The Use of Glenoid Structural Allografts for Glenoid Bone Defects in Reverse Shoulder Arthroplasty
Abstract
1. Introduction
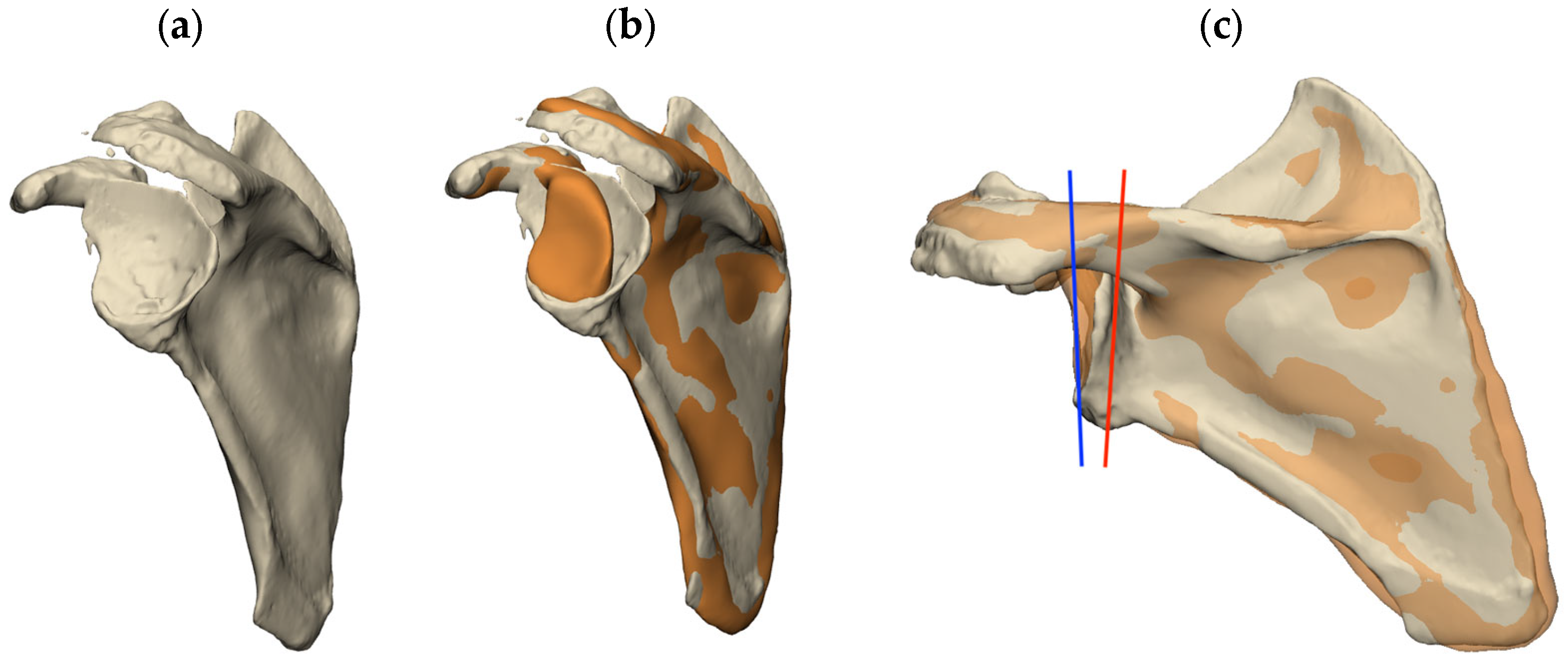
2. Glenoid Anatomy, Wear, and Defects
2.1. Types of Glenoid Bone Defects
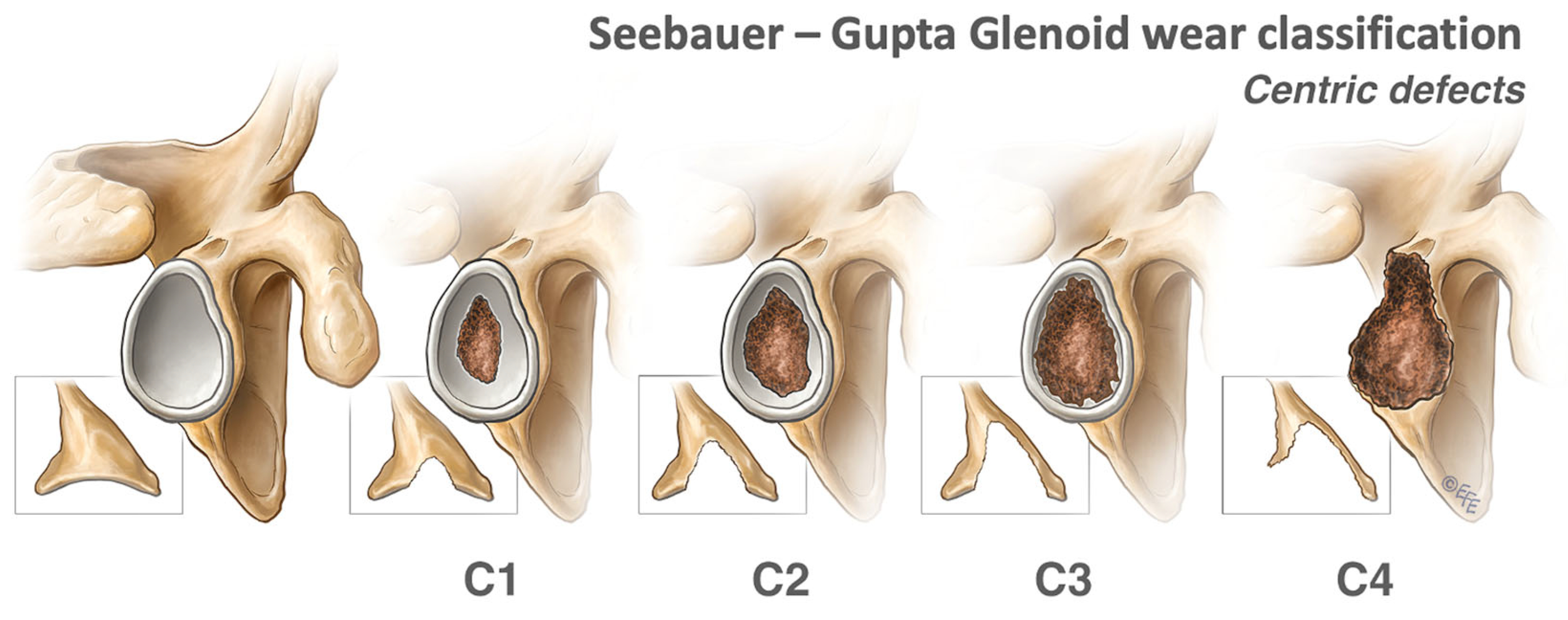
2.1.1. Centric Defects
2.1.2. Eccentric Defects
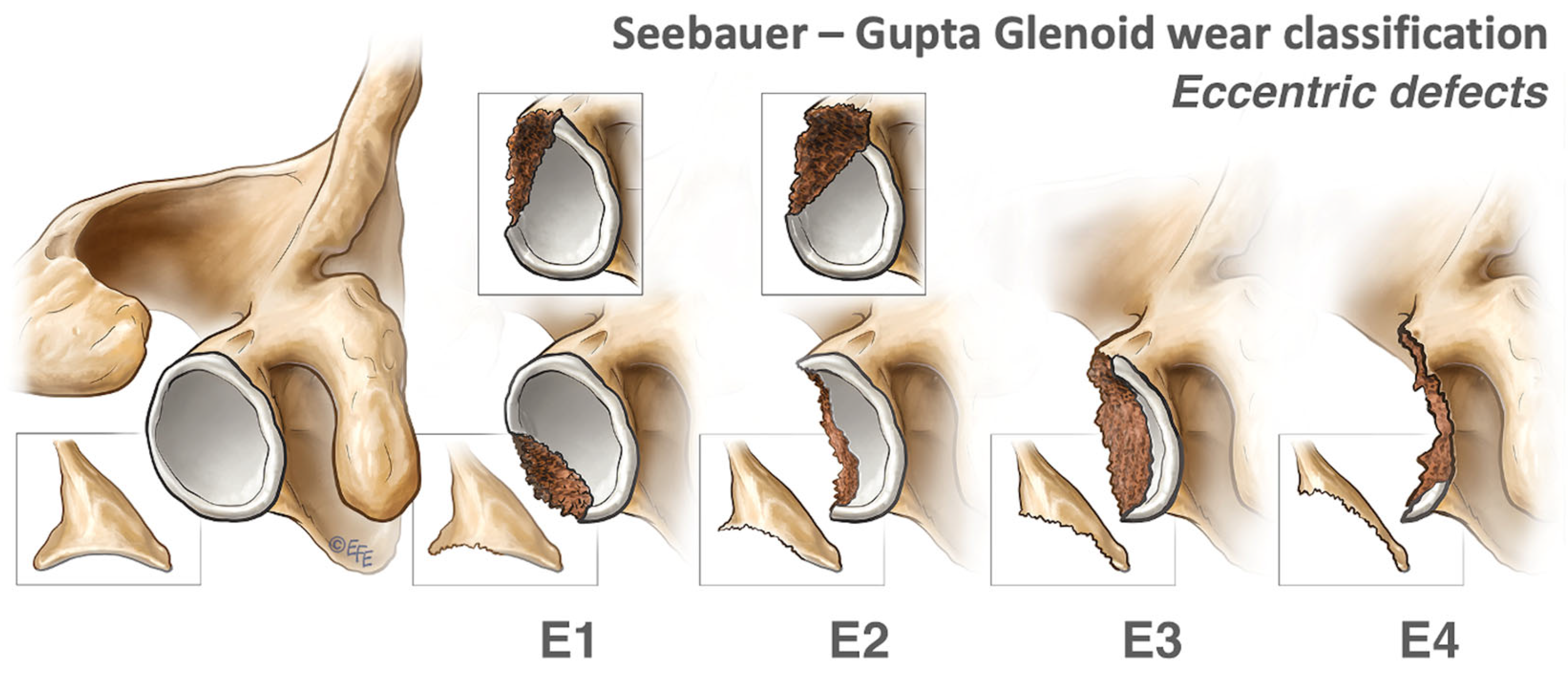
2.2. Indications for Glenoid Grafting
2.3. Joint Line Restoration
2.3.1. Lateralization
2.3.2. Version and Inclination
3. Allograft Types
3.1. Non-Structural Allografts
3.2. Structural Allografts
Composite Grafts
3.3. Bone Types
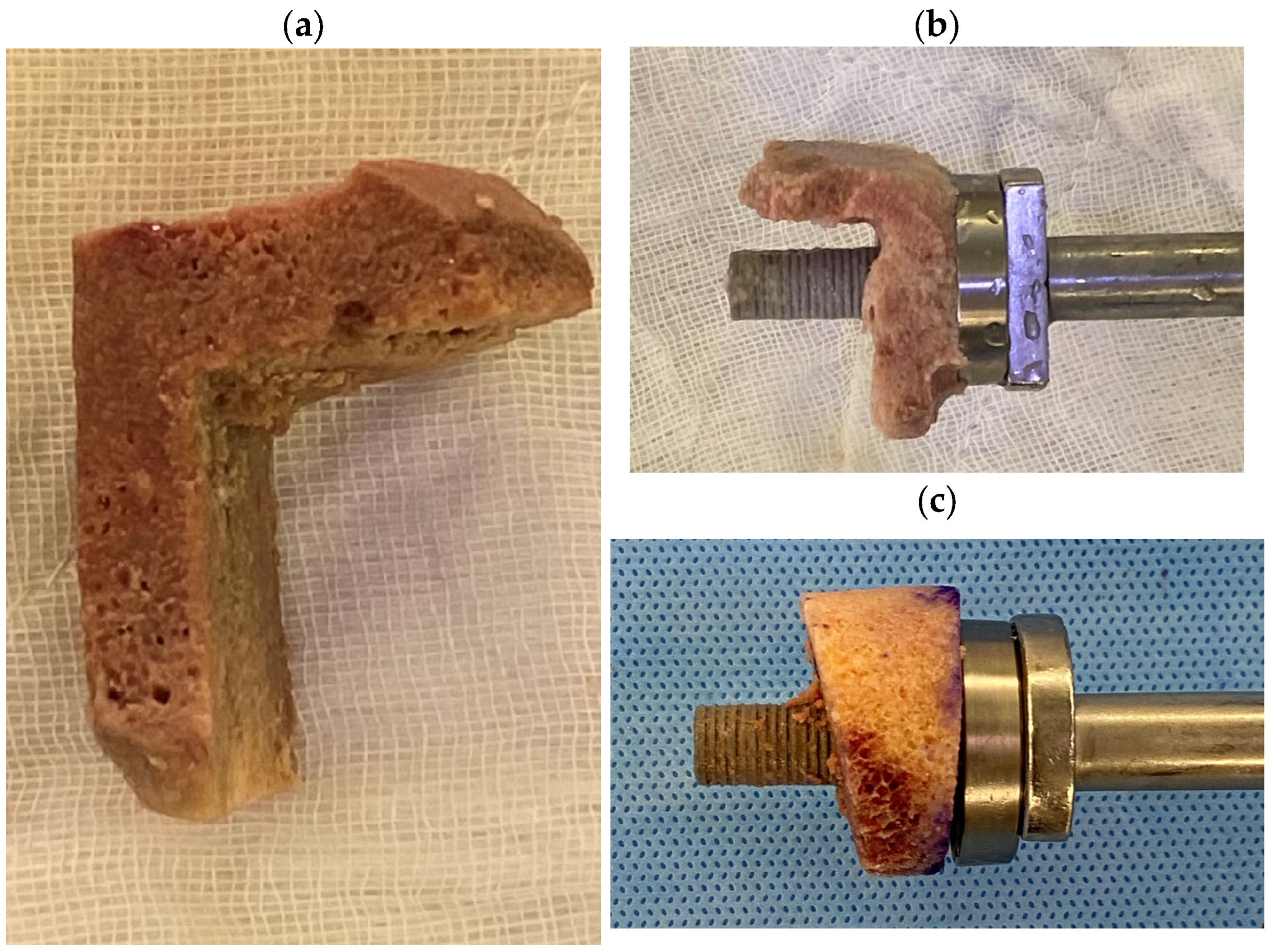
3.4. Allograft Preparation
3.5. Contraindications to Bone Grafting
4. Preoperative Planning and Surgical Technique
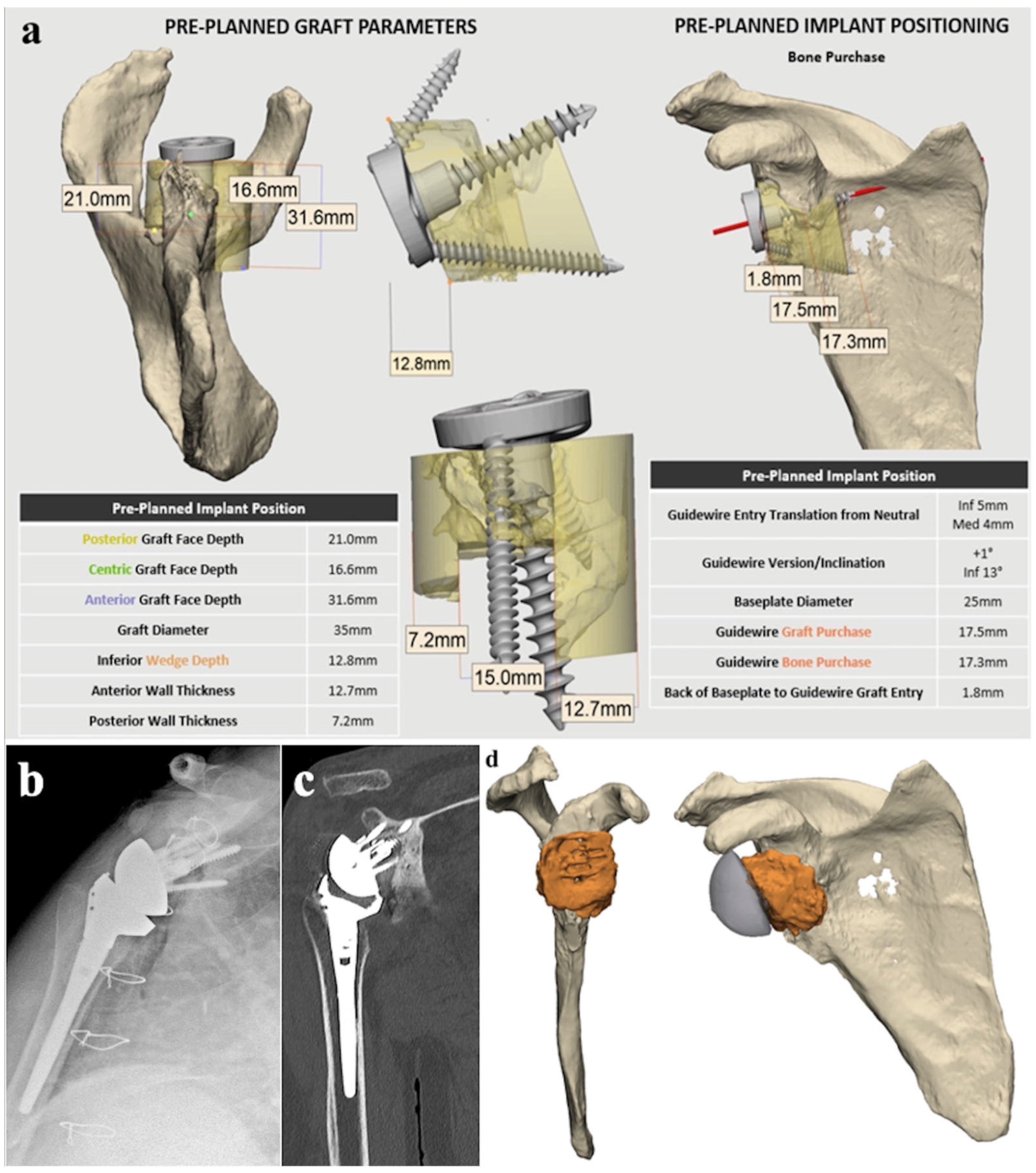
4.1. Preoperative Planning Technique
4.2. Surgical Technique

4.2.1. Glenoid Preparation
4.2.2. Baseplate
4.2.3. Preparation of the Structural Allograft
Cylindrical and Wedge Grafts
“Figure 7” Grafts
4.2.4. Graft Fixation and Position Considerations
4.2.5. Postoperative Protocol
5. Preliminary Results
5.1. Clinical Follow-Up
5.2. Radiographic Follow-Up
5.3. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, E.; Houdek, M.T.; Elhassan, B.T.; Sanchez-Sotelo, J.; Sperling, J.W.; Cofield, R.H. Glenoid Bone-Grafting in Revision to a Reverse Total Shoulder Arthroplasty: Surgical Technique. JBJS Essent. Surg. Tech. 2016, 6, e35. [Google Scholar] [CrossRef] [PubMed]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts. J. Bone Jt. Surg. Br. Vol. 2007, 89-B, 574–580. [Google Scholar] [CrossRef]
- Iannotti, J.P.; Frangiamore, S.J. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J. Shoulder Elb. Surg. 2012, 21, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Collotte, P.; Gauci, M.-O.; Vieira, T.D.; Walch, G. Bony increased-offset reverse total shoulder arthroplasty (BIO-RSA) associated with an eccentric glenosphere and an onlay 135° humeral component: Clinical and radiological outcomes at a minimum 2-year follow-up. JSES Int. 2022, 6, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Walch, G.; Badet, R.; Boulahia, A.; Khoury, A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J. Arthroplast. 1999, 14, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Bercik, M.J.; Kruse, K., 2nd; Yalizis, M.; Gauci, M.O.; Chaoui, J.; Walch, G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J. Shoulder Elb. Surg. 2016, 25, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Thussbas, C.; Koch, M.; Seebauer, L. Management of glenoid bone defects with reverse shoulder arthroplasty-surgical technique and clinical outcomes. J. Shoulder Elb. Surg. 2018, 27, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony Increased Offset-Reversed Shoulder Arthroplasty (BIO-RSA). JBJS Essent. Surg. Tech. 2017, 7, e37. [Google Scholar] [CrossRef] [PubMed]
- Italia, K.; Launay, M.; Gilliland, L.; Nielsen, J.; Pareyon, R.; Hollman, F.; Salhi, A.; Maharaj, J.; Jomaa, M.; Cutbush, K.; et al. Single-Stage Revision Reverse Shoulder Arthroplasty: Preoperative Planning, Surgical Technique, and Mixed Reality Execution. J. Clin. Med. 2022, 11, 7422. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Nicholson, G.P. Management of Glenoid Bone Loss in Primary Reverse Total Shoulder Arthroplasty. Curr. Rev. Musculoskelet. Med. 2023, 16, 358–370. [Google Scholar] [CrossRef]
- Van de Kleut, M.L.; Yuan, X.; Teeter, M.G.; Athwal, G.S. Bony increased-offset reverse shoulder arthroplasty vs. metal augments in reverse shoulder arthroplasty: A prospective, randomized clinical trial with 2-year follow-up. J. Shoulder Elb. Surg. 2022, 31, 591–600. [Google Scholar] [CrossRef]
- Gilliland, L.; Launay, M.; Salhi, A.; Green, N.; Maharaj, J.; Italia, K.R.; Cutbush, K.; Gupta, A. Restoration of glenoid joint line: A three-dimensional analysis of scapular landmarks. JSES Int. 2023, 7, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony increased-offset reversed shoulder arthroplasty: Minimizing scapular impingement while maximizing glenoid fixation. Clin. Orthop. Relat. Res. 2011, 469, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.J.; Kirsch, J.M.; Gates, S.; Patel, M.S.; Joyce, C.D.; Hill, B.W.; Gutman, M.J.; Williams, G.R.; Namdari, S. Three-dimensional measures of posterior bone loss and retroversion in Walch B2 glenoids predict the need for an augmented anatomic glenoid component. J. Shoulder Elb. Surg. 2021, 30, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.R.; Muniz, A.R.; Chang, M.J.; Hunt, T.; Welp, K.M.; Woodmass, J.M.; Higgins, L.; Chen, N. Neuroapraxia and early complications after reverse shoulder arthroplasty with glenoid bone grafting. J. Shoulder Elb. Surg. 2021, 30, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Italia, K.R.; Green, N.; Maharaj, J.; Launay, M.; Gupta, A. Computed tomographic evaluation of glenoid joint line restoration with glenoid bone grafting and reverse shoulder arthroplasty in patients with significant glenoid bone loss. J. Shoulder Elb. Surg. 2021, 30, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.N.G.D.; McAuliffe, M.J.; McDougall, C.; Stoney, J.D.; Vertullo, C.J.; Wall, C.J.; Corfield, S.; Page, R.; Cuthbert, A.R.; Du, P.; et al. . Hip, Knee and Shoulder Arthroplasty: 2023 Annual Report, Australian Orthopaedic Association National Joint Replacement Registry; AOA: Adelaide, South Australia, 2023. [Google Scholar]
- Favard, L.; Lautmann, S.; Sirveaux, F.; Oudet, D.; Kerjean, Y.; Huguet, D. Hemiarthroplasty versus reverse arthroplasty in the treatment of osteoarthritis with massive rotator cuff tear. In 2000 shoulder prostheses. Two to ten year follow-up; Walch, G., Boileau, P., Molé, D., Eds.; Sauramps Medical: Montepellier, France, 2001; pp. 261–268. [Google Scholar]
- Tashjian, R.Z.; Beck, L.; Stertz, I.; Chalmers, P.N. Preoperative three-dimensional computer planning for reverse total shoulder arthroplasty and bone grafting for severe glenoid deformity. Shoulder Elb. 2021, 13, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Favard, L.; Berhouet, J.; Walch, G.; Chaoui, J.; Lévigne, C. Superior glenoid inclination and glenoid bone loss: Definition, assessment, biomechanical consequences, and surgical options. Orthopade 2017, 46, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Rachuene, P.A.; Dey, R.; Sivarasu, S.; du Plessis, J.P.; Roche, S.; Vrettos, B. A narrative review of treatment strategies for major glenoid defects during primary reverse shoulder arthroplasty, with a focus on the use of structural bone graft. EFORT Open Rev. 2023, 8, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Peidro, L.; Segur, J.M.; Poggio, D.; de Retana, P.F. Use of freeze-dried bone allograft with platelet-derived growth factor for revision of a glenoid component. J. Bone Jt. Surg. Br. 2006, 88, 1228–1231. [Google Scholar] [CrossRef]
- Di Felice Ardente, P.; Fusaro, F.M.; Abad, M.P.; Soldado, F.; Coll, J.Q. The utilization of computer planning and 3D-printed guide in the surgical management of a reverse Hill-Sachs lesion. JSES Int. 2020, 4, 569–573. [Google Scholar] [CrossRef]
- Smucny, M.; Miniaci, A. Pre-shaped Allograft for Glenoid Reconstruction in Anterior Shoulder Instability. Arthrosc. Tech. 2018, 7, e343–e348. [Google Scholar] [CrossRef] [PubMed]
- Scalise, J.J.; Iannotti, J.P. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, S.E.; Sadeghpour, R.; Norris, T.R. Revision shoulder arthroplasty with a reverse shoulder prosthesis: Use of structural allograft for glenoid bone loss. Orthopade 2017, 46, 1055–1062. [Google Scholar] [CrossRef]
- Lopiz, Y.; García-Fernández, C.; Arriaza, A.; Rizo, B.; Marcelo, H.; Marco, F. Midterm outcomes of bone grafting in glenoid defects treated with reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 1581–1588. [Google Scholar] [CrossRef]
- Costain, D.J.; Crawford, R.W. Fresh-frozen vs. irradiated allograft bone in orthopaedic reconstructive surgery. Injury 2009, 40, 1260–1264. [Google Scholar] [CrossRef]
- Yu, D.; Panesar, P.S.; Delman, C.; Van, B.W.; Wilson, M.D.; Le, H.V.; Roberto, R.; Javidan, Y.; Klineberg, E.O. Comparing Fusion Rates Between Fresh-Frozen and Freeze-Dried Allografts in Anterior Cervical Discectomy and Fusion. World Neurosurg. X 2022, 16, 100126. [Google Scholar] [CrossRef]
- An, H.S.; Lynch, K.; Toth, J. Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: Differences among freeze-dried, frozen, and mixed grafts. Clin. Spine Surg. 1995, 8, 131–135. [Google Scholar] [CrossRef]
- Wu, G.; van der Helm, F.C.; Veeger, H.E.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]
- Castricini, R.; Mercurio, M.; Galasso, O.; Sanzo, V.; De Gori, M.; De Benedetto, M.; Orlando, N.; Gasparini, G. Femoral head allograft for glenoid bone loss in primary reverse shoulder arthroplasty: Functional and radiological outcomes. J. Shoulder Elb. Surg. 2023, 33, e58–e67. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Broschinsky, K.; Stertz, I.; Chalmers, P.N. Structural glenoid allograft reconstruction during reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 534–540. [Google Scholar] [CrossRef]
- Ho, J.C.; Thakar, O.; Chan, W.W.; Nicholson, T.; Williams, G.R.; Namdari, S. Early radiographic failure of reverse total shoulder arthroplasty with structural bone graft for glenoid bone loss. J. Shoulder Elb. Surg. 2020, 29, 550–560. [Google Scholar] [CrossRef] [PubMed]


| Contraindications to Bone Graft Implantation | Relative Contraindications to Humeral Autograft Harvest | Absolute Contraindications to Humeral Autograft Harvest |
|---|---|---|
| Denosumab | Osteopenia | Tumour |
| Active infection | Previous cuff repair | Infection |
| Proximal humeral fracture | ||
| Avascular necrosis |
| Variable | n (%) |
|---|---|
| Sex | |
| Male Female | 15 (54%) 13 (46%) |
| Surgery | |
| Primary One-stage bone grafting procedure Two-stage bone grafting procedure Revision One-stage revision Two-stage revision | 13 (45%) 12 (92%) 1 (8%) 16 (55%) 12 (75%) 4 (25%) |
| Seebauer-Gupta Classification C0 E0 C1 E1 C1 E2 C2 E1 C2 E2 C2 E3 C3 E0 C3 E1 C3 E2 C3 E3 C3 E4 C4 E2 | 1 (3%) 4 (14%) 2 (7%) 2 (2%) 5 (17%) 2 (7%) 2 (7%) 1 (3%) 1 (3%) 4 (14%) 3 (10%) 2 (7%) |
| Type of Graft “Figure 7” Wedge | 5 (17%) 24 (83%) |
| Diagnosis Primary Osteoarthritis Cuff tear arthropathy Rheumatoid arthritis Proximal humerus fracture Fracture malunion Irreducible anterior dislocation Revision Infection Implant loosening Failed anatomic TSA Failed hemiarthroplasty Component malposition Pain Instability Periprosthetic fracture | 4 (30.8%) 4 (30.8%) 1 (7.7%) 2 (15.4%) 1 (7.7%) 1 (7.7%) 6 (37.5%) 2 (12.5%) 2 (12.5%) 1 (6.3%) 1 (6.3%) 2 (12.5%) 1 (6.3%) 1 (6.3%) |
| Parameters | Preoperative | Postoperative | p Value |
|---|---|---|---|
| VAS (mean ± SD) | 5 ± 4 | 1 ± 2 | 0.014 |
| Range of Motion (mean ± SD) | |||
| Forward Flexion (deg) Lateral Elevation (deg) External Rotation (deg) Internal Rotation (deg) | 69 ± 39 64 ± 36 7 ± 16 12 ± 15 | 141 ± 32 122 ± 30 35 ± 20 49 ± 20 | <0.001 0.002 <0.001 <0.001 |
| PROMs (mean ± SD) ASES Constant Score | 34 ± 23 22 ± 12 | 73 ± 22 58 ± 14 | <0.001 <0.001 |
| Satisfaction (%) | 10% | 76% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingoe, H.; Italia, K.; Gilliland, L.; Kang, H.W.; Karel, M.; Maharaj, J.; Cutbush, K.; Gupta, A. The Use of Glenoid Structural Allografts for Glenoid Bone Defects in Reverse Shoulder Arthroplasty. J. Clin. Med. 2024, 13, 2008. https://doi.org/10.3390/jcm13072008
Ingoe H, Italia K, Gilliland L, Kang HW, Karel M, Maharaj J, Cutbush K, Gupta A. The Use of Glenoid Structural Allografts for Glenoid Bone Defects in Reverse Shoulder Arthroplasty. Journal of Clinical Medicine. 2024; 13(7):2008. https://doi.org/10.3390/jcm13072008
Chicago/Turabian StyleIngoe, Helen, Kristine Italia, Luke Gilliland, Hean Wu Kang, Mirek Karel, Jashint Maharaj, Kenneth Cutbush, and Ashish Gupta. 2024. "The Use of Glenoid Structural Allografts for Glenoid Bone Defects in Reverse Shoulder Arthroplasty" Journal of Clinical Medicine 13, no. 7: 2008. https://doi.org/10.3390/jcm13072008
APA StyleIngoe, H., Italia, K., Gilliland, L., Kang, H. W., Karel, M., Maharaj, J., Cutbush, K., & Gupta, A. (2024). The Use of Glenoid Structural Allografts for Glenoid Bone Defects in Reverse Shoulder Arthroplasty. Journal of Clinical Medicine, 13(7), 2008. https://doi.org/10.3390/jcm13072008






