The Role of Index of Microcirculatory Resistance in Left Anterior Descending Artery ST Segment Elevation Myocardial Infarction Patients after Primary Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Definition of Variables and Measurements
2.3. Intra Coronary Physiologic Measurements
2.4. Endpoints Determination and Follow-Up Data Acquisition
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Angiographic Characteristics
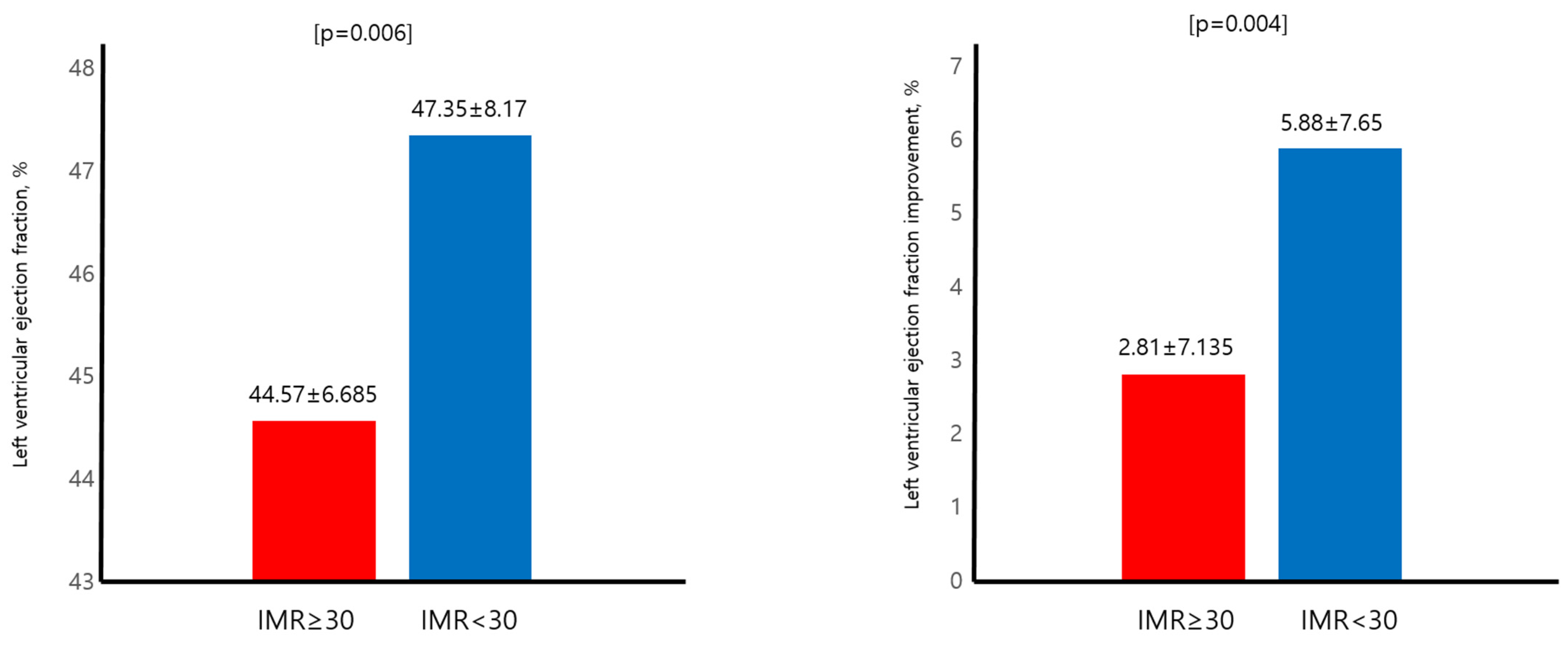
3.3. Primary Endpoint and LVEF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foerst, J.; Vorpahl, M.; Engelhardt, M.; Koehler, T.; Tiroch, K.; Wessely, R. Evolution of coronary stents: From bare-metal stents to fully biodegradable, drug-eluting stents. Comb. Prod. Ther. 2013, 3, 9–24. [Google Scholar] [CrossRef]
- Rodriguez, F.; Mahaffey, K.W. Management of patients with NSTE-ACS: A comparison of the recent AHA/ACC and ESC guidelines. J. Am. Coll. Cardiol. 2016, 68, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wessler, J.D.; Stant, J.; Duru, S.; Rabbani, L.; Kirtane, A.J. Updates to the ACCF/AHA and ESC STEMI and NSTEMI guidelines: Putting guidelines into clinical practice. Am. J. Cardiol. 2015, 115, 23A–28A. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Cohen, M.; Bernink, P.J.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI risk score for unstable angina/non–ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000, 284, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.W.; Wong, C.-K.; Herbison, P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am. Heart J. 2007, 153, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Jeong, M.H.; Ahn, Y.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; Choi, D.H. Hospital discharge risk score system for the assessment of clinical outcomes in patients with acute myocardial infarction (Korea Acute Myocardial Infarction Registry [KAMIR] score). Am. J. Cardiol. 2011, 107, 965–971.e1. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.O.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- Suda, A.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Shindo, T.; Ikeda, S.; Sato, K.; Sugisawa, J.; Matsumoto, Y.; Miyata, S.; et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J. Am. Coll. Cardiol. 2019, 74, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of microcirculatory resistance as a tool to characterize microvascular obstruction and to predict infarct size regression in patients with STEMI undergoing primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamagishi, M.; Ueno, T.; Hara, K.; Ishiwata, S.; Itoh, T.; Hamanaka, I.; Wakatsuki, T.; Sugano, T.; Kawai, K.; et al. Prevalence of visual–functional mismatch regarding coronary artery stenosis in the CVIT-DEFER registry. Cardiovasc. Interv. Ther. 2014, 29, 300–308. [Google Scholar] [CrossRef]
- Park, S.-J.; Kang, S.-J.; Ahn, J.-M.; Shim, E.B.; Kim, Y.-T.; Yun, S.-C.; Song, H.; Lee, J.-Y.; Kim, W.-J.; Park, D.-W.; et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc. Interv. 2012, 5, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- ESC Committee for Practice Guidelines (CPG); Bax, J.J.; Baumgartner, H.; Ceconi, C.; Dean, V.; Deaton, C.; Fagard, R.; Funck-Brentano, C.; Hasdai, D.; Hoes, A. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar]
- Wu, Z.; Ye, F.; You, W.; Zhang, J.; Xie, D.; Chen, S. Microcirculatory significance of periprocedural myocardial necrosis after percutaneous coronary intervention assessed by the index of microcirculatory resistance. Int. J. Cardiovasc. Imaging 2014, 30, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Terentes-Printzios, D.; Shanmuganathan, M.; Marin, F.; Scarsini, R.; Bradley-Watson, J.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.; Kharbanda, R.K.; et al. Long-term clinical outcomes in patients with an acute ST-segment-elevation myocardial infarction stratified by angiography-derived index of microcirculatory resistance. Front. Cardiovasc. Med. 2021, 8, 717114. [Google Scholar] [CrossRef]
- McAlindon, E.; Pufulete, M.; Harris, J.; Lawton, C.; Johnson, T.; Strange, J.; Baumbach, A.; Bucciarelli-Ducci, C. Microvascular dysfunction determines infarct characteristics in patients with reperfused ST-segment elevation myocardial infarction: The MICROcirculation in Acute Myocardial Infarction (MICRO-AMI) study. PLoS ONE 2018, 13, e0203750. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Che, W.; Liu, L.; Zhang, W.; Yin, G.; Xu, B.; Xu, Y.; Duan, S.; Yu, H.; Li, C.; et al. Diagnostic value of angiography-derived IMR for coronary microcirculation and its prognostic implication after PCI. Front. Cardiovasc. Med. 2021, 8, 735743. [Google Scholar] [CrossRef] [PubMed]
- Grüntzig, A. Transluminal dilatation of coronary-artery stenosis. Lancet 1978, 311, 263. [Google Scholar] [CrossRef] [PubMed]
- Canfield, J.; Totary-Jain, H. 40 years of percutaneous coronary intervention: History and future directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Giannitsis, E.; Blankenberg, S.; Christenson, R.H.; Frey, N.; von Haehling, S.; Hamm, C.W.; Inoue, K.; Katus, H.A.; Lee, C.-C.; McCord, J.; et al. Critical appraisal of the 2020 ESC guideline recommendations on diagnosis and risk assessment in patients with suspected non-ST-segment elevation acute coronary syndrome. Clin. Res. Cardiol. 2021, 110, 1353–1368. [Google Scholar] [CrossRef]
- Prejean, S.P.; Din, M.; Reyes, E.; Hage, F.G. Guidelines in review: Comparison of the 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes and the 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. J. Nucl. Cardiol. 2018, 25, 769–776. [Google Scholar]
- Grech, E.D. Pathophysiology and investigation of coronary artery disease. BMJ 2003, 326, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Murthy, V.L.; Taqueti, V.R.; Foster, C.R.; Klein, J.; Garber, M.; Dorbala, S.; Hainer, J.; Blankstein, R.; Resnic, F.; et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J. Nucl. Med. 2014, 55, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hennigan, B.; Layland, J.; Fearon, W.F.; Oldroyd, K.G. Fractional flow reserve and the index of microvascular resistance in patients with acute coronary syndromes. EuroIntervention 2014, 10, T55–T63. [Google Scholar] [CrossRef]
- Williams, R.P.; de Waard, G.A.; De Silva, K.; Lumley, M.; Asrress, K.; Arri, S.; Ellis, H.; Mir, A.; Clapp, B.; Chiribiri, A.; et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am. J. Cardiol. 2018, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.-S.; Ahn, S.G.; Woo, S.-I.; Yoon, M.H.; Lee, M.-J.; Choi, S.H.; Seo, J.-Y.; Kwon, S.W.; Park, S.-D.; Seo, K.-W. The Index of Microcirculatory Resistance after Primary Percutaneous Coronary Intervention Predicts Long-Term Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction. J. Clin. Med. 2021, 10, 4752. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Foin, N.; Cabrera-Fuentes, H.A.; Yeo, K.K.; Wong, A.S.; Fam, J.M.; Wong, P.E.; Tan, J.W.; Low, A.F.; Hausenloy, D.J. Index of microvascular resistance and microvascular obstruction in patients with acute myocardial infarction. Cardiovasc. Interv. 2016, 9, 2172–2174. [Google Scholar] [CrossRef]
- Wijnbergen, I.; van’t Veer, M.; Lammers, J.; Ubachs, J.; Pijls, N.H. Absolute coronary blood flow measurement and microvascular resistance in ST-elevation myocardial infarction in the acute and subacute phase. Cardiovasc. Revasculariz. Med. 2016, 17, 81–87. [Google Scholar] [CrossRef]


| Baseline Characteristics | Total Population (246) | IMR ≥ 30 (93) | IMR < 30 (153) | p Value |
|---|---|---|---|---|
| Age, years | 55.47 ± 11.30 | 57.91 ± 11.99 | 54 ± 10.63 | 0.008 |
| Gender (male), n% | 216 (87.8%) | 76 (81.7%) | 140 (91.5%) | 0.023 |
| BMI, kg/m2 | 24.45 ± 3.01 | 24.52 ± 3.50 | 24.39 ± 2.68 | 0.741 |
| SBP, mmHg | 133.44 ± 23.12 | 136.93 ± 25.56 | 131.55 ± 21.56 | 0.117 |
| DBP, mmHg | 82.64 ± 15.95 | 84 ± 18.1 | 81.91 ± 14.74 | 0.377 |
| Heart rate, bpm | 79.06 ± 15.15 | 77.61 ± 13.77 | 79.83 ± 15.83 | 0.325 |
| HTN | 91 (36.8%) | 37 (39.8%) | 54 (35.1%) | 0.456 |
| DM | 61 (24.7%) | 24 (25.8%) | 37 (24%) | 0.753 |
| Dyslipidemia | 115 (46.6%) | 28 (30.1%) | 87 (56.5%) | <0.001 |
| Smoking | 194 (78.5%) | 70 (75.3%) | 124 (80.5%) | 0.33 |
| Prior PCI | 2 (1.3%) | 1 (1.5%) | 1 (1.1%) | 0.831 |
| Door-to-balloon time, min | 81.27 ± 89.29 | 80.45 ± 82.55 | 81.78 ± 93.49 | 0.911 |
| Symptom-to-balloon time, min | 357.47 ± 917.75 | 412.05 ± 1040.84 | 323.54 ± 833.71 | 0.469 |
| Symptom-to-door time, min | 322.10 ± 1140.32 | 386.72 ± 1248.12 | 275.10 ± 1059.958 | 0.553 |
| Serum creatinin | 1.00 ± 0.29 | 0.98 ± 0.34 | 1.02 ± 0.26 | 0.418 |
| Ntpro BNP | 929.51 ± 3719.96 | 1501.97 ± 5059.89 | 547.87 ± 2455.89 | 0.28 |
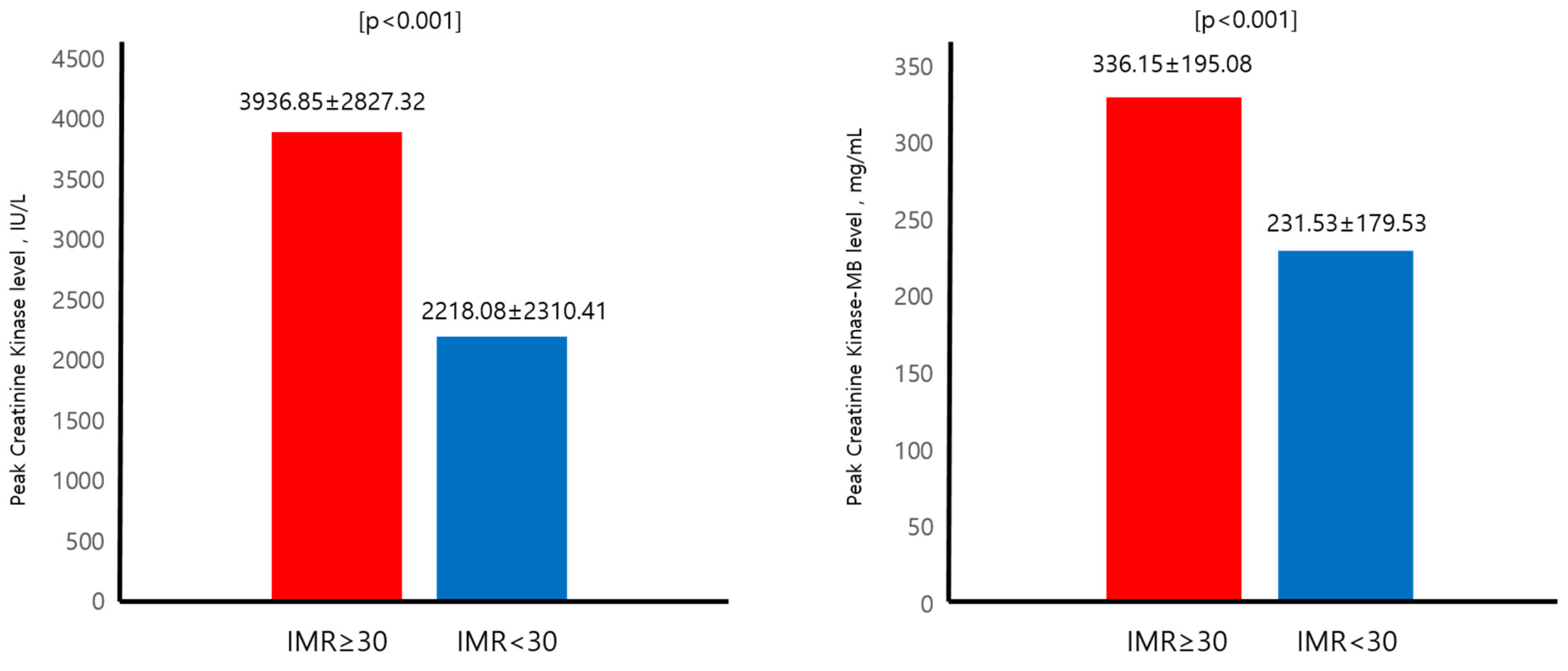
| Peak CK, IU/L | 2808.36 ± 2623.66 | 3936.85 ± 2827.32 | 2218.08 ± 2310.41 | <0.001 |
| Peak CK-MB, mg/mL | 270.66 ± 191.93 | 336.15 ± 195.08 | 231.53 ± 179.53 | <0.001 |
| Peak Trop-I, ng/mL | 66.13 ± 73.53 | 76.15 ± 82.15 | 60.39 ± 67.74 | 0.122 |
| IMR | 29.70 ± 20.68 | 49.92 ± 20.91 | 17.56 ± 5.44 | <0.001 |
| All-cause mortality | 9 (3.7%) | 7 (7.5%) | 2 (1.3%) | 0.012 |
| Medication | ||||
| clopidogrel | 210 (85.3%) | 80 (86%) | 130 (84.4%) | 0.732 |
| ticagrelor | 30 (12.2%) | 10 (10.7%) | 20 (13%) | 0.393 |
| prasugrel | 5 (2%) | 1 (1%) | 4 (3%) | 0.299 |
| ARB/ACEi | 127 (51.2%) | 46 (49.5%) | 81 (52%) | 0.842 |
| B-blocker | 132 (53.2%) | 46 (49.5%) | 86 (56%) | 0.231 |
| Statin | 138 (55.65) | 50 (53.7%) | 88 (57%) | 0.452 |
| Baseline Characteristics | IMR ≥ 30 (93) | IMR < 30 (153) | p Value |
|---|---|---|---|
| Number of vessels, n (%) | 0.017 | ||
| 1 | 53 (57.6%) | 110 (71.4%) | |
| 2 | 35 (38%) | 33 (21.4%) | |
| 3 | 4 (4.3%) | 11 (7.1%) | |
| TIMI grade before PCI, n (%) | 0.001 | ||
| 0 | 51 (57.3%) | 51 (36.7%) | |
| 1 | 19 (21.3%) | 29 (20.9%) | |
| 2 | 17 (19.1%) | 38 (27.3%) | |
| 3 | 2 (2.2%) | 21 (15.1%) | |
| DES characteristics | |||
| Stent diameter, mm | 3.19 ± 0.38 | 3.18 ± 0.32 | 0.81 |
| Stent length, mm | 26.61 ± 9.06 | 25.37 ± 9.9 | 0.327 |
| TMP grade after PCI, n (%) | <0.001 | ||
| 0 | 8 (9.2%) | 0 (0%) | |
| 1 | 16 (18.4%) | 1 (0.8%) | |
| 2 | 31 (35.6%) | 47 (35.6%) | |
| 3 | 32 (36.8%) | 84 (63.6%) | |
| TIMI grade after PCI, n (%) | <0.001 | ||
| 0/1 | 0 | 0 | |
| 2 | 22 (25.6%) | 4 (3.1%) | |
| 3 | 64 (74.4%) | 123 (96.9%) |
| Variables | Univariate Analysis | Mutivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.056 | 0.999–1.117 | 0.055 | |||
| Gender | 0.488 | 0.101–2.350 | 0.371 | |||
| Dyslipidemia | 1.047 | 0.280–3.909 | 0.946 | |||
| BMI | 0.829 | 0.684–1.005 | 0.057 | |||
| Hypertension | 3.719 | 0.929–14.879 | 0.063 | |||
| Diabetes | 2.499 | 0.670–9.319 | 0.173 | |||
| Current smoking | 0.492 | 0.123–1.973 | 0.317 | |||
| Serum CK mb | 1.001 | 0.998–1.004 | 0.413 | |||
| Serum trop | 1.008 | 1.001–1.015 | 0.028 | |||
| Multi vessel disease | 1.667 | 0.447–6.212 | 0.447 | |||
| Initail TIMI flow 0 | 2.531 | 0.633–10.122 | 0.189 | |||
| Symptom to balloon time | 1.000 | 0.994–1.002 | 0.789 | |||
| Door to balloon time | 0.987 | 0.960–1.016 | 0.386 | |||
| LV ejection fraction | 0.871 | 0.784–0.968 | 0.01 | 0.856 | 0.749–0.980 | 0.024 |
| IMR ≥ 30 | 5.755 | 1.195–27.717 | 0.029 | 5.151 | 1.062–24.987 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.H.; Ahn, S.G.; Yoon, M.H.; Seo, K.-W.; Lee, K.-J.; Kwon, S.W.; Park, S.-D.; Woo, S.-I. The Role of Index of Microcirculatory Resistance in Left Anterior Descending Artery ST Segment Elevation Myocardial Infarction Patients after Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 1989. https://doi.org/10.3390/jcm13071989
Choi SH, Ahn SG, Yoon MH, Seo K-W, Lee K-J, Kwon SW, Park S-D, Woo S-I. The Role of Index of Microcirculatory Resistance in Left Anterior Descending Artery ST Segment Elevation Myocardial Infarction Patients after Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2024; 13(7):1989. https://doi.org/10.3390/jcm13071989
Chicago/Turabian StyleChoi, Seong Huan, Sung Gyun Ahn, Myeong Ho Yoon, Kyoung-Woo Seo, Ki-Jeung Lee, Sung Woo Kwon, Sang-Don Park, and Seong-Ill Woo. 2024. "The Role of Index of Microcirculatory Resistance in Left Anterior Descending Artery ST Segment Elevation Myocardial Infarction Patients after Primary Percutaneous Coronary Intervention" Journal of Clinical Medicine 13, no. 7: 1989. https://doi.org/10.3390/jcm13071989
APA StyleChoi, S. H., Ahn, S. G., Yoon, M. H., Seo, K.-W., Lee, K.-J., Kwon, S. W., Park, S.-D., & Woo, S.-I. (2024). The Role of Index of Microcirculatory Resistance in Left Anterior Descending Artery ST Segment Elevation Myocardial Infarction Patients after Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine, 13(7), 1989. https://doi.org/10.3390/jcm13071989





