of oxidized ascorbic acid and Cu/Zn superoxide dismutase, an oxidative stress marker. Nephron Clin. Pract. 2008, 109, c49–c54. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.M.; Pansani, T.N.; Hebling, J.; de Souza Costa, C.A.; Basso, F.G. Chemotherapy drugs and inflammatory cytokines enhance matrix metalloproteinases expression by oral mucosa cells. Arch. Oral Biol. 2021, 127, 105159. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Taruya, A.; Ozaki, M.; Tanaka, A.; Mukaida, N.; Kondo, T. Crucial Involvement of IL-6 in Thrombus Resolution in Mice via Macrophage Recruitment and the Induction of Proteolytic Enzymes. Front. Immunol. 2020, 10, 3150. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.J. Collagenases and tissue inhibitors of metalloproteinases: A functional balance in tissue degradation. Oral Dis. 1996, 2, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kobusiak-Prokopowicz, M.; Krzysztofik, J.; Kaaz, K.; Jolda-Mydlowska, B.; Mysiak, A. MMP-2 and TIMP-2 in patients with heart failure and chronic kidney disease. Open Med. 2018, 13, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Squire, I.B.; Evans, J.; Ng, L.L.; Loftus, I.M.; Thompson, M.M. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: Correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J. Card. Fail. 2004, 10, 228–233. [Google Scholar] [CrossRef]
- Hsu, T.W.; Kuo, K.L.; Hung, S.C.; Huang, P.H.; Chen, J.W.; Tarng, D.C. Progression of kidney disease in non-diabetic patients with coronary artery disease: Predictive role of circulating matrix metalloproteinase-2,-3, and -9. PLoS ONE 2013, 8, e70132. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Fukami, K.; Yamagishi, S.; Ueda, S.; Kaida, Y.; Matsumoto, T.; Yoshimura, J.; Hazama, T.; Takamiya, Y.; Kusumoto, T.; et al. Circulating matrix metalloproteinase-2 is an independent correlate of proteinuria in patients with chronic kidney disease. Am. J. Nephrol. 2009, 29, 109–115. [Google Scholar] [CrossRef]
- Borden, P.; Heller, R.A. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit. Rev. Eukaryot. Gene Expr. 1997, 7, 159–178. [Google Scholar] [CrossRef]
- Valentin, F.; Bueb, J.L.; Kieffer, A.P.; Tschirhart, B.E.; Atkinson, A.J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam. Clin. Pharmacol. 2005, 19, 661–667. [Google Scholar] [CrossRef]
- Tan, S.; Zhou, F.; Zhang, Z.; Wang, J.; Xu, J.; Zhuang, Q.; Meng, Q.; Xi, Q.; Jiang, Y.; Wu, G. Beta-1 blocker reduces inflammation and preserves intestinal barrier function after open abdominal surgery. Surgery 2021, 169, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Ackland, G.L.; Yao, S.T.; Rudiger, A.; Dyson, A.; Stidwill, R.; Poputnikov, D.; Singer, M.; Gourine, A.V. Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit. Care Med. 2010, 38, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and specificity: Insights from the interleukin 6 family of cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, G.S.; Nagoor Meeran, M.F.; Selvaraj, P. Activation of β1-adrenoceptor triggers oxidative stress mediated myocardial membrane destabilization in isoproterenol induced myocardial infarcted rats: 7-hydroxycoumarin and its counter action. Eur. J. Pharmacol. 2016, 777, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Seeland, U.; Selejan, S.; Engelhardt, S.; Muller, P.; Lohse, M.J.; Bohm, M. Interstitial remodelingin β1-adrenergic receptor transgenic mice. Basic Res. Cardiol. 2007, 102, 183–193. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Doerries, C.; Manes, C.; Speer, T.; Dessy, C.; Lobysheva, I.; Mohmand, W.; Akbar, R.; Bahlmann, F.; Besler, C.; et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J. Am. Coll. Cardiol. 2011, 57, 601–611. [Google Scholar] [CrossRef]
- Fang, Y.; Nicol, L.; Harouki, N.; Monteil, C.; Wecker, D.; Debunne, M.; Bauer, F.; Lallemand, F.; Richard, V.; Thuillez, C.; et al. Improvement of left ventricular diastolic function induced by beta-blockade: A comparison between nebivolol and metoprolol. J. Mol. Cell. Cardiol. 2011, 51, 168–176. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Barany, P. Anaemia, rHuEPO resistance, and cardiovascular disease in end-stage renal failure: Links to inflammation and oxidative stress. Nephrol. Dial. Transplant. 2002, 17, 32–37. [Google Scholar] [CrossRef]
- Kaysen, G.A. The microinflammatory state in uremia: Causes and potential consequences. J. Am. Soc. Nephrol. 2001, 12, 1549–1557. [Google Scholar] [CrossRef]
- Rysz, J.; Banach, M.; Stolarek, R.A.; Pasnik, J.; Cialkowska-Rysz, A.; Koktysz, R.; Piechota, M.; Baj, Z. Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J. Nephrol. 2007, 20, 444–452. [Google Scholar]
- Kim, S.S.; Shin, N.; Bae, S.S.; Lee, M.Y.; Rhee, H.; Kim, I.Y.; Seong, E.Y.; Lee, D.W.; Lee, S.B.; Kwak, I.S.; et al. Enhanced expression of two discrete isoforms of matrix metalloproteinase-2 in experimental and human diabetic nephropathy. PLoS ONE 2017, 12, e0171625. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Nakabayashi, K.; Sekiuchi, M.; Kuroda, T.; Soejima, A.; Yamada, A. Matrix metalloproteinase-2, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in the peripheral blood of patients with various glomerular diseases and their implication in pathogenetic lesions: Study based on an enzyme-linked assay and immunohistochemical staining. Clin. Exp. Nephrol. 2006, 10, 253–261. [Google Scholar] [PubMed]
- Marçal, D.M.; Rizzi, E.; Martins-Oliveira, A.; Ceron, C.S.; Guimaraes, D.A.; Gerlach, R.F.; Tanus-Santos, J.E. Comparative study on antioxidant effects and vascular matrix metalloproteinase-2 downregulation by dihydropyridines in renovascular hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 35–44. [Google Scholar] [CrossRef]
- Mendes, A.S.; Blascke de Mello, M.M.; Parente, J.M.; Omoto, A.C.M.; Neto-Neves, E.M.; Fazan, R., Jr.; Tanus-Santos, J.E.; Castro, M.M. Verapamil decreases calpain-1 and matrix metalloproteinase-2 activities and improves hypertension-induced hypertrophic cardiac remodeling in rats. Life Sci. 2020, 244, 117153. [Google Scholar] [CrossRef]
- Martinez, M.L.; Castro, M.M.; Rizzi, E.; Fernandes, K.; Demacq, C.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Lercanidipine reduces matrix metalloproteinase-2 activity and reverses vascular dysfunction in renovascular hypertensive rats. Eur. J. Pharmacol. 2008, 591, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zervoudaki, A.; Economou, E.; Pitsavos, C.; Vasiliadou, K.; Aggeli, C.; Tsioufis, K.; Toutouza, M.; Stefanadis, C.; Toutouzas, P. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase-2 and -9 in essential hypertension. Am. J. Hypertens. 2004, 17, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Zervoudaki, A.; Economou, E.; Stefanadis, C.; Pitsavos, C.; Tsioufis, K.; Aggeli, C.; Vasiliadou, K.; Toutouza, M.; Toutouzas, P. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J. Hum. Hypertens. 2003, 17, 119–124. [Google Scholar] [CrossRef]

| β-Blockers (+), n = 23 | β-Blockers (−), n = 27 | p Value | |
|---|---|---|---|
| Age, years | 53.43 ± 12.70 | 53.48 ± 17.74 | 0.897 |
| Male sex, % | 57 | 59 | 0.886 |
| eGFR, mL/min/1.73 m2 | 17.5 (9.7–42.6) | 18.7 (12.8–33.8) | 0.535 |
| CKD, stage 1, % | 0 | 4 | 0.332 |
| CKD, stage 2, % | 17 | 15 | 0.849 |
| CKD, stage 3, % | 17 | 19 | 0.856 |
| CKD, stage 4, % | 17 | 19 | 0.856 |
| CKD, stage 5, % | 48 | 42 | 0.673 |
| SBP, mm Hg | 136.90 ± 11.23 | 134.23 ± 14.26 | 0.471 |
| DBP, mm Hg | 88.57 ± 7.27 | 84.62 ± 7.20 | 0.695 |
| Heart rate, bpm | 73.3 ± 8.9 | 68.8 ± 9.5 | 0.562 |
| RBC, 1012/L | 3.63 ± 0.72 | 3.45 ± 0.57 | 0.334 |
| Hemoglobin, g/dL | 11.13 ± 2.35 | 10.70 ± 1.98 | 0.490 |
| WBC, 109/L | 6.41 ± 1.95 | 6.49 ± 2.62 | 0.560 |
| Glucose, mg/dL | 90.0 (81.0–100.0) | 93.5 (85.0–117.0) | 0.474 |
| Albumin, g/dL | 3.12 ± 0.71 | 3.07 ± 0.77 | 0.814 |
| Total protein, g/dL | 6.10 ± 1.22 | 6.10 ± 1.17 | 0.999 |
| Total cholesterol, mg/dL | 208.0 (175.0–223.0) | 190.0 (170.0–219.0) | 0.338 |
| Triglycerides, mg/dL | 170 (115–265) | 152 (80–291) | 0.448 |
| Cardiovascular disease, % | 48 | 37 | 0.432 |
| Smokers, % | 9 | 19 | 0.316 |
| Diabetic nephropathy, % | 37 | 30 | 0.652 |
| Glomerulonephritis, % | 37 | 47 | 0.475 |
| Polycystic kidney disease, % | 17 | 19 | 0.854 |
| Hypertensive nephropathy, % | 9 | 4 | 0.234 |
| Diuretics, % | 26 | 15 | 0.166 |
| Converting enzyme inhibitors/sartans % | 65 | 52 | 0.176 |
| Calcium channel antagonists, % | 74 | 52 | 0.110 |
| α-adrenoceptor antagonists, % | 13 | 4 | 0.123 |
| Statin, % | 9 | 19 | 0.316 |
| β-Blockers (+) | β-Blockers (−) | |||
|---|---|---|---|---|
| MMP-2 | TIMP-2 | MMP-2 | TIMP-2 | |
| Sex, male = 1 | R = 0.031 NS | R = 0.008 NS | χ2 = 4.518 p = 0.034 | χ2 = 2.706 NS |
| SBP | R = 0.163 NS | R = 0.463 p = 0.035 | R = 0.180 NS | R = 0.150 NS |
| RBC | R = −0.500 p = 0.015 | R = −0.509 p = 0.013 | R = −0.142 NS | R = −0.075 NS |
| Hemoglobin | R = −0.510 p = 0.013 | R = −0.556 p = 0.006 | R = −0.102 NS | R = −0.046 NS |
| Interleukin 6 | R = 0.458 p = 0.028 | R = 0.390 p = 0.066 | R = 0.101 NS | R = 0.061 NS |
| TNF-α | R = 0.419 p = 0.046 | R = 0.230 NS | R = 0.042 NS | R = 0.062 NS |
| Cu/Zn SOD | R = −0.066 NS | R = 0.077 NS | R = 0.382 p = 0.049 | R = 0.549 p = 0.003 |
| Albumin | R = −0.586 p = 0.002 | R = −0.290 NS | R = −0.471 p = 0.030 | R = −0.300 NS |
| Total protein | R = −0.544 p = 0.008 | R = −0.082 NS | R = −0.273 NS | R = −0.056 NS |
| Diabetic nephropathy | χ2 = 0.016 NS | χ2 = 0.020 NS | χ2 = 6.497 p = 0.011 | χ2 = 6.618 p = 0.010 |
| Glomerulonephritis | χ2 = 4.422 p = 0.035 | χ2 = 2.782 NS | χ2 = 6.253 p = 0.010 | χ2 = 6.367 p = 0.012 |
| Calcium channel antagonists | χ2 = 6.199 p = 0.013 | χ2 = 7.475 p = 0.006 | χ2 = 0.514 NS | χ2 = 0.019 NS |
| Independent Variable | Regression Coefficient (β) | Standard Error | p Values | |
|---|---|---|---|---|
| MMP-2 | albumin | −0.553 | 0.181 | <0.001 |
| RBC | −0.512 | 0.178 | 0.016 | |
| TIMP-2 * | MMP-2 | 0.740 | 0.132 | <0.001 |
| SBP | 0.297 | 0.132 | 0.038 |
| Independent Variable | Regression Coefficient (β) | Standard Error | p Values | |
|---|---|---|---|---|
| MMP-2 | Cu/Zn SOD | 0.734 | 0.208 | 0.005 |
| albumin | −0.682 | 0.242 | 0.018 | |
| TIMP-2 * | MMP-2 | 0.739 | 0.113 | <0.001 |
| glomerulonephritis | −0.264 | 0.112 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopańko, M.; Zabłudowska, M.; Pawlak, D.; Sieklucka, B.; Krupa, A.; Sokołowska, K.; Ziemińska, M.; Pawlak, K. The Possible Effect of β-Blocker Use on the Circulating MMP-2/TIMP-2 System in Patients with Chronic Kidney Disease on Conservative Treatment. J. Clin. Med. 2024, 13, 1847. https://doi.org/10.3390/jcm13071847
Kopańko M, Zabłudowska M, Pawlak D, Sieklucka B, Krupa A, Sokołowska K, Ziemińska M, Pawlak K. The Possible Effect of β-Blocker Use on the Circulating MMP-2/TIMP-2 System in Patients with Chronic Kidney Disease on Conservative Treatment. Journal of Clinical Medicine. 2024; 13(7):1847. https://doi.org/10.3390/jcm13071847
Chicago/Turabian StyleKopańko, Magdalena, Magdalena Zabłudowska, Dariusz Pawlak, Beata Sieklucka, Anna Krupa, Katarzyna Sokołowska, Marta Ziemińska, and Krystyna Pawlak. 2024. "The Possible Effect of β-Blocker Use on the Circulating MMP-2/TIMP-2 System in Patients with Chronic Kidney Disease on Conservative Treatment" Journal of Clinical Medicine 13, no. 7: 1847. https://doi.org/10.3390/jcm13071847





