Repurposing the Fibrosis-4 Score in Rheumatoid Arthritis: Data from the ESPOIR Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Main Study Outcomes
2.2. FIB-4 Score
2.3. Patient and Public Involvement
2.4. Ethics Approval and Consent to Participate
2.5. Statistical Methods
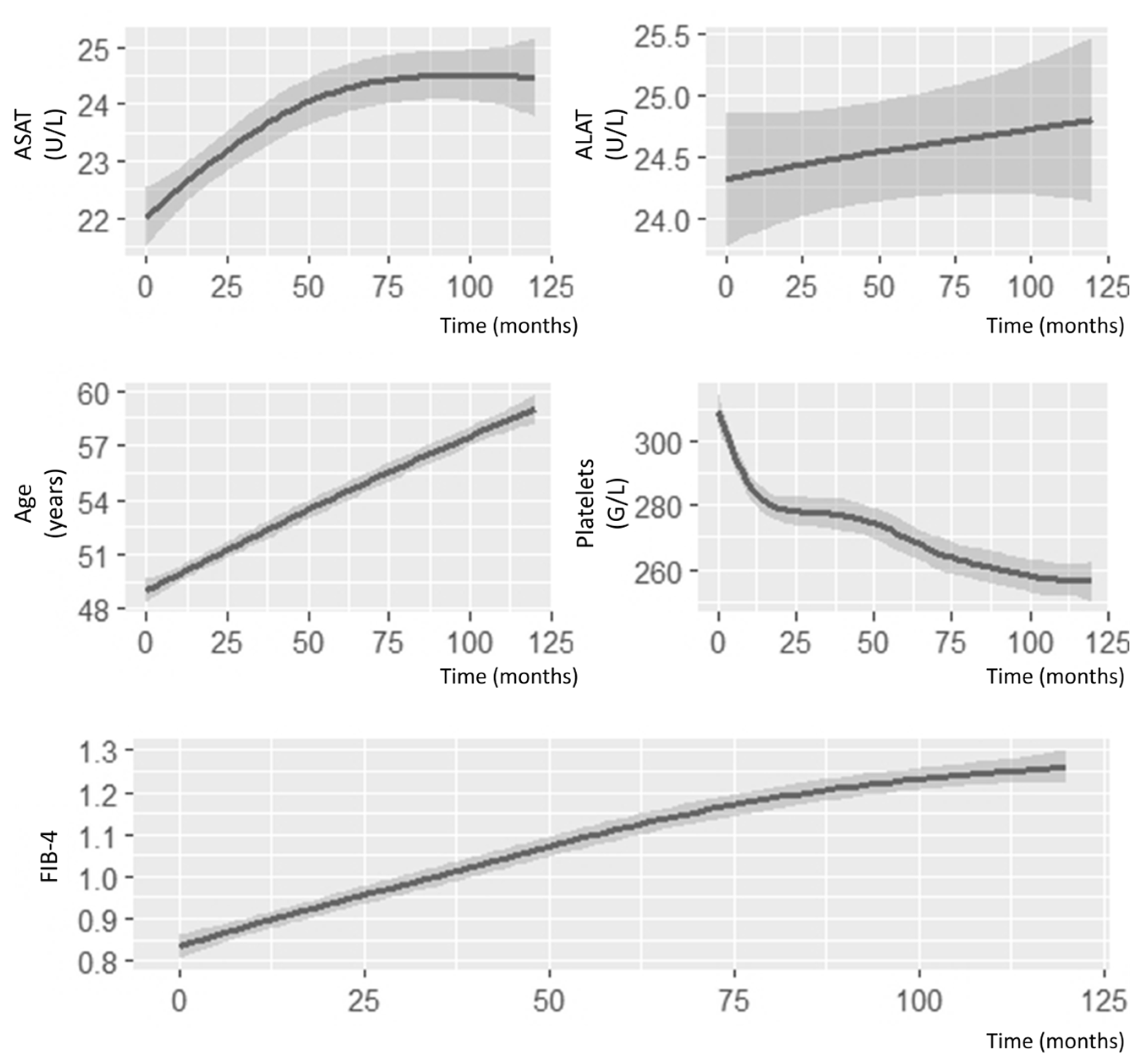
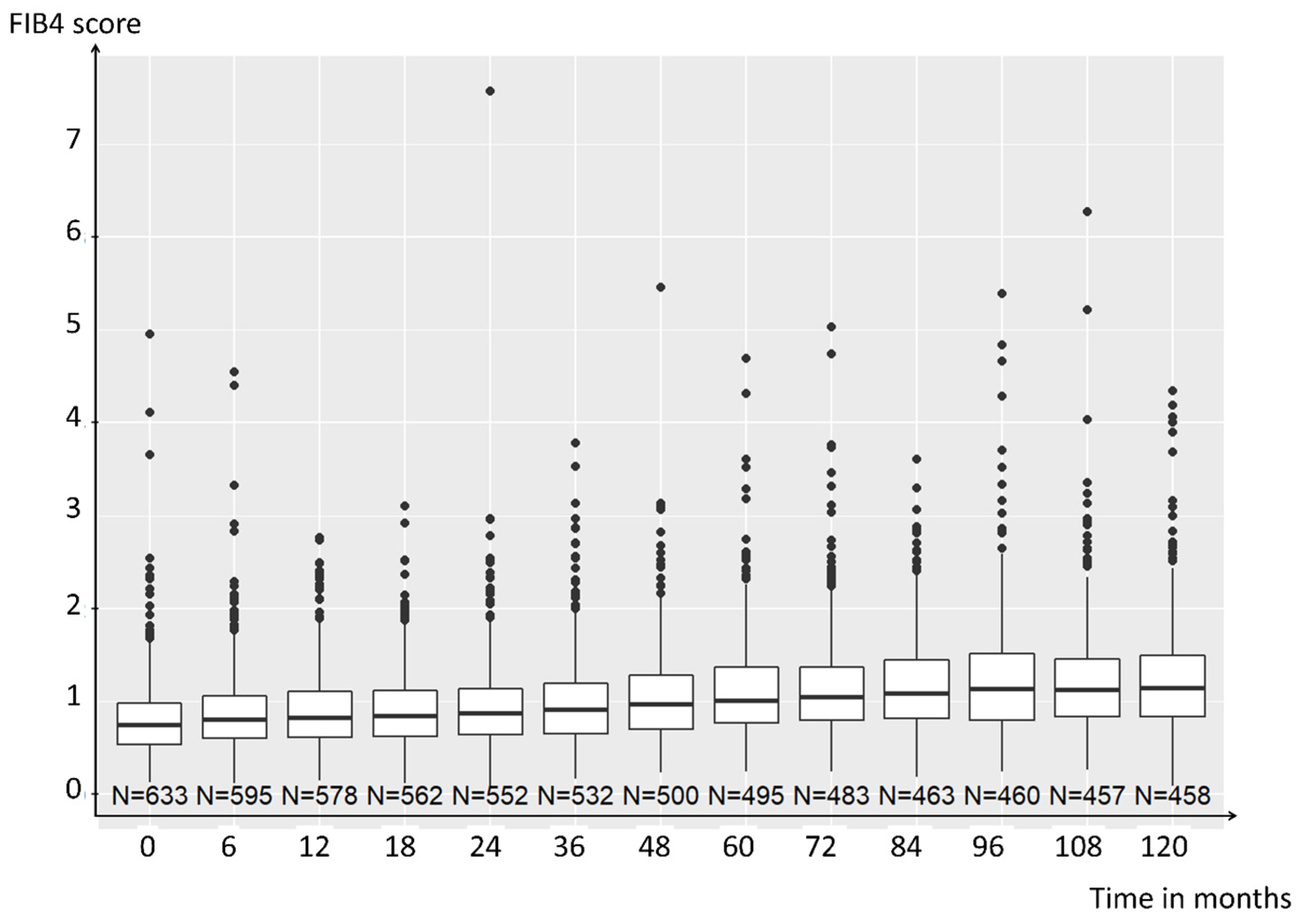
3. Results
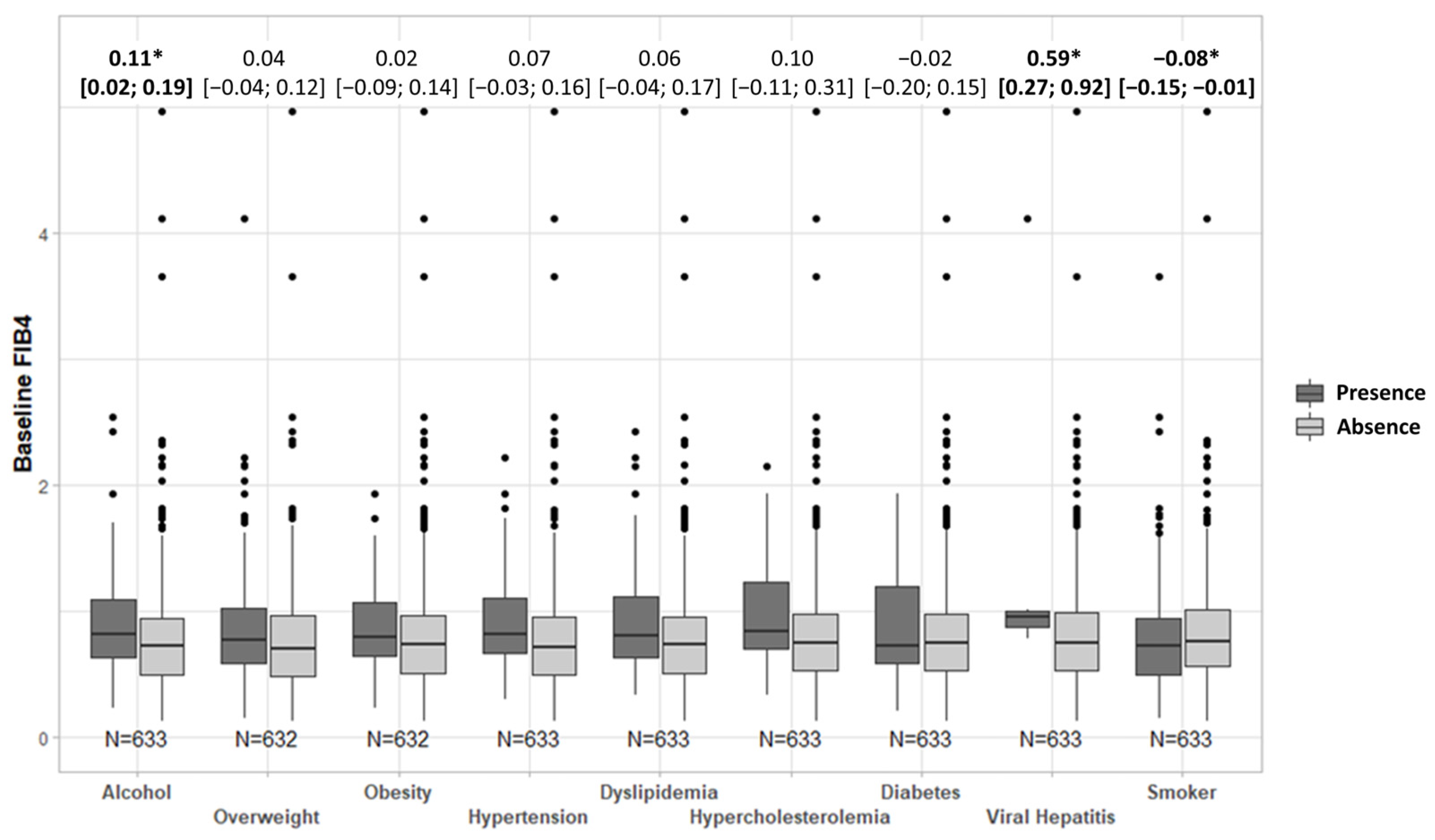
3.1. FIB-4 Score Association with Comorbidities
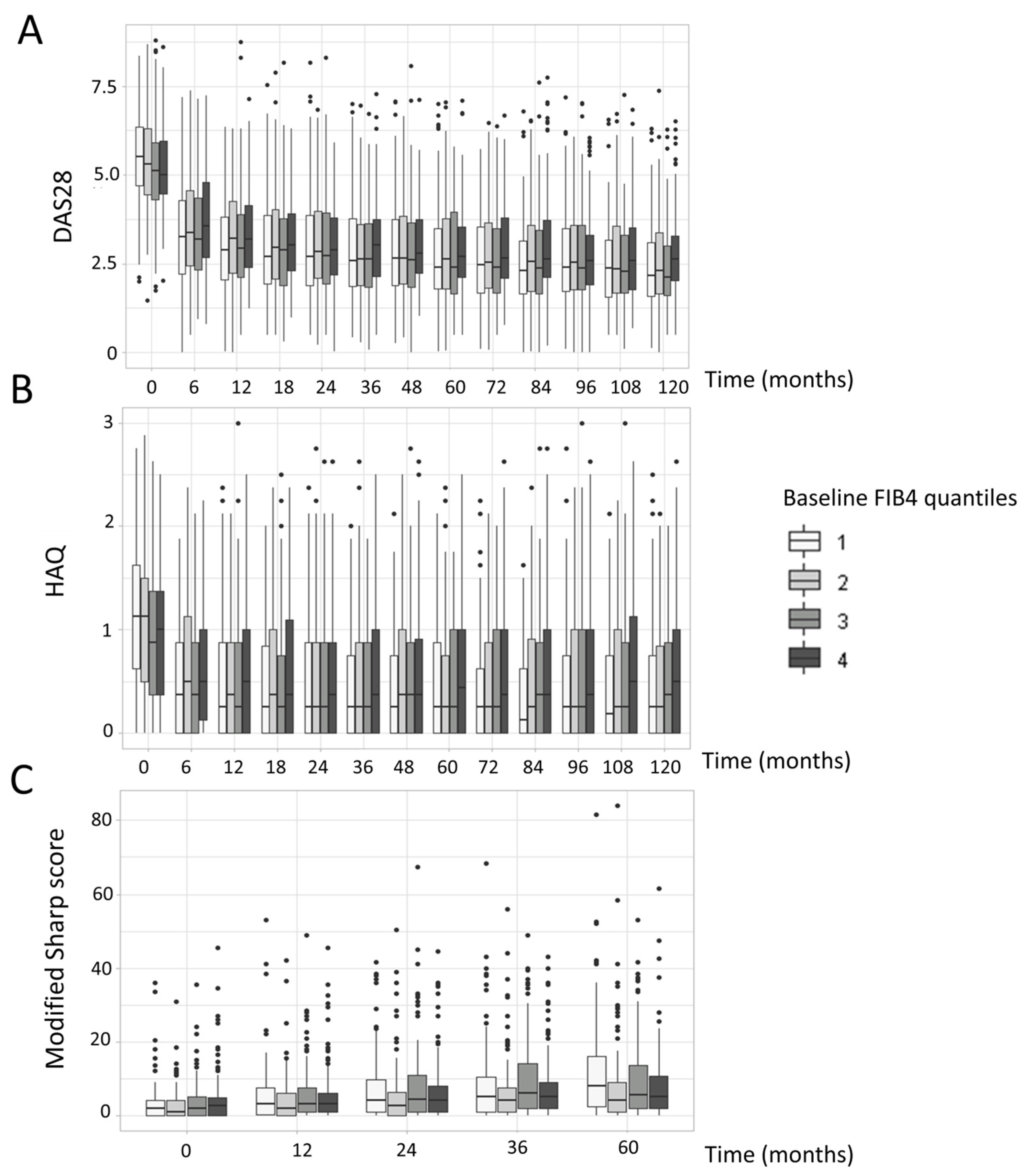
3.2. FIB-4 Score Association with Disease Activity and Radiographic Progression
3.3. FIB-4 Association with Comorbidities and Mortality
3.4. Effect of Treatments on FIB-4 Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Nash Clinical Research Network Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Boehmer, E.D.; Kovacs, E.J. The aging innate immune system. Curr. Opin. Immunol. 2005, 17, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Crowson, C.S.; Matteson, E.L.; Myasoedova, E.; Michet, C.J.; Ernste, F.C.; Warrington, K.J.; Davis, J.M.; Hunder, G.G.; Therneau, T.M.; Gabriel, S.E. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011, 63, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.M.H.; Maier, A.B.; Stinissen, P.; Masson, P.; Lories, R.J.; De Keyser, F. The influence of ageing on the development and management of rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 9, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Olumuyiwa-Akeredolu, O.-O.; Page, M.J.; Soma, P.; Pretorius, E. Platelets: Emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Stavropoulos-Kalinoglou, A.; Mikhailidis, D.P.; Douglas, K.M.J.; Kitas, G.D. Platelet function in rheumatoid arthritis: Arthritic and cardiovascular implications. Rheumatol. Int. 2011, 31, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Yazici, S.; Yazici, M.; Erer, B.; Erer, B.; Calik, Y.; Ozhan, H.; Ataoglu, S. The platelet indices in patients with rheumatoid arthritis: Mean platelet volume reflects disease activity. Platelets 2010, 21, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Baillet, A.; Gossec, L.; Carmona, L.; de Wit, M.; van Eijk-Hustings, Y.; Bertheussen, H.; Alison, K.; Toft, M.; Kouloumas, M.; Ferreira, R.J.O.; et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: A EULAR initiative. Ann. Rheum. Dis. 2016, 75, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Ortiz-Brizuela, E.; Vargas-Vázquez, A.; González-Lara, M.F.; Leon, A.P.D.; Sifuentes-Osornio, J.; Aguilar-Salinas, C.A. Validation and repurposing of the MSLCOVID- 19 score for prediction of severe COVID-19 using simple clinical predictors in a triage setting: The Nutri-CoV score. PLoS ONE 2020, 15, e0244051. [Google Scholar] [CrossRef] [PubMed]
- Sakthiswary, R.; Chan, G.Y.L.; Koh, E.T.; Leong, K.P.; Thong, B.Y.H. Methotrexate-associated nonalcoholic fatty liver disease with transaminitis in rheumatoid arthritis. Sci. World J. 2014, 2014, 823763. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Park, Y.-B.; Han, K.-H.; Lee, S.-W. Fibrosis-4 index at diagnosis can predict all-cause mortality in patients with rheumatoid arthritis: A retrospective monocentric study. Mod. Rheumatol. 2020, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Combe, B.; Benessiano, J.; Berenbaum, F.; Cantagrel, A.; Daurès, J.-P.; Dougados, M.; Fardellone, P.; Fautrel, B.; Flipo, R.-M.; Goupille, P.; et al. The ESPOIR cohort: A ten-year follow-up of early arthritis in France: Methodology and baseline characteristics of the 813 included patients. Jt. Bone Spine 2007, 74, 440–445. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.M.; van Leeuwen, M.A.; van Riel, P.L.; van de Putte, L.B. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp’s method (van der Heijde modification). J Rheumatol 1995, 22, 1792–1796. [Google Scholar] [PubMed]
- Fransen, J.; van Riel, P.L.C.M. The Disease Activity Score and the EULAR response criteria. Clin. Exp. Rheumatol. 2005, 23, S93–S99. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Degrave, R.; Vergneault, H.; Combier, A.; Wanono, S.; Boisson, M.; Frantz, C.; Allanore, Y. Risk of liver fibrosis induced by methotrexate and other rheumatoid arthritis medications according to the Fibrosis-4 Index. Clin. Exp. Rheumatol. 2021, 140, 150–157. [Google Scholar] [CrossRef] [PubMed]
| Age, years, median (IQR) | 51.0 (40.4–57.5) |
| Female, n (%) | 500 (77.3%) |
| Male, n (%) | 147 (22.7%) |
| FIB-4 score, median (IQR) * | 0.75 (0.53–0.99) |
| High FIB-4 score **, n (%) | 61 (9.6%) |
| Follow-up in months | |
| Mean (±SD) | 102.4 (±35.4) |
| Median (IQR) | 120 (108–120) |
| Swollen joint count, median (IQR | 6 (4–11) |
| Tender joint count, median (IQR) | 7 (4–13) |
| DAS28-ESR, median (IQR) | 5.2 (4.5–6.1) |
| ESR, mm at 1st hour, median (IQR) | 24 (12–39) |
| CRP level, mg/l, median (IQR) | 10 (5–26) |
| Modified total Sharp score,median (IQR) | 2 (0–4) |
| Rheumatoid factor, IgM,median (IQR) | 12 (4–72.5) |
| HAQ score,median (IQR) | 1.0 (0.5–1.5) |
| Positive for rheumatoid factor, n (%) | 325 (50.2%) |
| Anti-citrullinated protein antibodies, median (IQR) | 0 (0–501.5) |
| Positive for anti-citrullinated protein antibodies, n (%) | 286 (44.6%) |
| Weight, kg,median (IQR) | 66.3 (58.0–76.0) |
| Height, m,median (IQR) | 1.64 (1.59–1.70) |
| BMI, kg/m2,median (IQR) | 24.3 (21.9–27.7) |
| Overweight (BMI > 25 kg/m2), n (%) | 274 (42.3%) |
| Obese (BMI > 30 kg/m2), n (%) | 89 (13.8%) |
| History of myocardial infarction, n (%) | 6 (0.9%) |
| History of stroke, n (%) | 4 (0.6%) |
| History of major cardiovascular event (MACEs), n (%) | 10 (1.5%) |
| History of viral hepatitis | 7 (1.1%) |
| Hypertension, n (%) | 117 (18.1%) |
| Hypercholesterolemia, n (%) | 98 (15.1%) |
| Hypertriglyceridemia, n (%) | 21 (3.2%) |
| Triglycerides, mmol/L | 1.0 (0.8–1.5) |
| Total cholesterol, mmol/L | 5.2 (4.5–6.0) |
| HDL cholesterol, mmol/L | 1.4 (1.2–1.8) |
| Smoker, n (%) | |
| Ever | 313 (48.4%) |
| Never | 334 (51.6%) |
| Current | 145 (22.4%) |
| Diabetes, n (%) | 27 (4.2%) |
| Chronic alcohol consumption, n (%) | 119 (18.4%) |
| Alcohol consumption, if any, g per day,median (IQR) | 10 (7–25) |
| Variable | Variables Included in Model | Mean Difference | 95% CI | p-Value |
|---|---|---|---|---|
| DAS28-ESR | Time | −1.40 × 10−1 | −0.19; −0.097 | <0.0001 |
| Baseline age | 2.00 × 10−4 | −0.0095; 0.0099 | 0.97 | |
| Baseline number of swollen joints | 1.20 × 10−1 | 0.11; 0.14 | <0.0001 | |
| Baseline rheumatoid factor | 7.50 × 10−5 | −1.5 × 10−4; 3.0 × 10−4 | 0.51 | |
| Baseline ACPA (presence) | −3.80 × 10−3 | −0.21; 0.20 | 0.97 | |
| Baseline CRP | 1.10 × 10−2 | 0.0074; 0.014 | <0.0001 | |
| Baseline modified Sharp score > 0 | 1.60 × 10−1 | −0.056; 0.38 | 0.15 | |
| Baseline FIB-4 | −1.50 × 10−1 | −0.40; 0.11 | 0.26 | |
| Baseline age: time | 7.10 × 10−4 | −0.00031; 0.0017 | 0.17 | |
| Baseline number of swollen joints: time | −9.40 × 10−3 | −0.011; −0.0075 | <0.0001 | |
| Baseline rheumatoid factor: time | 1.10 × 10−6 | −2.2 × 10−5; 2.4 × 10−5 | 0.92 | |
| Baseline ACPA (presence): time | 8.00 × 10−3 | −0.014; 0.030 | 0.47 | |
| Baseline CRP: time | −9.40 × 10−4 | −0.0013; −0.00060 | <0.0001 | |
| Baseline modified Sharp score >0: time | 2.10 × 10−2 | −0.0018; 0.044 | 0.071 | |
| Baseline FIB-4: time | 2.10 × 10−2 | −0.0053; 0.048 | 0.21 | |
| Modified Sharp score | Time | 4.30 × 10−2 | −0.00038; 0.086 | 0.052 |
| Baseline age | 1.10 × 10−1 | 0.049; 0.17 | 0.0005 | |
| Baseline number of swollen joints | 5.50 × 10−2 | −0.067; 0.18 | 0.38 | |
| Baseline rheumatoid factor | −3.80 × 10−4 | −0.0019; 0.0011 | 0.61 | |
| Baseline ACPA (presence) | 1.70 | 0.38; 3.1 | 0.012 | |
| Baseline CRP level | 2.10 × 10−3 | −0.017; 0.022 | 0.84 | |
| Baseline FIB-4 | −1.00 | −2.7; 0.69 | 0.25 | |
| Baseline age: time | 7.50 × 10−4 | −0.00017; 0.0017 | 0.11 | |
| Baseline number of swollen joints: time | 3.20 × 10−4 | −0.0014; 0.0020 | 0.71 | |
| Baseline rheumatoid factor: time | −4.80 × 10−6 | −2.4 × 10−5; 1.4 × 10−5 | 0.62 | |
| Baseline ACPA (presence): time | 5.00 × 10−2 | 0.031; 0.069 | <0.0001 | |
| Baseline CRP level: time | 6.40 × 10−4 | 0.00035; 0.00093 | <0.0001 | |
| Baseline FIB-4: time | −1.90 × 10−2 | −0.043; 0.0040 | 0.11 |
| Characteristic/Treatment | Mean Difference | 95% CI | p-Value |
|---|---|---|---|
| Time | 0.418 | 0.33; 0.511 | <0.001 |
| Sex | 0.121 | 0.04; 0.206 | 0.005 |
| Methotrexate | −0.129 | −0.19; −0.073 | <0.001 |
| Leflunomide | −0.039 | −0.12; 0.042 | 0.344 |
| NSAIDs | −0.001 | −0.04; 0.037 | 0.950 |
| bDMARDs | 0.078 | 0.01; 0.142 | 0.015 |
| Tocilizumab | −0.105 | −0.82; 0.606 | 0.772 |
| Methotrexate: time | 0.080 | −0.01; 0.172 | 0.086 |
| Leflunomide: time | 0.163 | 0.02; 0.305 | 0.024 |
| NSAIDs: time | −0.058 | −0.13; 0.009 | 0.091 |
| bDMARDs: time | −0.061 | −0.16; 0.033 | 0.204 |
| Tocilizumab: time | 0.493 | −0.32; 1.307 | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felten, R.; Fabacher, T.; Sedmak, N.; Sibilia, J.; Sordet, C.; Chatelus, E.; Berenbaum, F.; Combe, B.; Ruyssen-Witrand, A.; Vittecoq, O.; et al. Repurposing the Fibrosis-4 Score in Rheumatoid Arthritis: Data from the ESPOIR Cohort. J. Clin. Med. 2024, 13, 1905. https://doi.org/10.3390/jcm13071905
Felten R, Fabacher T, Sedmak N, Sibilia J, Sordet C, Chatelus E, Berenbaum F, Combe B, Ruyssen-Witrand A, Vittecoq O, et al. Repurposing the Fibrosis-4 Score in Rheumatoid Arthritis: Data from the ESPOIR Cohort. Journal of Clinical Medicine. 2024; 13(7):1905. https://doi.org/10.3390/jcm13071905
Chicago/Turabian StyleFelten, Renaud, Thibaut Fabacher, Nathanaël Sedmak, Jean Sibilia, Christelle Sordet, Emmanuel Chatelus, Francis Berenbaum, Bernard Combe, Adeline Ruyssen-Witrand, Olivier Vittecoq, and et al. 2024. "Repurposing the Fibrosis-4 Score in Rheumatoid Arthritis: Data from the ESPOIR Cohort" Journal of Clinical Medicine 13, no. 7: 1905. https://doi.org/10.3390/jcm13071905





