Prognostic Value of Postneoadjuvant Chemotherapy Neutrophil-to-Lymphocyte Ratio in Patients undergoing Radical Cystectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Inclusion and Exclusion Criteria
2.3. Measures and Outcomes
2.4. Statistical Analysis
3. Results
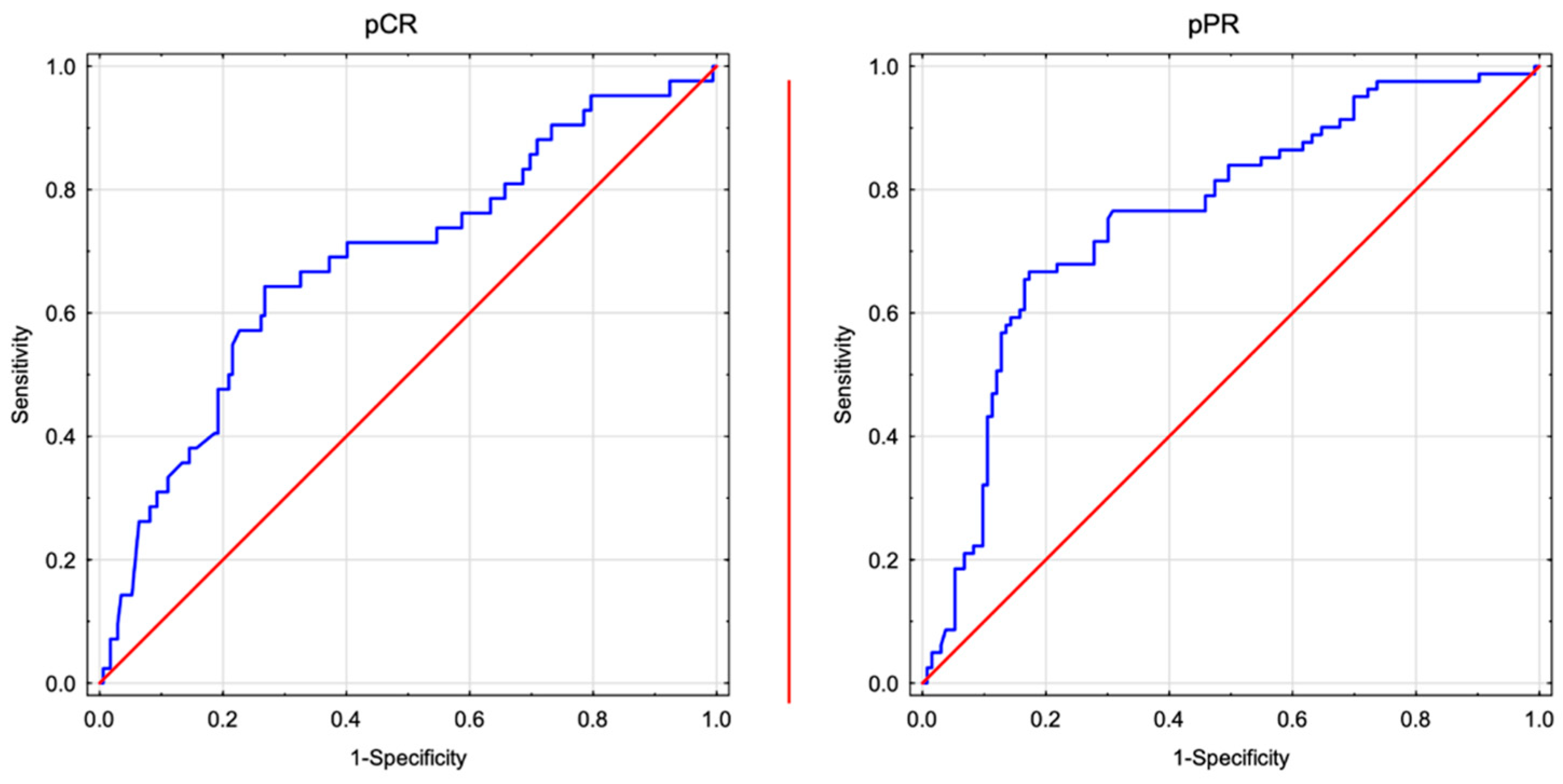
3.1. ROC Analysis
3.2. Comparison of Analysed Groups
3.3. High post-NAC NLR Is Associated with a Worse Local Response
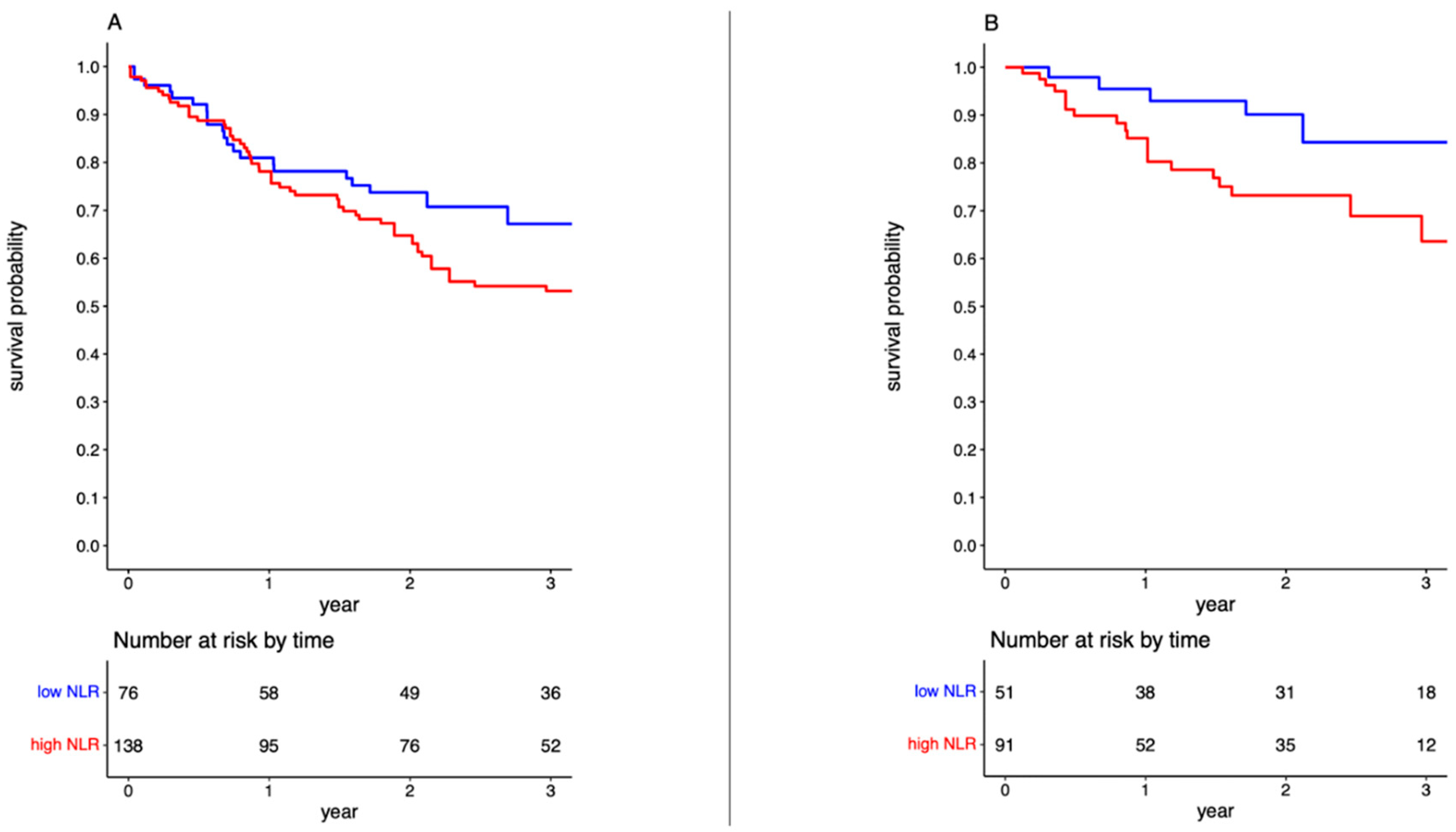
3.4. High post-NAC NLR Is Associated with Worse Overall Survival
3.5. High post-NAC NLR Is Associated with Worse Cancer-Specific Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, K.A.; Milowsky, M.I.; Ritchey, J.; Carroll, P.R.; Nanus, D.M. Low incidence of perioperative chemotherapy for Stage III bladder cancer 1998 to 2003: A report from the national cancer database. J. Urol. 2007, 178, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Fransen van de Putte, E.E.; Horenblas, S.; Drabick, J.J. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: A systematic review and two-step meta-analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef] [PubMed]
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group; Griffiths, G.; Hall, R.; et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Lemiński, A.; Kaczmarek, K.; Byrski, T.; Słojewski, M. Neoadjuvant chemotherapy with dose-dense MVAC is associated with improved survival after radical cystectomy compared to other cytotoxic regimens: A tertiary centre experience. PLoS ONE 2021, 16, e0259526. [Google Scholar] [CrossRef] [PubMed]
- Advanced Bladder Cancer (ABC). Meta-Analysis Neoadjuvant chemotherapy in invasive bladder cancer: An update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 2005, 48, 202–205; discussion 205. [Google Scholar] [CrossRef] [PubMed]
- Lemiński, A.; Michalski, W.; Masojć, B.; Kaczmarek, K.; Małkiewicz, B.; Kienitz, J.; Zawisza-Lemińska, B.; Falco, M.; Słojewski, M. Combined modality bladder-sparing therapy for muscle-invasive bladder cancer: How (should) we do it? Nnarrative reviews. J. Clin. Med. 2023, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J. Clin. Oncol. 2022, 40, 2013–2022. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 34, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Horwich, A.; Babjuk, M.; Bellmunt, J.; Bruins, H.M.; De Reijke, T.M.; De Santis, M.; Gillessen, S.; James, N.; Maclennan, S.; Palou, J.; et al. EAU–ESMO consensus statements on the management of advanced and variant bladder cancer: An international collaborative multi-stakeholder effort under the auspices of the EAU and ESMO Guidelines Committees. Ann. Oncol. 2019, 30, 1697–1727. [Google Scholar] [CrossRef]
- Russo, P.; Marino, F.; Rossi, F.; Bizzarri, F.P.; Ragonese, M.; Dibitetto, F.; Filomena, G.B.; Marafon, D.P.; Ciccarese, C.; Iacovelli, R.; et al. Is Systemic Immune-Inflammation Index a Real Non-Invasive Biomarker to Predict Oncological Outcomes in Patients Eligible for Radical Cystectomy? Medicina 2023, 59, 2063. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Lucca, I.; Jichlinski, P.; Shariat, S.F.; Rouprêt, M.; Rieken, M.; Kluth, L.A.; Rink, M.; Mathieu, R.; Mbeutcha, A.; Maj-Hes, A.; et al. Nneutrophil-to-lymphocyte ratio as a prognostic factor in patients with urothelial bladder carcinoma of the bladder following radical cystectomy: Validation and meta-analysis. Eur. Urol. Focus. 2016, 2, 79–85. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, Y.S.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Pretreatment lymphocytopenia is an adverse prognostic biomarker in advanced stage ovarian cancer. Cancer Med. 2019, 8, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Favilla, V.; Castelli, T.; Urzì, D.; Reale, G.; Privitera, S.; Salici, A.; Russo, G.I.; Cimino, S.; Morgia, G. Neutrophil to lymphocyte ratio, a biomarker in non-muscle invasive bladder cancer: A single-institutional longitudinal study. Int. Braz. J. Urol. 2016, 42, 685–693. [Google Scholar] [CrossRef]
- Sudoł, D.; Widz, D.; Mitura, P.; Płaza, P.; Godzisz, M.; Kuliniec, I.; Yadlos, A.; Cabanek, M.; Bar, M.; Bar, K. Neutrophil-to-lymphocyte ratio as a predictor of overall survival and cancer advancement in patients undergoing radical cystectomy for bladder cancer. Cent. Eur. J. Urol. 2022, 75, 41–46. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.; Bajorin, D.F.; et al. Consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011, 12, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomised, multinational, multicentre phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Plimack, E.R.; Hoffman-Censits, J.H.; Viterbo, R.; Trabulsi, E.J.; Ross, E.A.; Greenberg, R.E.; Chen, D.Y.; Lallas, C.D.; Wong, Y.N.; Lin, J.; et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J. Clin. Oncol. 2014, 32, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Fléchon, A.; Soulié, M.; Guy, L.; Laguerre, B.; Mottet, N.; Joly, F.; Allory, Y.; Harter, V.; et al. Phase III Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Muscle-invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur. Urol. 2021, 79, 214–221. [Google Scholar] [CrossRef]
- Albers, P.; Park, S.I.; Niegisch, G.; Fechner, G.; Steiner, U.; Lehmann, J.; Heimbach, D.; Heidenreich, A.; Fimmers, R.; Siener, R.; et al. Randomised phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: Short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann. Oncol. 2011, 22, 288–294. [Google Scholar] [CrossRef]
- Kawahara, T.; Furuya, K.; Nakamura, M.; Sakamaki, K.; Osaka, K.; Ito, H.; Ito, Y.; Izumi, K.; Ohtake, S.; Miyoshi, Y.; et al. The NLR is a prognostic marker in patients with bladder cancer after radical cystectomy. BMC Cancer 2016, 16, 185. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jiang, Y.Z.; Qian, W.H. Prognostic role of NLR in urinary cancers: A meta-analysis. PLoS ONE 2014, 9, e92079. [Google Scholar] [CrossRef] [PubMed]
- Black, A.J.; Zargar, H.; Zargar-Shoshtari, K.; Fairey, A.S.; Mertens, L.S.; Dinney, C.P.; Mir, M.C.; Krabbe, L.M.; Cookson, M.S.; Jacobsen, N.E.; et al. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy and radical cystectomy. Urol. Oncol. 2020, 38, 3.e17–3.e27. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Tanaka, K.; Yamasaki, M.; Yamashita, K.; Makino, T.; Saito, T.; Takahashi, T.; Kurokawa, Y.; Motoori, M.; Kimura, Y.; et al. Neutrophil-to-lymphocyte ratio after neoadjuvant chemotherapy is an independent prognostic factor in patients with oesophageal squamous cell carcinoma. Oncol. Lett. 2023, 25, 58. [Google Scholar] [CrossRef]
- Herzberg, H.; Lifshitz, K.; Golan, S.; Baniel, J.; Malshy, K.; Hoffman, A.; Amiel, G.E.; Zreik, R.; Freifeld, Y.; Dekel, Y.; et al. Association between early changes in neutrophil-to-lymphocyte ratio after radical cystectomy and treatment outcomes. BJU Int. 2022, 130, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Li, H.; North, S.A.; Leibowitz-Amit, R.; Seah, J.A.; Morshed, N.; Chau, C.; Lee-Ying, R.; Heng, D.Y.C.; Sridhar, S.; et al. The Prognostic Role of the Change in Neutrophil-to-Lymphocyte Ratio During Neoadjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer: A Retrospective, Multi-Institutional Study. Bladder Cancer 2018, 26, 185–194. [Google Scholar] [CrossRef]
- Sanna, E.; Tanca, L.; Cherchi, C.; Gramignano, G.; Oppi, S.; Chiai, M.G.; Macciò, A.; Madeddu, C. Decrease in neutrophil-to-lymphocyte ratio during neoadjuvant chemotherapy as a predictive and prognostic marker in advanced ovarian cancer. Diagnostics 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.H.; Han, J.Y.; Kim, H.T.; Yun, T.; Lee, J.S. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J. Cancer Res. Clin. Oncol. 2012, 138, 2009–2016. [Google Scholar] [CrossRef]
- Gondo, T.; Nakashima, J.; Ohno, Y.; Choichiro, O.; Horiguchi, Y.; Namiki, K.; Yoshioka, K.; Ohori, M.; Hatano, T.; Tachibana, M. Prognostic value of the neutrophil-to-lymphocyte ratio and establishment of a novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 2012, 79, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, T.; Bhindi, B.; Wei, Y.; Yu, J.; Noon, A.P.; Richard, P.O.; Bhatt, J.R.; Almatar, A.; Jewett, M.A.; Fleshner, N.E.; et al. Pre-treatment neutrophil-to-lymphocyte ratio as a predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br. J. Cancer 2014, 111, 444–451. [Google Scholar] [CrossRef]
- Krane, L.S.; Richards, K.A.; Kader, A.K.; Davis, R.; Balaji, K.C.; Hemal, A.K. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J. Endourol. 2013, 27, 1046–1050. [Google Scholar] [CrossRef]
- Viers, B.R.; Boorjian, S.A.; Frank, I.; Tarrell, R.F.; Thapa, P.; Karnes, R.J.; Thompson, R.H.; Tollefson, M.K. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur. Urol. 2014, 66, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Nagai, Y.; Shutta, R.; Masuda, D.; Yamashita, S.; Seo, M.; Yamada, T.; Nakagawa, A.; Yasumura, Y.; Nakagawa, Y.; et al. Combination of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as a Novel Predictor of Cardiac Death in Patients with Acute Decompensated Heart Failure With Preserved Left Ventricular Ejection Fraction: A Multicenter Study. J. Am. Heart Assoc. 2023, 12, e026326. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Fang, S.; Chen, Y.; Zhang, W.; Xia, F.; Wang, N.; Lu, Y. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 6219545. [Google Scholar] [CrossRef]
- Barone, B.; Calogero, A.; Scafuri, L.; Ferro, M.; Lucarelli, G.; Di Zazzo, E.; Sicignano, E.; Falcone, A.; Romano, L.; De Luca, L.; et al. Immune checkpoint inhibitors as a neoadjuvant/adjuvant treatment of muscle-invasive bladder cancer: A systematic review. Cancers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, M.L.; Miceli, C.C.; De Felice, M.; Barone, B.; Pompella, L.; Chiancone, F.; Di Zazzo, E.; Tirino, G.; Della Corte, C.M.; Imbimbo, C.; et al. Novel therapeutic opportunities in neoadjuvant setting in urothelial cancers: A new horizon opened by molecular classification and immune checkpoint inhibitors. Int. J. Mol. Sci. 2022, 23, 1133. [Google Scholar] [CrossRef]
- Tufano, A.; Napolitano, L.; Barone, B.; Pezone, G.; Alvino, P.; Cilio, S.; Buonerba, C.; Canciello, G.; Passaro, F.; Perdonà, S. Preoperative Albumin-to-Alkaline Phosphatase Ratio as an Independent Predictor of Lymph Node Involvement in Penile Cancer. Medicina 2024, 60, 414. [Google Scholar] [CrossRef]

| Variable | NLR ≤ 1.75 | NLR > 1.75 | p-Value |
|---|---|---|---|
| Totals, No. | 76 | 138 | |
| Age, years | 0.290 | ||
| Mean | 65.48 | 66.54 | |
| SD | 6.73 | 7.06 | |
| Sex, No. | 0.587 | ||
| Male | 57 | 108 | |
| Female | 19 | 30 | |
| BMI, No. | 0.125 | ||
| <30 kg/m2 | 58 | 117 | |
| ≥30 kg/m2 | 18 | 21 | |
| ASA score, No. | 0.151 | ||
| 1 | 3 | 11 | |
| 2 | 47 | 75 | |
| 3 | 24 | 50 | |
| 4 | 1 | 0 | |
| Smoking status, No. | 0.281 | ||
| Never | 18 | 23 | |
| Former | 34 | 75 | |
| Current | 21 | 38 | |
| Clinical T stage, No. | 0.193 | ||
| cT2 | 46 | 67 | |
| cT3 | 15 | 30 | |
| cT4 | 15 | 41 | |
| Pathological T stage, No. | <0.001 | ||
| ypT0 | 27 | 13 | |
| ypTis/Ta/T1 | 26 | 9 | |
| ypT2 | 5 | 49 | |
| ypT3 | 10 | 30 | |
| ypT4 | 8 | 37 | |
| Pathological N stage, No. | 0.003 | ||
| ypN0 | 65 | 92 | |
| ypN+ | 11 | 46 | |
| Cancer grade, No. | 0.567 | ||
| Low grade | 4 | 5 | |
| High grade | 72 | 133 | |
| Chemotherapy regimen, No. | 0.876 | ||
| ddMVAC | 42 | 82 | |
| Gemcitabine–cisplatin | 27 | 44 | |
| Gemcitabine–carboplatin | 2 | 5 | |
| Gemcitabine–paclitaxel | 5 | 7 |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI Lower | 95% CI Upper | p-Value | OR | 95% CI Lower | 95% CI Upper | p-Value | |
| Age | 1.007 | 0.967 | 1.049 | 0.730 | 1.049 | 0.984 | 1.119 | 0.145 |
| Sex | ||||||||
| Male | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Female | 1.100 | 0.567 | 2.136 | 0.778 | 0.683 | 0.256 | 1.823 | 0.446 |
| BMI | ||||||||
| <30 kg/m2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| ≥30 kg/m2 | 1.559 | 0.769 | 3.161 | 0.218 | 0.933 | 0.302 | 2.879 | 0.904 |
| ASA score | ||||||||
| 1–2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 3–4 | 0.787 | 0.429 | 1.445 | 0.440 | 1.000 | 0.402 | 2.487 | 0.999 |
| Smoking status | ||||||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Former | 0.613 | 0.291 | 1.290 | 0.197 | 0.257 | 0.084 | 0.790 | 0.018 |
| Current | 0.902 | 0.400 | 2.032 | 0.803 | 0.537 | 0.165 | 1.751 | 0.302 |
| Clinical T stage | ||||||||
| cT2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| cT3 | 0.184 | 0.079 | 0.431 | <0.001 | 0.064 | 0.018 | 0.228 | <0.001 |
| cT4 | 0.102 | 0.041 | 0.258 | <0.001 | 0.030 | 0.008 | 0.118 | <0.001 |
| NLR | ||||||||
| NLR ≤ 1.75 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| NLR > 1.75 | 0.082 | 0.042 | 0.161 | <0.001 | 0.045 | 0.017 | 0.119 | <0.001 |
| Chemotherapy regimen | ||||||||
| ddMVAC | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Gemcitabine–cisplatin | 1.024 | 0.555 | 1.887 | 0.940 | 1.086 | 0.442 | 2.672 | 0.857 |
| Gemcitabine–carboplatin | 0.753 | 0.140 | 4.047 | 0.741 | 0.490 | 0.048 | 4.957 | 0.545 |
| Gemcitabine–paclitaxel | 1.346 | 0.403 | 4.493 | 0.629 | 1.253 | 0.233 | 6.749 | 0.793 |
| Overall Survival | Cancer-Specific Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI Lower | 95% CI Upper | p-Value | HR | 95% CI Lower | 95% CI Upper | p-Value | |
| Age | 1.009 | 0.981 | 1.038 | 0.534 | 1.028 | 0.959 | 1.103 | 0.432 |
| Sex | ||||||||
| Male | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Female | 1.627 | 0.985 | 2.689 | 0.057 | 0.391 | 0.102 | 1.507 | 0.173 |
| BMI | ||||||||
| <30 kg/m2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| ≥30 kg/m2 | 0.958 | 0.571 | 1.607 | 0.871 | 0.170 | 0.041 | 0.701 | 0.014 |
| ASA score | ||||||||
| 1–2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 3–4 | 1.243 | 0.807 | 1.913 | 0.324 | 8.161 | 2.983 | 22.331 | <0.001 |
| Smoking status | ||||||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Former | 3.570 | 1.952 | 6.530 | <0.001 | 0.788 | 0.151 | 4.107 | 0.777 |
| Current | 2.639 | 1.429 | 4.871 | 0.002 | 0.680 | 0.104 | 4.454 | 0.687 |
| Clinical T stage | ||||||||
| cT2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| cT3 | 1.874 | 1.146 | 3.064 | 0.012 | 8.161 | 2.983 | 22.331 | <0.001 |
| cT4 | 2.737 | 1.697 | 4.413 | <0.001 | 5.522 | 1.985 | 15.360 | 0.001 |
| NLR | ||||||||
| NLR ≤ 1.75 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| NLR > 1.75 | 1.686 | 1.049 | 2.711 | 0.031 | 2.069 | 0.789 | 5.427 | 0.140 |
| Chemotherapy regimen | ||||||||
| ddMVAC | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Gemcitabine–cisplatin | 1.347 | 0.906 | 2.002 | 0.141 | 0.814 | 0.317 | 2.086 | 0.667 |
| Gemcitabine–carboplatin | 1.949 | 0.586 | 6.481 | 0.276 | 0.895 | 0.106 | 7.535 | 0.919 |
| Gemcitabine–paclitaxel | 0.579 | 0.244 | 1.376 | 0.216 | n/a | n/a | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, K.; Małkiewicz, B.; Gurwin, A.; Krawczyk, W.M.; Skonieczna-Żydecka, K.; Lemiński, A. Prognostic Value of Postneoadjuvant Chemotherapy Neutrophil-to-Lymphocyte Ratio in Patients undergoing Radical Cystectomy. J. Clin. Med. 2024, 13, 1953. https://doi.org/10.3390/jcm13071953
Kaczmarek K, Małkiewicz B, Gurwin A, Krawczyk WM, Skonieczna-Żydecka K, Lemiński A. Prognostic Value of Postneoadjuvant Chemotherapy Neutrophil-to-Lymphocyte Ratio in Patients undergoing Radical Cystectomy. Journal of Clinical Medicine. 2024; 13(7):1953. https://doi.org/10.3390/jcm13071953
Chicago/Turabian StyleKaczmarek, Krystian, Bartosz Małkiewicz, Adam Gurwin, Wiktor Mateusz Krawczyk, Karolina Skonieczna-Żydecka, and Artur Lemiński. 2024. "Prognostic Value of Postneoadjuvant Chemotherapy Neutrophil-to-Lymphocyte Ratio in Patients undergoing Radical Cystectomy" Journal of Clinical Medicine 13, no. 7: 1953. https://doi.org/10.3390/jcm13071953
APA StyleKaczmarek, K., Małkiewicz, B., Gurwin, A., Krawczyk, W. M., Skonieczna-Żydecka, K., & Lemiński, A. (2024). Prognostic Value of Postneoadjuvant Chemotherapy Neutrophil-to-Lymphocyte Ratio in Patients undergoing Radical Cystectomy. Journal of Clinical Medicine, 13(7), 1953. https://doi.org/10.3390/jcm13071953







