Changes in Body Weight in Severely Obese Patients Treated with the Anorexiant Mazindol
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Data
3.2. Baseline Characteristics
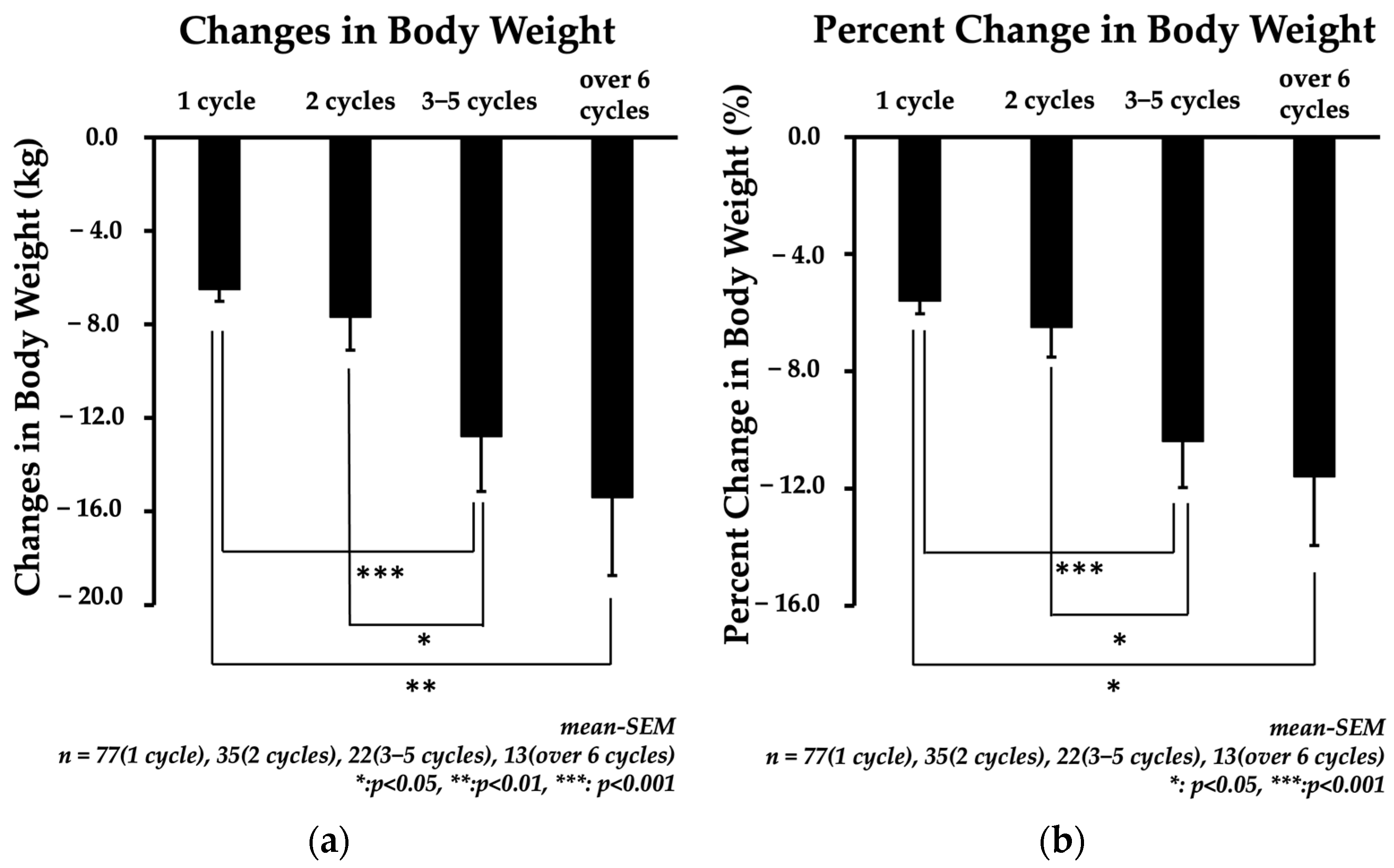
3.3. Change in Body Weight (CBW) and Percent Change in Body Weight (%CBW)
3.4. Factors Influencing CBW and %CBW
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, X.-M.T.; Lane, J.; Wang, P. Relationship Between Obesity and Diabetes in a US Adult Population: Findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes. Surg. 2011, 21, 351–355. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight Gain as a Risk Factor for Clinical Diabetes Mellitus in Women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef]
- Carmo, J.M.D.; da Silva, A.A.; Wang, Z.; Fang, T.; Aberdein, N.; de Lara Rodriguez, C.E.; Hall, J.E. Obesity-Induced Hypertension: Brain Signaling Pathways. Curr. Hypertens. Rep. 2016, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Palmer, T.M.; Benn, M.; Zacho, J.; Tybjærg-Hansen, A.; Smith, G.D.; Timpson, N.J. The Effect of Elevated Body Mass Index on Ischemic Heart Disease Risk: Causal Estimates from a Mendelian Randomisation Approach. PLoS Med. 2012, 9, e1001212. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Cause-Specific Excess Deaths Associated with Underweight, Overweight, and Obesity. JAMA 2007, 298, 2028–2037. [Google Scholar] [CrossRef]
- Balachandran, J.S.; Masa, J.F.; Mokhlesi, B. Obesity Hypoventilation Syndrome: Epidemiology and diagnosis. Sleep Med. Clin. 2014, 9, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Chihara, Y.; Azuma, M.; Murase, K.; Toyama, Y.; Yoshimura, C.; Oga, T.; Nakamura, H.; Mishima, M.; Chin, K.; et al. Obesity hypoventilation syndrome in Japan and independent determinants of arterial carbon dioxide levels. Respirology 2014, 19, 1233–1240. [Google Scholar] [CrossRef]
- Müller, T.D.; Clemmensen, C.; Finan, B.; DiMarchi, R.D.; Tschöp, M.H. Anti-Obesity Therapy: From Rainbow Pills to Polyagonists. Pharmacol. Rev. 2018, 70, 712–746. [Google Scholar] [CrossRef]
- Kimura, Y.; Fujishima, Y.; Nishizawa, H.; Saito, T.; Miyazaki, Y.; Shirahase, K.; Tokuzawa, C.; Nagai, N.; Fukuda, S.; Maeda, K.; et al. Changes in Eating Behaviors and Their Associations with Weight Loss in Japanese Patients Who Underwent Laparoscopic Sleeve Gastrectomy. Nutrients 2023, 15, 353. [Google Scholar] [CrossRef]
- Altieri, M.S.; Yang, J.; Nie, L.; Blackstone, R.; Spaniolas, K.; Pryor, A. Rate of revisions or conversion after bariatric surgery over 10 years in the state of New York. Surg. Obes. Relat. Dis. 2018, 14, 500–507. [Google Scholar] [CrossRef]
- Tolvanen, L.; Christenson, A.; Surkan, P.J.; Lagerros, Y.T. Patients’ Experiences of Weight Regain After Bariatric Surgery. Obes. Surg. 2022, 32, 1498–1507. [Google Scholar] [CrossRef]
- Alyahya, R.; A Alnujaidi, M. Prevalence and Outcomes of Depression After Bariatric Surgery: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e25651. [Google Scholar] [CrossRef]
- Adams, T.D.; Gress, R.E.; Smith, S.C.; Halverson, R.C.; Simper, S.C.; Rosamond, W.D.; LaMonte, M.J.; Stroup, A.M.; Hunt, S.C. Long-Term Mortality after Gastric Bypass Surgery. New Engl. J. Med. 2007, 357, 753–761. [Google Scholar] [CrossRef]
- Japan Society for the Study of Obesity. Guidelines for the Management of Obesity Disease 2022; Life Science Publishing Company: Tokyo, Japan, 2022. (In Japanese) [Google Scholar]
- Cunha, J.B.; Fialho, M.C.M.P.; Arruda, S.L.M.; Nóbrega, O.T.; Camargos, E.F. Clinical and Metabolic Improvement after Bariatric Surgery in Older Adults: A 6-Year Follow-Up. J. Nutr. Health Aging 2020, 24, 865–869. [Google Scholar] [CrossRef]
- Arguelles-Tello, F.A.; Kammar-García, A.; Trejo-Jasso, C.A.; Huerta-Cruz, J.C.; Barranco-Garduño, L.M.; Rocha-González, H.I.; Reyes-García, J.G. Metformin improves the weight reduction effect of mazindol in prediabetic obese Mexican subjects. Int. J. Clin. Pharmacol. Ther. 2022, 60, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cruz, J.C.; Rocha-González, H.I.; Kammar-García, A.; Canizales-Quinteros, S.; Barranco-Garduño, L.M.; Reyes-García, J.G. Combined First Month Body Weight Loss and Development of Tolerance as Predictors of 6-Month Efficacy of Mazindol in Mild and Moderate Obese Subjects. J. Clin. Med. 2022, 11, 3211. [Google Scholar] [CrossRef]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.W.; Ranson, S.W. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 1940, 78, 149–172. [Google Scholar] [CrossRef]
- Anand, B.K.; Brobeck, J.R. Localization of a “Feeding Center” in the Hypothalamus of the Rat. Exp. Biol. Med. 1951, 77, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Lee, W.S.; You, G.; Brown, D.; Hediger, M.a. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J. Clin. Investig. 1994, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Iizuka, T.; Omura, M.; Kuramoto, N.; Miki, T.; Ito, H.; Chiba, S. Effect of Mazindol on Body Weight and Insulin Sensitivity in Severely Obese Patients after a Very-Low-Calorie Diet Therapy. Endocr. J. 1996, 43, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sakane, N.; Umekawa, T.; Yoshioka, K.; Kondo, M.; Wakabayashi, Y. Usefulness of mazindol in combined diet therapy consisting of a low-calorie diet and Optifast in severely obese women. Int. J. Clin. Pharm. Res. 1994, 14, 125–132. [Google Scholar]



| Clinical Parameters | 1 Cycle | 2 Cycles | 3–5 Cycles | Over 6 Cycles | p-Value | Pairwise Comparison |
|---|---|---|---|---|---|---|
| n | 77 | 35 | 22 | 13 | − | |
| Age (years) | 44.1 ± 1.4 | 45.0 ± 2.1 | 44.8 ± 2.9 | 53.3 ± 3.4 | 0.14 | n.s. |
| Sex (male/female) | 41/36 | 23/12 | 13/9 | 4/9 | 0.55 | n.s. |
| Height (cm) | 164 ± 1.0 | 165 ± 1.7 | 165 ± 1.8 | 163 ± 2.1 | 0.86 | n.s. |
| Weight (kg) | 110 ± 2.6 | 111 ± 4.1 | 123 ± 6.6 | 124 ± 7.4 | 0.09 | n.s. |
| BMI | 40.8 ± 0.8 | 40.5 ± 0.9 | 44.5 ± 1.7 | 46.8 ± 2.3 | 0.004 | 1 cycle, 2 cycles < over 6 cycles * |
| BS (mg/dL) | 125 ± 5.6 | 106 ± 4.1 | 117 ± 9.4 | 132 ± 16.6 | 0.22 | n.s. |
| HbA1c (%) | 6.6 ± 0.2 | 6.1 ± 0.1 | 6.0 ± 0.3 | 6.6 ± 0.3 | 0.10 | n.s. |
| Systolic blood pressure (mmHg) | 140 ± 2.0 | 135 ± 3.1 | 140 ± 3.9 | 140 ± 3.6 | 0.68 | n.s. |
| Diastolic blood pressure (mmHg) | 85 ± 1.4 | 83 ± 1.8 | 87 ± 2.3 | 86 ± 3.7 | 0.98 | n.s. |
| LDL cholesterol (mg/dL) | 120 ± 4.1 | 123 ± 4.7 | 128 ± 6.4 | 115 ± 3.9 | 0.65 | n.s. |
| HDL cholesterol (mg/dL) | 48 ± 1.2 | 50 ± 1.8 | 49 ± 1.9 | 48 ± 2.9 | 0.71 | n.s. |
| Triglyceride (mg/dL) | 178 ± 11 | 178 ± 20 | 146 ± 15 | 145 ± 19 | 0.33 | n.s. |
| Uric acid (mg/dL) | 5.9 ± 0.2 | 6.2 ± 0.2 | 5.6 ± 0.3 | 5.5 ± 0.3 | 0.20 | n.s. |
| AST (IU/L) | 32 ± 1.7 | 42 ± 4.9 | 27 ± 2.4 | 30 ± 3.4 | 0.16 | n.s. |
| ALT (IU/L) | 44 ± 3.4 | 60 ± 8.9 | 34 ± 4.6 | 36 ± 3.5 | 0.60 | n.s. |
| Comorbidities | ||||||
| Diabetes mellitus (%) | 33(42.9) | 13(37.1) | 11(50.0) | 9(69.2) | 0.23 | n.s. |
| Hypertension (%) | 30(39.0) | 16(45.7) | 13(59.1) | 8(61.5) | 0.22 | n.s. |
| Hyperlipidemia (%) | 18(23.4) | 19(54.3) | 12(54.5) | 11(84.6) | <0.001 | 1 cycle < 2 cycles, 3–5 cycles *, 1 cycle < over 6 cycles *** |
| Sleep apnea syndrome (%) | 10(13.0) | 17(48.6) | 13(59.1) | 9(69.2) | <0.001 | 1 cycle < 2 cycles, 3–5 cycles, over 6 cycles *** |
| Medications | ||||||
| Biguanide (%) | 16(20.8) | 8(22.9) | 6(27.3) | 6(46.2) | 0.26 | n.s. |
| SGLT2 inhibitor (%) | 16(20.8) | 7(20.0) | 2(9.1) | 5(38.5) | 0.25 | n.s. |
| DPP4 inhibitor (%) | 7(9.1) | 3(8.6) | 2(9.1) | 3(23.1) | 0.45 | n.s. |
| GLP1 receptor agonist (%) | 5(6.5) | 4(11.4) | 3(13.6) | 2(15.4) | 0.45 | n.s. |
| SU (%) | 6(7.8) | 1(2.9) | 0(0) | 1(7.7) | 0.44 | n.s. |
| Insulin (%) | 6(7.8) | 0(0) | 0(0) | 2(15.4) | 0.06 | n.s. |
| Angiotensin receptor blocker (%) | 24(31.2) | 9(25.7) | 7(27.3) | 8(61.5) | 0.14 | n.s. |
| Calcium channel blocker (%) | 22(28.6) | 5(14.3) | 6(27.3) | 6(46.2) | 0.13 | n.s. |
| Statin (%) | 13(16.9) | 5(14.3) | 9(40.9) | 6(46.2) | 0.01 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Maeda, N.; Koseki, M.; Maeda, K. Changes in Body Weight in Severely Obese Patients Treated with the Anorexiant Mazindol. J. Clin. Med. 2024, 13, 1860. https://doi.org/10.3390/jcm13071860
Tanaka Y, Maeda N, Koseki M, Maeda K. Changes in Body Weight in Severely Obese Patients Treated with the Anorexiant Mazindol. Journal of Clinical Medicine. 2024; 13(7):1860. https://doi.org/10.3390/jcm13071860
Chicago/Turabian StyleTanaka, Yoshimitsu, Norikazu Maeda, Masahiro Koseki, and Kazuhisa Maeda. 2024. "Changes in Body Weight in Severely Obese Patients Treated with the Anorexiant Mazindol" Journal of Clinical Medicine 13, no. 7: 1860. https://doi.org/10.3390/jcm13071860
APA StyleTanaka, Y., Maeda, N., Koseki, M., & Maeda, K. (2024). Changes in Body Weight in Severely Obese Patients Treated with the Anorexiant Mazindol. Journal of Clinical Medicine, 13(7), 1860. https://doi.org/10.3390/jcm13071860





