Pre-Treatment and Post-Treatment I-131 Imaging in Differentiated Thyroid Carcinoma
Abstract
:1. Introduction
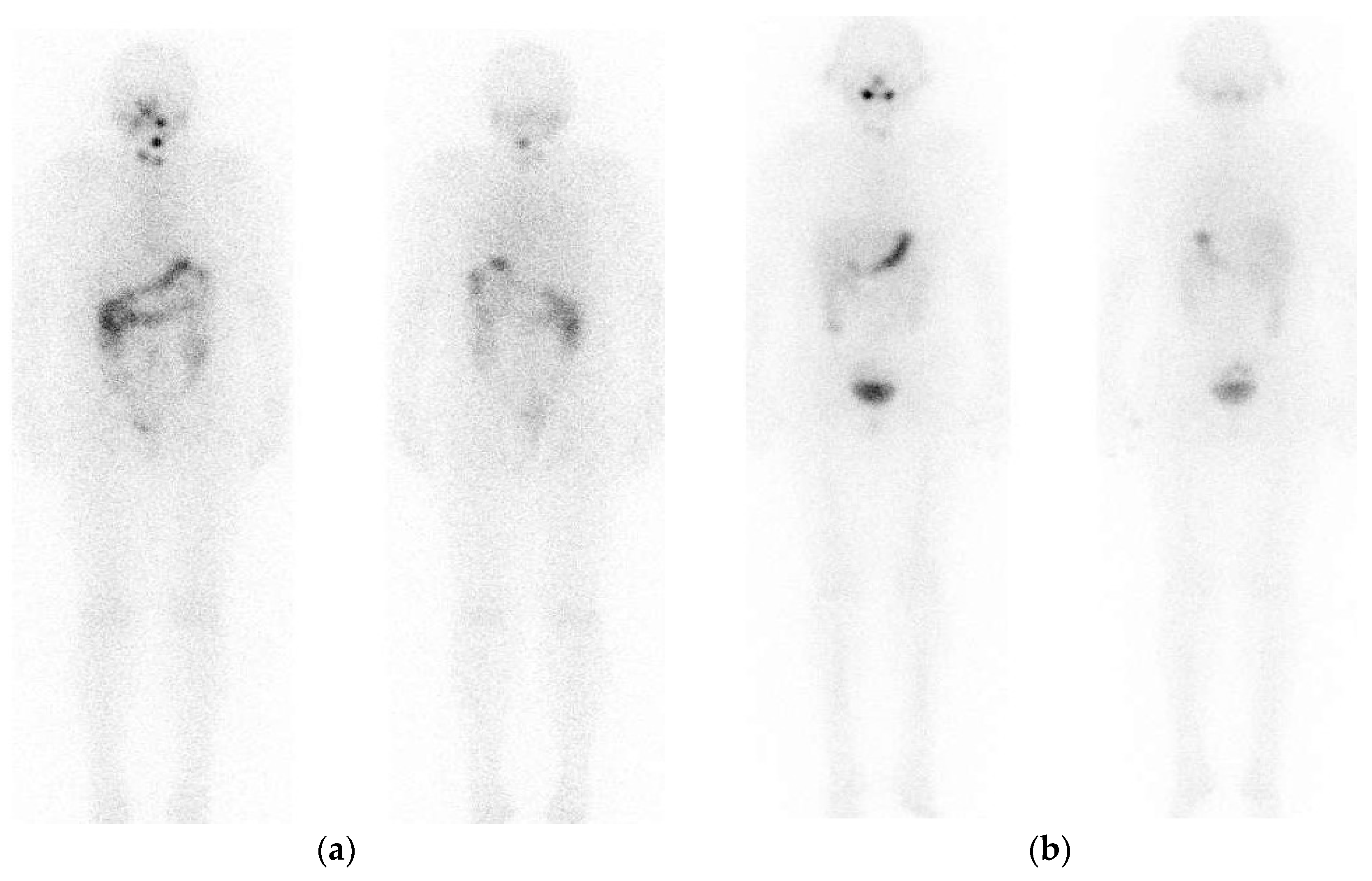
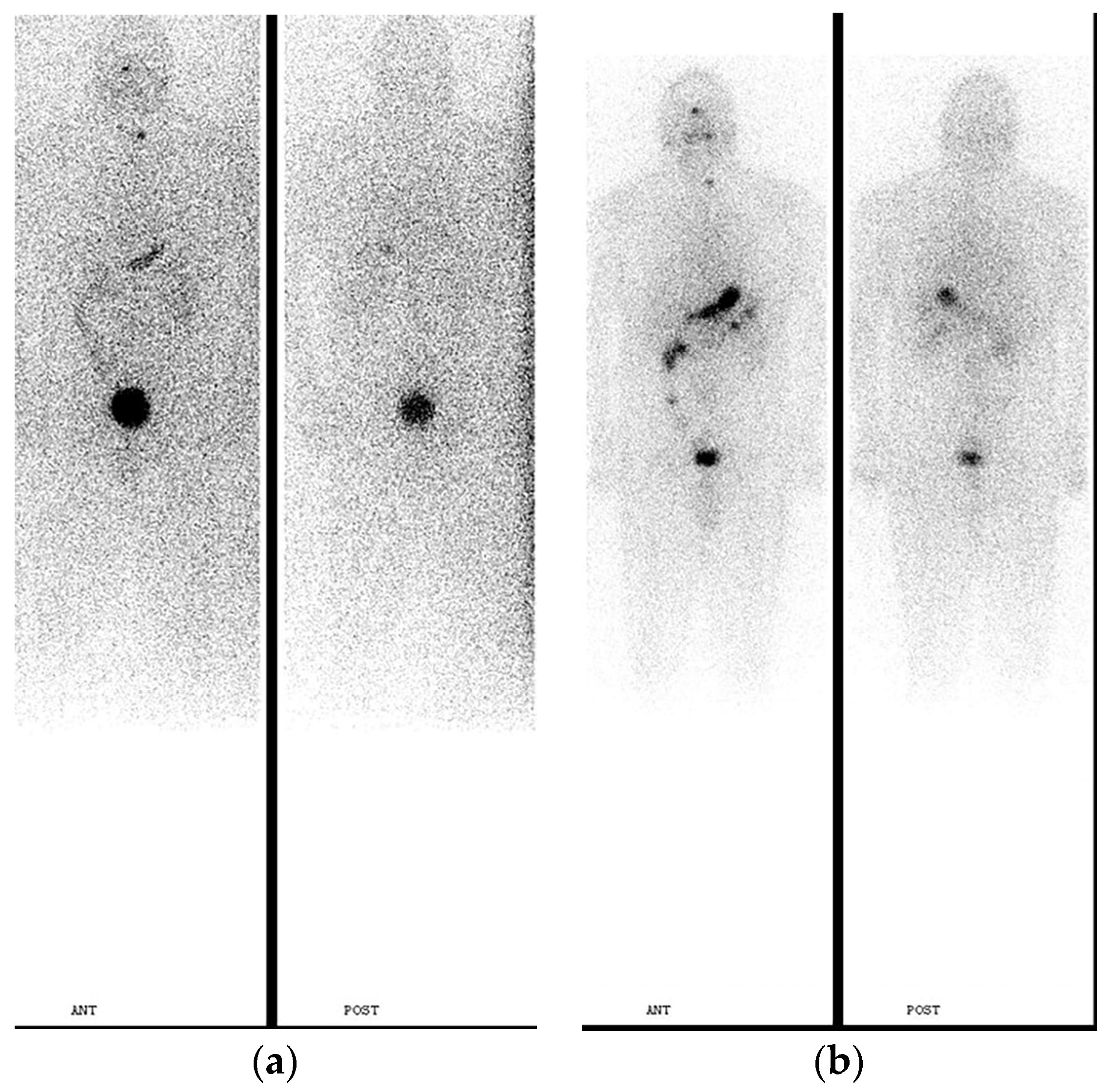
2. Pre-Treatment Whole-Body Imaging
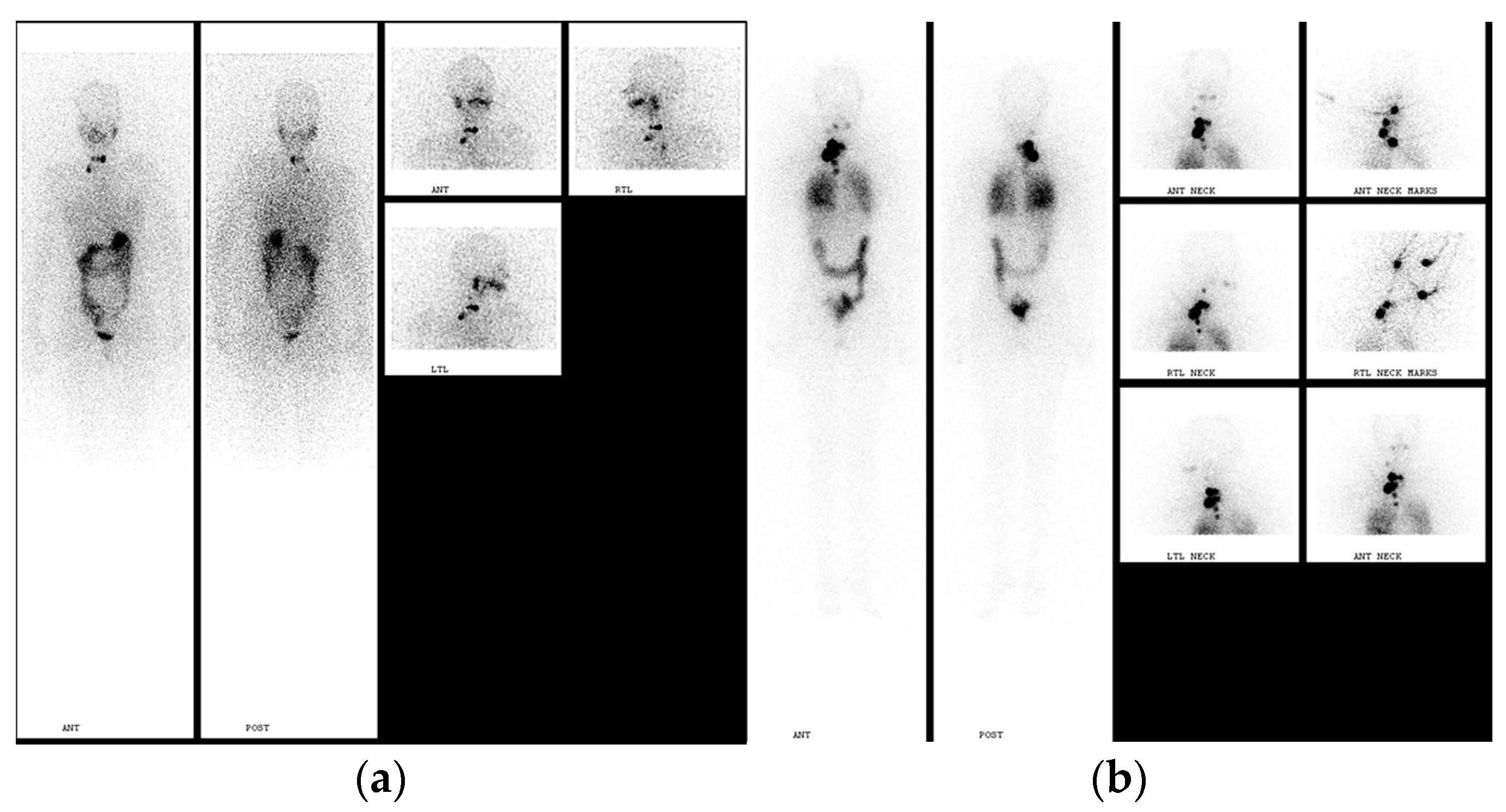
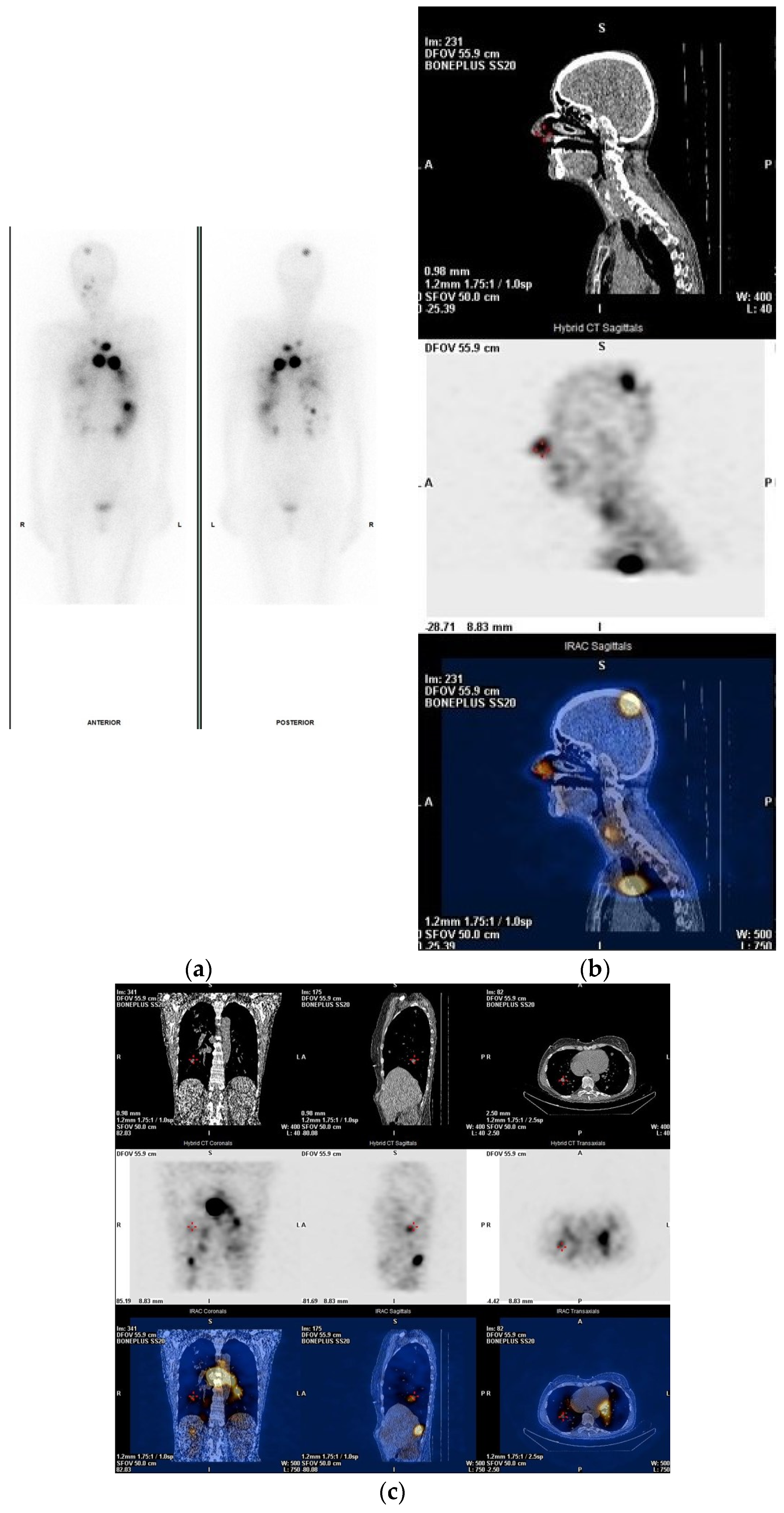
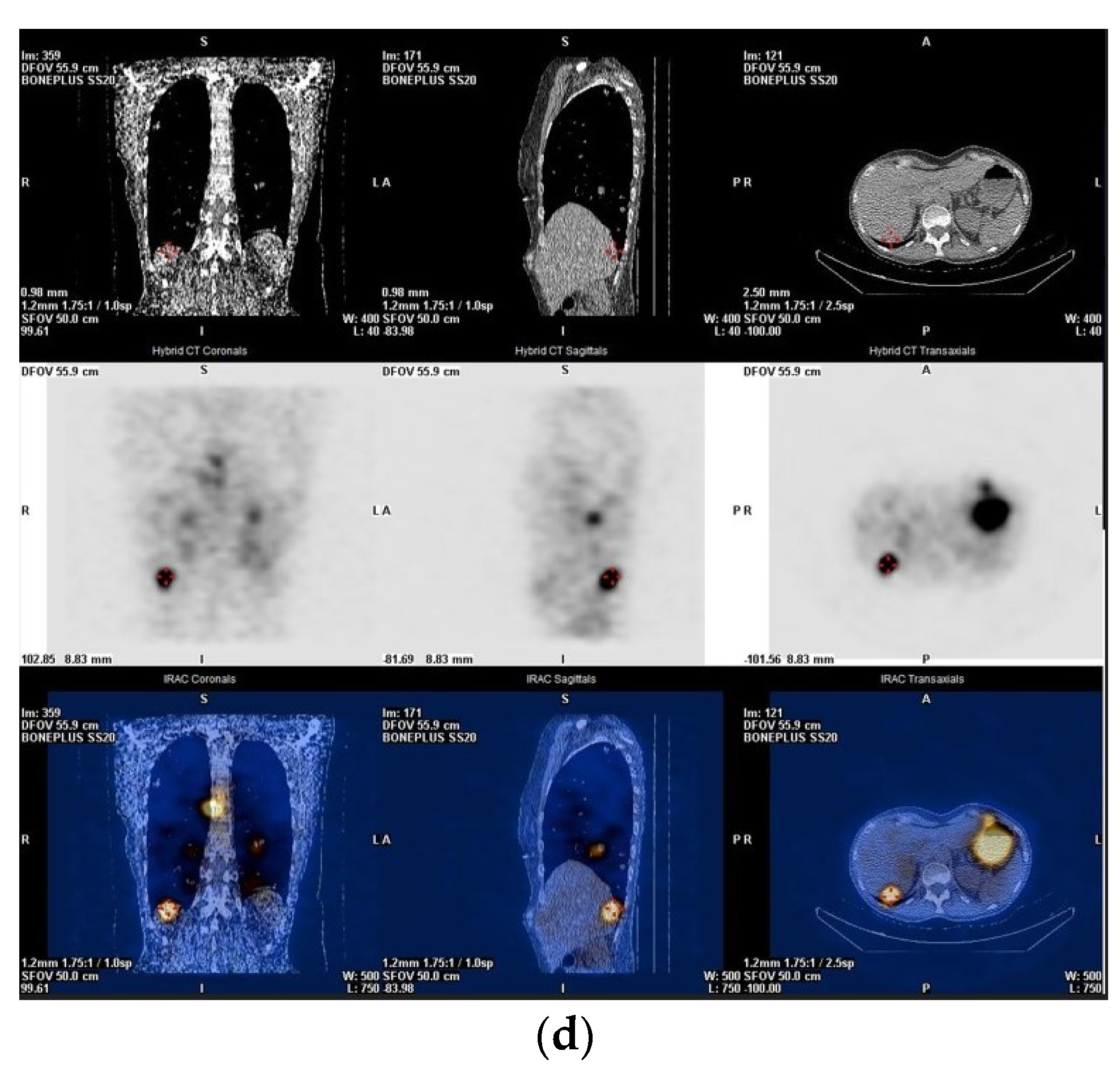
3. Post-Treatment Whole-Body Imaging
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Kuker, R. Radioactive Iodine Remnant Ablation: The Beta-knife Completion Thyroidectomy. Mol. Imaging. Radionucl. Ther. 2017, 26 (Suppl. S1), 16–23. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Pacini, F.; Wiersinga, W.M.; Toft, A.; Smit, J.W.; Sanchez Franco, F.; Lind, P.; Limbert, E.; Jarzab, B.; Jamar, F.; et al. Follow-up and management of differentiated thyroid carcinoma: A European perspective in clinical practice. Eur. J. Endocrinol. 2004, 151, 539–548. [Google Scholar] [CrossRef]
- Silberstein, E.B.; Alavi, A.; Balon, H.R.; Clarke, S.E.M.; Divgi, C.; Gelfand, M.J.; Goldsmith, S.J.; Jadvar, H.; Marcus, C.S.; Martin, W.H.; et al. The SNMMI Practice Guideline for therapy of thyroid disease with I-131 3.0. J. Nucl. Med. 2012, 53, 1633–1651. [Google Scholar] [CrossRef]
- Arnstein, N.B.; Carey, J.E.; Spaulding, S.A.; Sisson, J.C. Determination of iodine-131 diagnostic dose for imaging metastatic thyroid cancer. J. Nucl. Med. 1986, 27, 1764–1769. [Google Scholar] [PubMed]
- Coakley, A.J.; Page, C.J.; Croft, D. Scanning dose and detection of thyroid metastases. J. Nucl. Med. 1980, 21, 803–804. [Google Scholar]
- Waxman, A.; Ramanna, L.; Chapman, N.; Chapman, D.; Brachman, M.; Tanasescu, D.; Berman, D.; Catz, B.; Braunstein, G. The significance of 131-I scan dose in patients with thyroid cancer: Determination of ablation. J. Nucl. Med. 1981, 22, 861–865. [Google Scholar]
- Nemec, J.; Rohling, S.; Zamrazil, V.; Pohunkova, D. Comparison of the distribution of diagnostic and thyroablative 131-I in the evaluation of differentiated thyroid cancers. J. Nucl. Med. 1979, 20, 92–97. [Google Scholar]
- Pacini, F.; Lippi, F.; Formica, N.; Elisei, R.; Anelli, S.; Ceccarelli, C.; Pinchera, A. Therapeutic doses of iodine-131 reveal undiagnosed metastases in thyroid cancer patients with detectable serum thyroglobulin levels. J. Nucl. Med. 1987, 28, 1888–1891. [Google Scholar]
- Rawson, R.W.; Rall, J.E.; Peacock, W. Limitations and indications in the treatment of cancer of the thyroid with radioactive iodine. J. Clin. Endocrinol. Metab. 1951, 11, 1128–1131. [Google Scholar] [CrossRef]
- Jeevenram, R.K.; Shah, D.H.; Sharma, S.M.; Ganatra, R.D. Influence of initial large dose on subsequent uptake of therapeutic radioiodine in thyroid cancer patients. Nucl. Med. Biol. 1986, 13, 277–279. [Google Scholar] [CrossRef]
- Park, H.M.; Perkins, O.W.; Edmondson, J.W.; Schnute, R.B.; Manatunga, A. Influence of diagnostic radioiodines on the uptake of ablative dose of iodine131. Thyroid 1994, 4, 49–54. [Google Scholar] [CrossRef]
- Park, H.M. Stunned thyroid after high-dose I-131 imaging. Clin. Nucl. Med. 1992, 17, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Coakley, A.J. Thyroid stunning. Eur. J. Nucl. Med. 1998, 25, 203–204. [Google Scholar] [CrossRef]
- Muratet, J.P.; Daver, A.; Minier, J.F.; Larra, F. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. J. Nucl. Med. 1998, 39, 1546–1550. [Google Scholar]
- Lees, W.; Mansberg, R.; Roberts, J.; Towson, J.; Chua, E.; Turtle, J. The clinical effects of thyroid stunning after diagnostic whole-body scanning with 185 MBq I-131. Eur. J. Nucl. Med. 2002, 29, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Verkoiijen, R.B.; Stokkel, M.P.; van Isselt, J.W. The success of 131-I ablation in thyroid cancer patients is significantly reduced after a diagnostic activity of 40 MBq 131-I. Nuklearmedizin-NuclearMedicine 2009, 48, 138–142. [Google Scholar]
- Van Nostrand, D.; Aiken, M.; Atkins, F.; Moreau, S.; Garcia, C.; Acio, E.; Burman, K.; Wartofsky, L. The Utility of Radioiodine Scans Prior to 131-I Ablation in Patients with Well-Differentiated Thyroid Cancer. Thyroid 2009, 19, 849–855. [Google Scholar] [CrossRef] [PubMed]
- van der Boom, T.; Zandee, W.T.; Dekkers, C.C.J.; van der Horst-Schrivers, A.N.A.; Jansen, L.; Kruijff, S.; Brouwers, A.H.; Links, T.P. The Value of Pre-Ablative I-131 Scan for Clinical Management in Patients with Differentiated Thyroid Carcinoma. Front. Endocrinol. 2021, 12, 655676. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.J.; Shankar, L.K.; Benard, F.; Yamamoto, A.; Alavi, A. Superiority of iodine-123 compared with iodine-131 scanning for thyroid remnants in patients with differentiated thyroid cancer. Clin. Nucl. Med. 2001, 26, 6–9. [Google Scholar] [CrossRef]
- Park, H.M.; Gerard, S.K. Stunning. In Thyroid Cancer: A Comprehensive Guide to Clinical Management, 2nd ed.; Wartofsky, L., Van Nostrand, D., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 337–344. [Google Scholar]
- Alazhrani, A.S.; Bakheet, S.; Mandil, M.A.; Al-Hajjaj, A.; Almahfouz, A.; Al-Haj, A. 123-I Isotope as a Diagnostic Agent in the Follow-Up of Patients with Differentiated Thyroid Cancer: Comparison with Post 131-I Therapy Whole Body Scanning. J. Clin. Endocrinol. Metab. 2001, 86, 5294–5300. [Google Scholar] [CrossRef]
- Chen, M.K.; Yasrebi, M.; Samii, J.; Staib, L.H.; Doddamane, I.; Cheng, D.W. The Utility of I-123 Pretherapy Scan in I-131 Radioiodine Therapy for Thyroid Cancer. Thyroid 2012, 22, 304–309. [Google Scholar] [CrossRef]
- Song, H.; Mosci, C.; Akatsu, H.; Basina, M.; Dosiou, C.; Iagaru, A. Diagnostic 123-I whole body scan prior to ablation of thyroid remnant in patients with papillary thyroid cancer: Implications for clinical management. Clin. Nucl. Med. 2018, 43, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.A.; Kuker, R.A.; Goryawala, M.; Fernandez, C.; Perez, R.; Khan-Ghany, A.; Apaza, A.; Harja, E.; Harrell, M. (124)-I PET/CT in Patients with Differentiated Thyroid Cancer: Clinical and Quantitative Image Analysis. Thyroid 2016, 26, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M. Radioiodine scintigraphy with SPECT/CT: An important diagnostic tool for thyroid cancer staging and risk stratification. J. Nucl. Med. Technol. 2014, 42, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Sisson, J.C.; Koral, K.F.; Frey, K.A.; Avram, A.M. Staging of differentiated thyroid carcinoma using diagnostic 131-I SPECT/CT. Am. J. Roentgenol. 2010, 195, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Fig, L.M.; Frey, K.A.; Gross, M.D.; Wong, K.K. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: What is the impact on staging? J. Clin. Endocrinol. Metab. 2013, 98, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Esfandiari, N.H.; Wong, K.K. Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J. Clin. Endocrinol. Metab. 2015, 100, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Danilovic, D.L.; Coura-Filho, G.B.; Recchia, G.M.; Castroneves, L.A.; Mariu, S.; Buchpiguel, A.C.; Hoff, A.O.; Kopp, P. Is there a role for diagnostic scans in the management of intermediate-risk thyroid cancer? Endocr. Relat. Cancer 2022, 29, 475–483. [Google Scholar] [CrossRef]
- Avram, A.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Petranović Ovčariček, P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI procedure standard/EANM practice guideline for nuclear medicine evaluation and therapy of differentiated thyroid cancer: Abbreviated version. J. Nucl. Med. Mol. Imaging 2022, 63, 15N–35N. [Google Scholar]
- Luster, M.; Clarke, S.E.; Dietlin, M.; Lassman, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E.; European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard, B.G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81 (Suppl. S1), 1–122. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thyroid Carcinoma Version 4.2023; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2023; Available online: https://www.nccn.org/guidelines/nccn-guidelines (accessed on 24 December 2023).
- Schlumberger, M.J.; Leboulleux, S.M.; Pacini, F. Follow-up of patients with differentiated thyroid carcinoma. In Practical Management of Thyroid Cancer; Mazzaferri, E.L., Harmer, C., Mallick, U.J., Kendall-Taylor, P., Eds.; Springer: New York, NY, USA, 2006; pp. 229–236. [Google Scholar]
- Amdur, R.J.; Mazzaferri, E.L. The role of a diagnostic radioiodine whole body scan (DxWBS). In Essentials of Thyroid Cancer Management; Amdur, R.J., Mazzaferri, E.L., Eds.; Springer: New York, NY, USA, 2005; pp. 61–64. [Google Scholar]
- Arjani, S.; Quinn, P.L.; Chokshi, R.J. Preablation Diagnostic Whole-body Scan vs empiric radioactive iodine ablation in differentiated thyroid cancer: Cost-effectiveness analysis. Otolaryngol. Head Neck Surg. 2021, 164, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.A.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Draganescu, C.; Elisei, R.; Giovanella, L.; Grant, F.; Greenspan, B.; et al. A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on Current Diagnostic and Theranostic Approaches in the Management of Thyroid Cancer. Thyroid 2021, 31, 1009–1019. [Google Scholar]
- Van Nostrand, D.; Veytsman, I.; Kulkarni, K.; Heimlich, L.; Burman, K.D. Redifferentiation of Differentiated Thyroid Cancer: Clinical Insights from a Narrative Review of Literature. Thyroid 2023, 33, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Genzyme. Thyrogen; [Package Insert]; Genzyme: Cambridge, MA, USA, 2008. [Google Scholar]
- Ladenson, P.W.; Braverman, L.E.; Mazzaferri, E.L.; Brucker-Davis, F.; Cooper, D.S.; Garber, J.R.; Wondisford, F.E.; Davies, T.F.; DeGroot, L.J.; Daniels, G.H.; et al. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N. Engl. J. Med. 1997, 337, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Pacini, F.; Reiners, C.; Schlumberger, M.; Ladenson, P.W.; Sherman, S.I.; Cooper, D.S.; Graham, K.E.; Braverman, L.E.; Skarulis, M.C.; et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J. Clin. Endocrinol. Metab. 1999, 84, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyr. J. 2022, 11, e210046. [Google Scholar] [CrossRef] [PubMed]
- Zeuren, R.; Biagini, A.; Grewal, R.K.; Randolph, G.W.; Kamani, D.; Sabra, M.M.; Shaha, A.R.; Tuttle, R.M. RAI thyroid bed uptake after total thyroidectomy: A novel SPECT-CT anatomic classification system. Laryngoscope 2015, 125, 2417–2424. [Google Scholar]
- Seregni, E.; Mallia, A.; Chiesa, C.; Scaramellini, G.; Massimino, M.; Bombardieri, E. Radioiodine therapy of differentiated thyroid cancer. In Nuclear Medicine Therapy. Principles and Clinical Applications; Kumali, A., Goldsmith, S.J.G., Eds.; Springer Science + Business: New York, NY, USA, 2013; pp. 133–153. [Google Scholar]
- Benua, R.S.; Cicale, N.R.; Sonenberg, M.; Rawson, R.W. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1962, 87, 171–182. [Google Scholar] [PubMed]
- Lassmann, M.; Hänscheid, H.; Chiesa, C.; Hindorf, C.; Flux, G.; Luster, M. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1405–1412. [Google Scholar] [CrossRef]
- Souza Rosario, P.W.; Barroso, A.L.; Rezende, L.L.; Padrao, E.L.; Fagundes, T.A.; Penna, G.C.; Purisch, S. Post I-131 therapy scanning in patients with thyroid carcinoma metastases: An unnecessary cost or a relevant contribution? Clin. Nucl. Med. 2004, 29, 795–798. [Google Scholar] [CrossRef]
- Sherman, S.I.; Tielens, E.T.; Sostre, S.; WharamJr, M.D.; Ladenson, P.W. Clinical utility of post-treatment radioiodine scans in the management of patients with thyroid carcinoma. J. Clin. Endocrinol. Metab. 1994, 78, 629–634. [Google Scholar]
- Agate, L.; Bianchi, F.; Brozzi, F.; Santini, P.; Molinaro, E.; Bottici, V.; Viola, D.; Lorusso, L.; Vitti, P.; Elisei, R. Less than 2% of the low- and intermediate-risk differentiated thyroid cancers show distant metastases at post-ablation whole-body scan. Eur. Thyr. J. 2019, 8, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.T.; Huang, S.H.; Huang, Y.E.; Wang, P.W. Appropriate time for post-therapeutic I-131 whole body scan. Clin. Nucl. Med. 2009, 34, 339–342. [Google Scholar] [CrossRef]
- Salvatori, M.; Perotti, G.; Villani, M.F.; Mazza, R.; Maussier, M.L.; Indovina, L.; Sigismondi, A.; Dottorini, M.E.; Giordano, A. Determining the appropriate time of execution of an I-131 post-therapy whole-body scan: Comparison between early and late imaging. Nucl. Med. Commun. 2013, 34, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.; Song, H.C.; Min, J.J.; Jeong, S.Y.; Ha, J.M.; Kim, J.; Yoo, S.U.; Oh, J.R.; Bom, H.S. Improved detection of lung or bone metastases with an I-131 whole body scan on the 7th day after high-dose I-131 therapy in patients with thyroid cancer. Nucl. Med. Mol. Imaging 2010, 44, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kodani, N.; Okuyama, C.; Aibe, N.; Matsushima, S.; Yamazaki, H. Utility of additional delayed post-therapeutic 131-I whole-body scanning in patients with thyroid cancer. Clin. Nucl. Med. 2012, 37, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Ruf, J.; Lehmkuhl, L.; Bertram, H.; Sandrock, D.; Amthauer, H.; Humplik, B.; Munz, D.L.; Felix, R. Impact of SPECT and integrated low-dose CT after radioiodine therapy on the management of patients with thyroid carcinoma. Nucl. Med. Commun. 2004, 25, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Tharp, K.; Israel, O.; Hausmann, J.; Bettman, L.; Martin, W.H.; Daitzchman, M.; Sandler, M.P.; Delbeke, D. Impact of 131I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1435–1442. [Google Scholar] [CrossRef]
- Aide, N.; Heutte, N.; Rame, J.P.; Rousseau, E.; Loiseau, C.; Henry-Amar, M.; Bardet, S. Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in postablation 131-I scintigraphy for thyroid cancer. J. Clin. Endocrinol. Metab. 2009, 94, 2075–2084. [Google Scholar] [CrossRef]
- Ciappuccini, R.; Heutte, N.; Trzepla, G.; Rame, J.P.; Vaur, D.; Aide, N.; Bardet, S. Post ablation 131-Iscintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur. J. Endocrinol. 2011, 164, 961–969. [Google Scholar] [CrossRef]
- Malamitsi, J.V.; Koutsikos, J.T.; Giourgouli, S.I.; Zachki, S.F.; Pipikos, T.A.; Vlacho, F.I.; Prassopoulos, V.K. I-131 postablation SPECT/CT predicts relapse of papillary thyroid carcinoma more accurately than whole body scan. In Vivo 2019, 33, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, H.; Mele, L.; De Vito, A.; Rondini, M.; Madeddu, G. The diagnostic usefulness of I-131 SPECT/CT at both radioiodine ablation and during long-term follow-up in patients thyroidectomized for differentiated thyroid carcinoma: Analysis of tissue risk factors ascertained at surgery and correlated with metastasis appearance. Diagnostics 2021, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Szujo, S.; Sira, L.; Bajnok, L.; Bodis, B.; Gyori, F.; Nemes, O.; Rucz, K.; Kenyeres, P.; Valkusz, Z.; Sepp, K.; et al. The impact of post-radioiodine therapy SPECT/CT on early risk stratification in differentiated thyroid cancer; a bi-institutional study. Oncotarget 2017, 8, 79825–79834. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.K.; Tuttle, R.M.; Fox, J.; Borkar, S.; Chou, J.F.; Gonen, M.; Strauss, H.W.; Larson, S.M.; Schoder, H. The effect of posttherapy 131-I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J. Nucl. Med. 2010, 51, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Szikszai, A.; Linke, R.; Bautz, W.; Kuwert, T. Impact of 131-I SPECT/spiral CT on nodal staging of differentiated hyroid carcinoma at the first radioablation. J. Nucl. Med. 2009, 50, 18–23. [Google Scholar] [CrossRef]
- Mustafa, M.; Kuwert, T.; Weber, K.; Knesewitsch, P.; Negele, T.; Haug, A.; Linke, R.; Bartenstein, P.; Schmidt, D. Regional lymph node involvement in T1 papillary thyroid carcinoma: A bicentric prospective SPECT/CT study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1462–1466. [Google Scholar] [CrossRef]
- Xue, Y.L.; Qiu, Z.L.; Song, H.J.; Luo, Q.Y. Value of 131-I SPECT/CT for the evaluation of differentiated thyroid cancer: A systematic review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Kohlfuerst, S.; Igerc, I.; Lobnig, M.; Gallowitsch, H.J.; Gomez-Segovia, I.; Matschnig, S.; Mayr, J.; Mikosch, P.; Beheshti, M.; Lind, P. Posttherapeutic (131)-I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Nekolla, S.; Ziegler, S.; Beer, A.; Krause, B.J.; Herrmann, K.; Scheidhauer, K.; Wester, H.J.; Rummeny, E.J.; Schwaiger, M.; et al. SPECT/CT. J. Nucl. Med. 2008, 49, 1305–1319. [Google Scholar] [CrossRef]
- Gelfand, M.J.; Lemen, L.C. PET/CT and SPECT/CT dosimetry in children: The challenge to the pediatric imager. Semin. Nucl. Med. 2007, 37, 391–398. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihailović, J. Pre-Treatment and Post-Treatment I-131 Imaging in Differentiated Thyroid Carcinoma. J. Clin. Med. 2024, 13, 1984. https://doi.org/10.3390/jcm13071984
Mihailović J. Pre-Treatment and Post-Treatment I-131 Imaging in Differentiated Thyroid Carcinoma. Journal of Clinical Medicine. 2024; 13(7):1984. https://doi.org/10.3390/jcm13071984
Chicago/Turabian StyleMihailović, Jasna. 2024. "Pre-Treatment and Post-Treatment I-131 Imaging in Differentiated Thyroid Carcinoma" Journal of Clinical Medicine 13, no. 7: 1984. https://doi.org/10.3390/jcm13071984
APA StyleMihailović, J. (2024). Pre-Treatment and Post-Treatment I-131 Imaging in Differentiated Thyroid Carcinoma. Journal of Clinical Medicine, 13(7), 1984. https://doi.org/10.3390/jcm13071984





