Abstract
Stroke is a severe injury of the central nervous system (CNS) and one of the leading causes of long-term disability and mortality. One of the main symptoms of neurological diseases is spasticity. This is defined as a motor condition characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks and resulting in the hyperexcitability of the stretch reflex. Rehabilitation after a stroke is focused on relearning lost skills and regaining independence. Many new methods in neurorehabilitation have been introduced. This review concentrates on the current evidence for extracorporeal shockwave therapy (ESWT) as a noninvasive alternative to treat spasticity. We present the effect of EWST and radial EWST interventions to post-stroke patients with spasticity in the upper limb. Our collected data suggest that different parameters of shockwaves can be used to achieve functional improvementsin the upper limb after a stroke. Our accumulated data imply that ESWT is safe and can be used for pain relief, reduced muscle tension, and an increased range of motion. According to many studies, complications after shockwave treatment are infrequent. Transient complications after shockwave therapy (ESWT) include redness, tingling, pain, and bruising. We reviewed clinical trials that present the possible benefits in upper-limb function after shockwave therapy for post-stroke patients. In this article, we used many database search engines, including PEDro. In the stroke rehabilitation literature, a key methodological problem is the design of double-blind studies, which very often are not feasible.
1. Introduction
Stroke is the second most common cause of death and the leading cause of disability worldwide [1]. According to analyses, more than 50 million people worldwide havepost-stroke disabilities [2]. By 2047, it is estimated that the number of people with a stroke diagnosis in the European Union will increase by 27%. The reason for this upward trend is an aging population and improved survival rates [3]. A common complication after stroke is spasticity [4]. Spasticity was first described by Lance. It is defined as a motor condition characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks and resulting in the hyperexcitability of the stretch reflex [5]. Spasticity is related to upper motor neuron syndrome (UMNS) [6]. This complication often occurs in neurological diseases, including after a stroke [7]. This condition reduces patients’ quality of life through complications such as contractures, pain, reduced joint range of motion, reduced motor function, reduced mobility, and skeletal deformities [8,9]. It affects about 17–43% of chronic stroke survivors [10]. Lack of movement as a result of paralysis, along with rigidity caused by spasticity in the limb, accelerates the formation of contractures [11]. According to Wissel et al. (2010), the point at which spasticity develops is variable, but it often occurs within the first 6 weeks after a stroke in 25% of patients and affects the elbow and wrist [12]. The incidence of spasticity increases with the length of time after a stroke’s onset [10]. Post-stroke patients often show the stereotypical pattern of upper-limb impairment: adducted arm and internal rotation of the arm, bent elbow, pronation of the forearm, wrist flexion, and clenched fist. This leads to a loss of coordination between joints and joint control [13]. The item described is called the Wernicke-Mann position [14].
To better understand spasticity, the term muscle hypertonia is important. This is a general term for the increased resistance of joints to passive stretching movements. A distinction is made between neuronal and non-neuronal components, and spasticity mainly has a neuronal origin [15]. One of the main factors contributing to the symptom of muscle hypertonia in neurological disorders is the hyperactivity of spinal motor neurons [16]. There is no single definition of spasticity. The term is also commonly used as a general description of the clinical phenomenon of increased resistance to passive stretching [15]. An expanded 2005 definition proposed by Pandian et al. (2005), indicates that spasticity is a disorder of sensorimotor control. The symptoms that accompany it are temporary or permanent, such as involuntary muscle activation [17]. The new terminology refers to the increased resistance of joints and limbs as well as amovement disorder [15]. The phenomenon of spasticity has many complex and diverse aspects in its definition, which contributes to the lack of a clear consensus in the literature. Some definitions can be problematic when used insomesituations of motor disorders in post-stroke patients, due to various complications like muscle weakness and abnormal reflex reactions [18].
Despite the wide range of proposed treatments, rehabilitation programs, and therapies, spasticity remains a problem [19]. Pharmacotherapy for the treatment of spasticity usually includes drugs like baclofen, tizanidine, or diazepam. Their actions focus on the central nervous system, which leads to a reduction in the muscle tone of excessively tense muscles. Pharmacological therapy is not without its drawbacks. Medications can cause systemic side effects that affect the patient’s daily functioning, such as drowsiness or lethargy [20]. One of the most used pharmacologic therapies for treating focal spasticity is botulinum toxin type A (BoNT-A). Important factors to consider are having a well-established treatment goal, the selection of the muscle injection site, the dose and method of administration, and muscle structure [7]. The mechanism of the protein neurotoxin is to selectively inhibit the release of acetylcholine at the neuromuscular junction [21]. Side effects include hematoma at the injection site, weakness of the limb [22], pain at the injection site, dysphagia, and fatigue [23,24]. Complications after the procedure occur most often when the dose is not optimally adjusted. With multiple injections, neutralizing antibodies can form [22].
There are many options for non-pharmacological interventions to treat spasticity. These include passive movements and stretching, direct current stimulation, transcutaneous electrical nerve stimulation, vibratory stimulation, ultrasound, acupuncture, orthoses, transcranial magnetic stimulation, thermotherapy, and cryotherapy [25]. Although there are many therapeutic approaches, there is a lack of high-quality scientific evidence for most of the above-mentioned interventions [26].
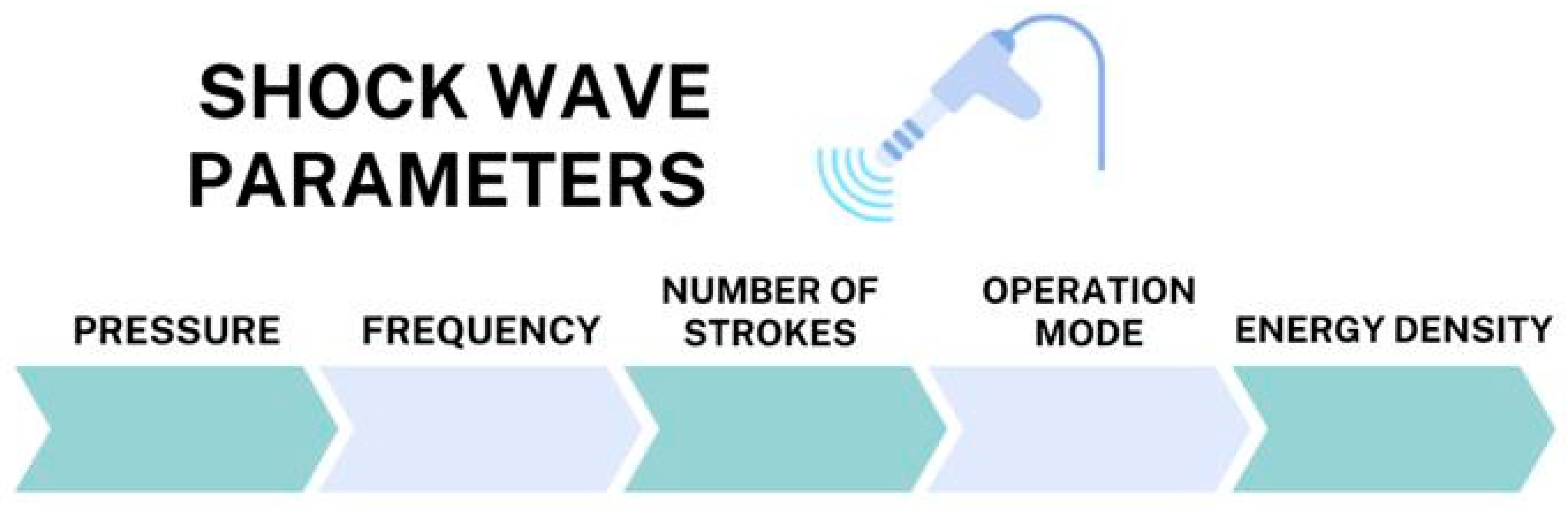
In the past decade, a non-pharmacological method gaining popularity in reducing muscle spasticity after a stroke is extracorporeal shockwave therapy (ESWT). This term refers to a treatment with a sequence of acoustic pulses characterized by a high peak pressure (100 MPa) and rapid pressure rise (<10 ns) and transmitted by a selected generator to a specific target area with an energy density in the range of 0.003–0.890 mJ/mm2 (Figure 1) [27]. Extracorporeal shockwave therapy (ESWT) is considered a noninvasive alternative for the treatment of spasticity. Publicly available data indicate that ESWT is a safe method and can be used to relieve pain, reduce muscle tension, and increase one’s range of motion.
Figure 1.
Shockwave parameters.
There are two types of shockwave generators used in clinical practice: focused (FSWT) and radial (rEWST). The two types differ in their physical properties of energy and energy propagation [28]. Focused shockwavescan be produced by electrohydraulic, electromagnetic, and piezoelectric generators. It is a more invasive intervention, due to its rapidly increasing pressure and depth of action of up to 12 cm [29]. Radial shockwaves are produced by a pneumatic source placed inside a generator in the device [30]. The greatest amount of energy is concentrated at the tip of the probe, which is most often in the form of a gun, which is then transmitted deep into the tissue [31]. The depth of penetration into the tissue is less than in the focused type and is up to 3–4 cm. The smaller level of penetration is better tolerated during the procedure by patients [32]. Treatment with radial shockwave therapy (rEWST) can cause less damage to the skin and soft tissues [31], which is reflected in the low incidence ofcomplications after therapy. According to a meta-analysis by Guo et al. (2017), complications after shockwave treatment are rare [33]. Transient complications after shockwave therapy (ESWT) include redness, tingling, pain, and bruising [34]. Shockwave treatment was first performed in 1980 to treat kidney stones [35]. Shockwave therapy (ESWT) has been successfully used in the field of orthopedics for more than 20 years. The most common conditions involve tendons and musculoskeletal pathologies [30].
2. Mechanism of Action of Radial Shockwaves
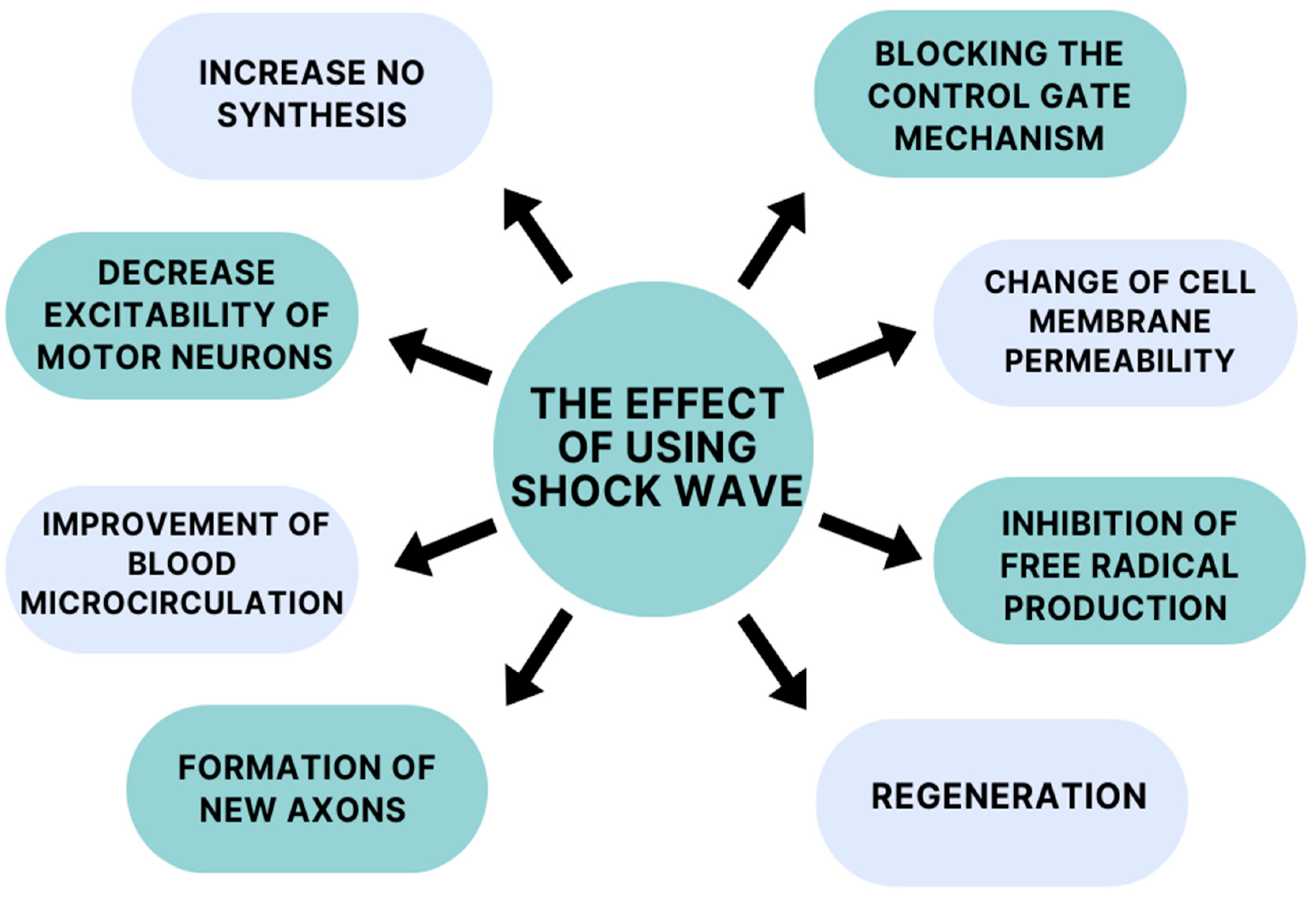
The mechanism of action of extracorporeal shockwave therapy remains incompletely understood, but it appears to demonstrate a broad spectrum of action (Figure 2). One mechanism of action of ESWT is to increase nitric oxide (NO) synthesis. This compound acts on both the peripheral and central nervous systems. In the case of the central nervous system (CNS), it affects one’s physiological functions, improving synaptic plasticity and neurotransmission. Activity in the peripheral nervous system is relatedtothe increased neovascularization of muscles and tendons [36,37]. NO is a desirable compound due to its anti-inflammatory properties. According to Mariotto (2005) et al., a molecular mechanism of anti-inflammatory action has been observed during ESWT (extracorporeal shockwave therapy), involving the tyrosine defosphorylation of endothelial nitric oxide synthase (eNOS), the successive augmentation of NO production, and the inhibition of nuclear factor kappa B (NF-κB) activation [38]. Kenmoku (2018) et al. and Jia (2020) at al. suggest in their studies that shockwave treatment can temporarily reduce acetylcholine at the neuromuscular junction, leading to a reduction in muscle spasticity [39,40].
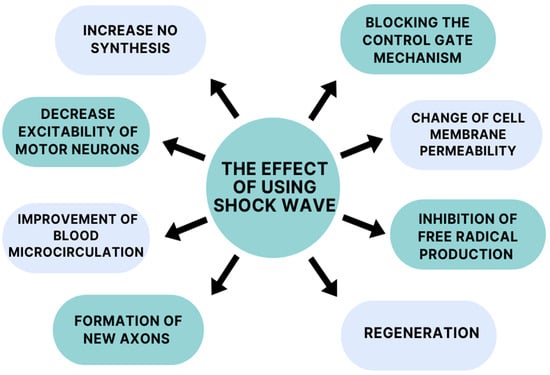
Figure 2.
Mechanisms of action of shockwaves.
Daliri et al. suggests in their study that shockwave therapy has a role in altering motor neuron excitability. Patients received one sham ESWT session during their study, followed by one active ESWT session 1 week later. The real session helped reduce patients’ wrist flexor spasticity and alpha motor neuron excitability. The excitability of motor neurons was measured by the neurophysiological Hmax/Mmax ratio via electromyography (EMG) [41]. According to one theory, a decrease in alpha motor neuron excitability can be caused by tendon pressure [42]. Goertz (2012) et al. used an extracorporeal shockwave treatment to accelerate the healing process in a burntmouse model. They found that the shockwaves had a positive effect on a number of burn-wound-healing parameters, in particular, thoseinregard to angiogenesis and leukocyte behavior. The number of rolling and sticking leukocytes increased, which improved the mice’s metabolism. These results imply that shockwave treatment leads to an increase in the efficiency of the tissue regeneration process by accelerating blood microcirculation and tissue rheology [43]. The basis for the initiation of the above studies was the previous confirmation by Kuo (2007) et al. of accelerated tissue repair in acute and chronic wounds with extracorporeal shockwave therapy. Furthermore, the findings presented by Link (2013) et al. also suggest the improved healing of distal wounds in horses. The reason for this is a reduction in granulation tissue production induced by the inhibition of TGF-β1 (transforming growth factor-β1) [44,45]. Kenmoku et al. (2012) conducted an extracorporeal shockwave study on rabbits. According to the researchers, the application of extracorporeal shockwaves to their muscles induced the transient dysfunction of nerve conduction at neuromuscular junctions. They found there was degeneration and a reduced number of acetylcholine receptors in the muscles, which contributed to the altered functional state. The low number of receptors is not a permanent effect; they rebuild in a short period of time. This is a possible reason for the short-term effect of extracorporeal shockwave therapy [46,47].
Neuronal effects after extracorporeal shockwave therapy have also been considered. A study by Lee et al. (2015) tells us that the early application of extracorporeal shockwaveincreases the expression of neurotrophin-3 and neurotrophin-3 mRNA. In addition, daily therapy sessions facilitated the activity of macrophages and Schwann cells, which affect neuronal survival and regeneration [48]. As we know, extracorporeal shockwave therapy reduces patients’ pain sensations. It causes the inhibition of pain fiber conduction by a altering cell membrane permeability and blocking the control gate mechanism due to the hyperstimulation of the peripheral nervous system [49]. Moreover, it shows a protective effect on neurons, due to the inhibition of the production of free radicals that lead to neuronal deformation. They positively influence the regeneration of damaged axons by stimulating one’s metabolism and the prevention of free radical damage, which is generated by axonotmesis. Extracorporeal shockwave therapy can effectively regenerate and redistribute sensory and motor fibers after a brain injury such as a stroke [50]. Many researchers have tested and applied radial shockwave treatments successfully to post-stroke patients with spasticity in the upper limb. They have reported pain relief, reduced muscle tension, and an increased range of motion [51,52,53].
2.1. Musculoskeletal Diseases and Spasticity
The mechanism of action of shockwaves in musculoskeletal disorders is related to multiple pathways of physiological responses, which are characterized by a final process of mechanotransduction. This is a physical-biological action during which shockwaves pass through the tissue causing high-pressure gradients [54]. Mechanotransduction affects the regulation of basic cellular functions and metabolism, such as migration, proliferation, differentiation, and apoptosis [55]. Shockwaves in musculoskeletal conditions are used to increase blood flow to the affected area by inducing healing via inflammation and secreted mediators, as well as inhibiting pain receptors and increasing angiogenesis [56]. Astur et al. (2015) performed shockwave therapy (ESWT) on patients with lower-extremity muscle injuries lasting longer than three weeks. After the treatment, they showed a reduction in pain sensations and an increase in muscle strength, allowing themtoplay sports again. To date, many biochemical studies on the effects of shockwaves on muscle tissue have been performed on mouse models in vitro and in vivo [57]. A study by Mattyasovszky et al. (2018) provides evidence of the potential of extracorporeal shockwave (rESWT) interventions to modulate the biological functions of primary human muscle cells. Shockwave treatments can affect the viability of human skeletal muscle cells and regulate the gene expression of muscle cell proteins in vitro, which can be explainedby the regenerative process [58]. Effective regeneration was observed on torn stage III muscles treated with rESWT in mice. Significant improvements in the muscle markers MyoD and myosin were observed. The presence of myosin gene expression indicated newly formed muscle fibers. This was confirmed by hematoxylin and eosin staining. Seven days after muscle injury, the number of mononuclear cells decreased, making it possible to visualize muscle fibers [59]. Speaking of muscle system regeneration, it is worth mentioning satellite cells, which are responsible for restoring function and producing a large number of new muscle fibers [60]. After anESWTintervention, the regeneration of skeletal muscle tissue is stimulated, resulting in accelerated repair processes. This is related to the increased number of satellite cells after ESWT therapy [61]. Spasticity, which is a neurological and muscular problem, is associated with the continuous stimulation of the neuromuscular plate. The effect of ESWT is similar to that of botulinum toxin A injection. In both cases, there is the destruction of endplates at neuromuscular junctions [62]. New research by Wu et al. (2019)points to the effectiveness of ESWT in treating calcified musculoskeletal structures. Functional improvements and reductionsin pain sensations in patients have been found [63]. An extensive review by Simplicio et al. (2020) demonstrates the use of shockwaves to trigger regenerative processes in the treatment of musculoskeletal disorders, including osteoarthritis [64]. In bone tissue after ESWT, the bone differentiation of stem cells and an increase in vascular endothelial growth factor (VEGF) expression are noted [65,66]. In cartilage tissue, only an increase in VEGF is noted [67]. Different tissues, whether nerve, muscle, or cartilage, respond differently to the same ESWT mechanical stimulus. Many mechanisms of ESWT remain unexplained [62].
2.2. Wound Healing
In aesthetic medicine, ESWT procedures are most commonly performed with the goal of treating cellulite and excess fat as well as body contouring [68]. The mechanisms used in aesthetic medicine are rooted in wound healing and regeneration. Cells respond to the indirect effects of the shockwave cavitation phenomenon, inducing changes that are beneficial in the treatment of cellulite, such as increasing local circulation and stimulating collagen production, leading to the restructuring of the dermis and epidermis, thereby restoring connective tissue elasticity and improving skin texture [68]. The phenomenon of cavitation can be explained as the formation of gas microbubbles in the fluids of biological tissues [69]. These are equally important processes in the healing of chronic wounds and burns. The first piece of supporting evidence for the healing of burn wounds and their complications usingESWTwas published in 2005 [70]. In the case of burns and hypertrophic scars following burns, the mechanotransduction process affects the transport of fibroblasts into the scar tissue, regulating the production of the molecules transforming growth factor-beta (TGF-β1), Smad, fibronectin, or collagen types I and III [54,71,72]. In addition to the process of angiogenesis, shockwavesalso affect the behavior and number of leukocytes. A study by Goertz et al. (2012) conducted on the ears of hairless mice demonstrates shockwave therapy accelerated angiogenesis, improved blood flow, and increased the leukocyte count [43]. Shockwave treatments may have a beneficial effect on the nature of hypertrophic scarring after a burn. The results of a study by Lee et al. (2021) report that ESWT interventions led to a significant reduction in scar erythema and an increase in sebum levels [73]. Changes also extend to the skin’s protective function in the stratum corneum barrier and the faster development of a uniform granulation layer [74,75]. During ESWT therapy, collagen fibers may be broken down, with consequences in terms ofscar remodeling [76]. ESWT treatments may be a promising treatment for patients with existing leg ulceration refractory to other treatments. In the results section of their study, Aschermann et al. (2017) noted morphological changes and the increased migration of keranocytes. Increased expression of the following genes responsible for cell cycle regulation was found: CCNA2, CCNB1, and CCNB2. In addition, increased secretion of pro-inflammatory cytokines responsible for accelerating wound healing and the pro-angiogenic activity of endothelial cells was noted [77].
3. Materials and Methods
The material collected for this review was sourced from the following databases: PubMed, PubMed Central, Cochrane Library, Medline, Embase, Web of Science, SCOPUS, and PEDro. Search topics included the following:
- Radial shockwave intervention in spasticity;
- Spasticity after stroke;
- Radial shockwave;
- Rehabilitation in spasticity;
- Stroke and shockwave.
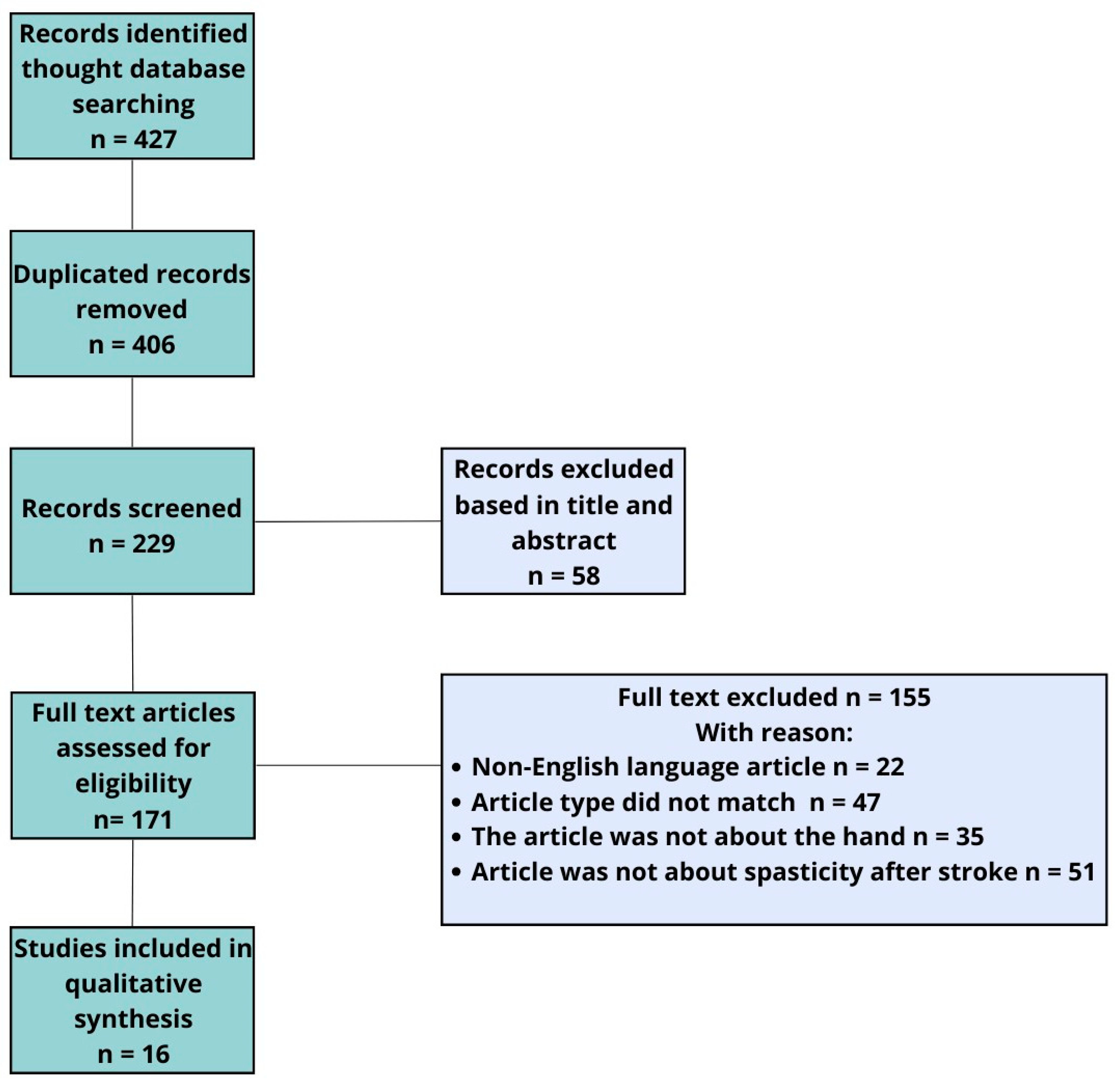
In addition to the main topics, ESWT and rEWST acronyms were also searched. A total of 71 articles were analyzed. In this study, we allowed the inclusion of articles mainly from the last 18 years. After removing duplicates, we qualified 14 articles and additional 2 articles found by performing a manual search of reference lists. We eliminated articles published in a language other than English, full-access articles, articles for which only the abstract was available, studies involving the lower limb, and studies combining several investigational therapies simultaneously. For this reason, only 16 studies that met all these requirements were accepted and included in our quality synthesis (Figure 3).
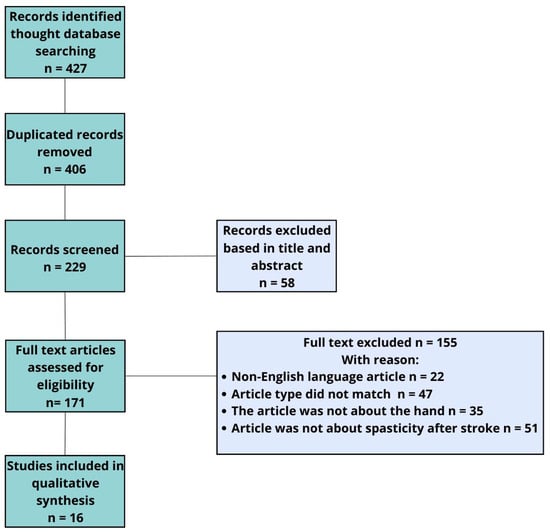
Figure 3.
Flowchart of study selection.
4. Benefits of Extracorporeal Shockwave Therapy (ESWT) forStroke Patients—Results
Motor dysfunction is mostly unilateral and is related to the location and severity of a brain injury [78]. Complications of spasticity include contractures, a decreased range of motion, decreased motor function, and pain [9]. Post-stroke patients often show increased muscle tone. This symptom develops in patients after a stroke within the first 6 weeks. This abnormal pattern affects both the lower and upper limbs. In the case of the upper limb, the biggest changes involve the elbow and wrist [10]. The stereotypical pattern of upper-limb impairment is an adducted arm and internal rotation of the arm, bent elbow, pronation of the forearm, wrist flexion, and clenched fist [13].
Extracorporeal shockwave therapy (ESWT) is amethod that is gaining popularity to decrease muscle spasticity after a stroke. ESWT is used to improve the motor function of the upper limb. Treatment with ESWT involves asequence of acoustic pulses characterized by a high peak pressure (100 MPa) and rapid pressure increase(<10 ns) and transmitted by a selected generator to a specific target area with an energy density in the range of 0.003–0.890 mJ/mm2 [27]. In general, most studies present models of therapy with differences in terms oftreatment, treatment area, application, frequency, pressure, number of shots, and bullet size.
The most common definition of spasticity is explained as a velocity-dependent increase in muscle tension due to the presence of enhanced stretch reflexes. Spasticity significantly limits patients’ function and mobility, which can worsen long-term disabilities [79]. Extracorporeal shockwave therapy is a non-invasive tool for the treatment of post-stroke motor dysfunction in the upper limb. Table 1 and Figure 4 summarize the possible benefits of ESWT in post-stroke patients. All included original articles were evaluated on the PEDro scale, which includes 10 items corresponding to their internal validity and interpretability to assess their methodological quality. The average methodological quality score of RCT reports is 6.4 points on the 0–10 PEDro scale.

Table 1.
Potential benefits in upper-limb motor function after post-stroke shockwave therapy with a valid measure of methodological quality of clinical trials using the PEDro scale.
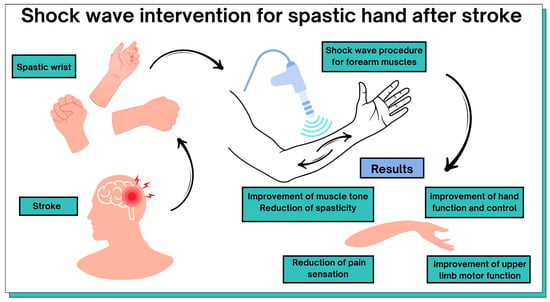
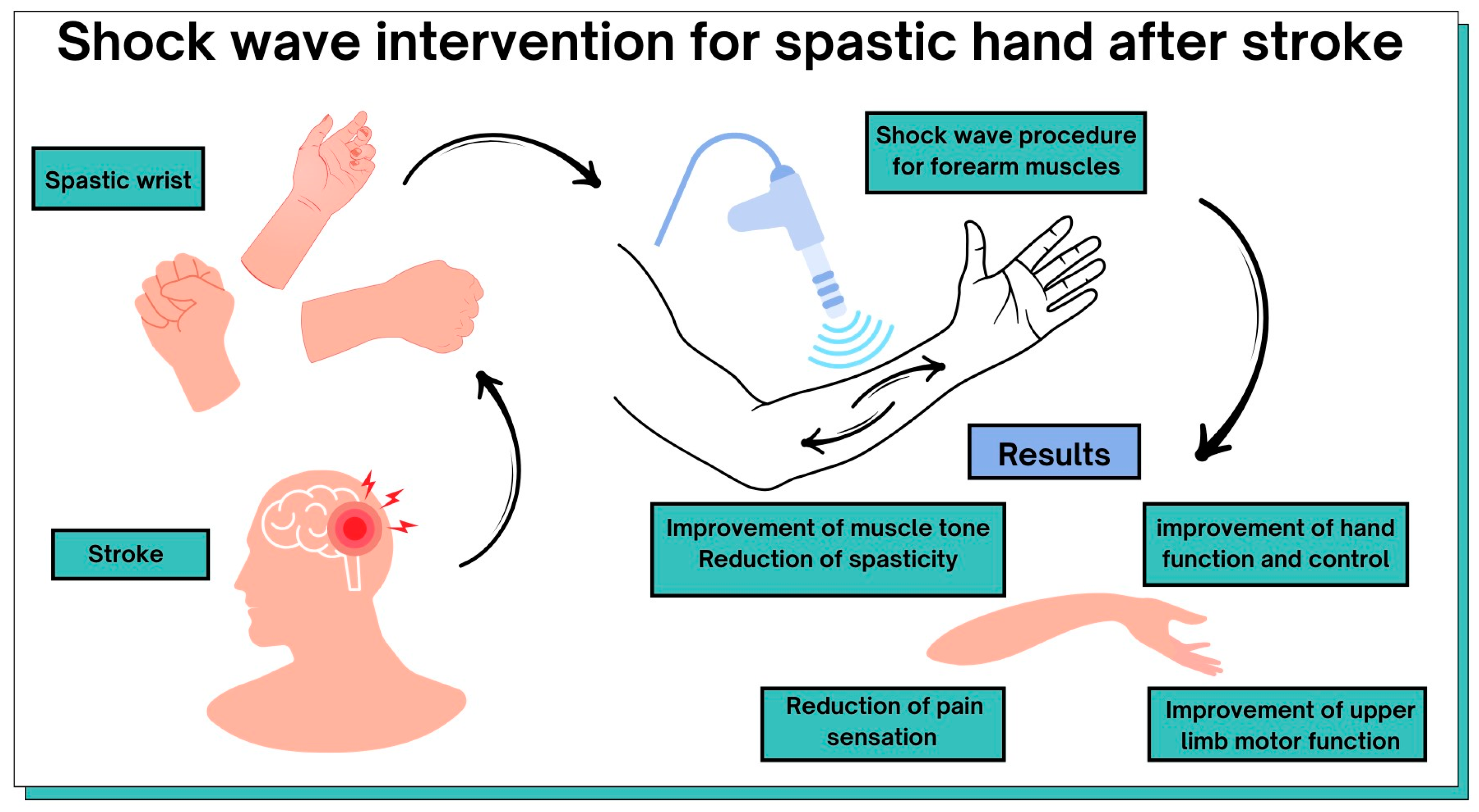
Figure 4.
Graphical representation of the results of this review.
5. Discussion
Spasticity can reduce post-stroke patients’quality of life, interfere with their ability to be independent, and lead to the development of depressive symptoms [93]. Treatment and rehabilitation in a patient with existing spasticity can be challenging [94]. Topical invasive treatments and medications often exhibit side effects and adverse reactions, limiting their effectiveness [95]. Recent studies, as well as systematic reviews and meta-analyses, indicate the positive effects of radial shockwave intervention. This treatment is safe and itseffects are impressive. Itreducesthe degree of spasticity, decreasesthe intensity ofpain, improvesthe motor function of the affected limb, and increases patientindependence [7].
The purpose of this systematic review was to identify current scientific evidence on the use of an intervention—extracorporeal shockwave therapy (ESWT)—as a non-invasive alternative for treating spasticity in post-stroke patients. We analyzed 16 scientific studies conducted between 2005 and 2023. As many as 14 studies involved patients after an ischemic stroke. Only one study, that by Guo et al. (2019), gathered a study group of more than 100 patients, more precisely, 120 patients [53]. The large majority of studies involved a smaller number of patients; the number ranged from 20 to 60 patients [29,32,34,80,81,82,83,85,88,90,91,92]. For oursystematic review, we also qualified a case study conducted on one patient [89]. To check upper-limb recovery, the researchers mainly used the Fugl-Meyer Assessment for Upper Extremity (FMA-UE) [29,34,53,85,88,91,92] and in some individual cases, the Action Research Arm Test (ARAT) was used [88]. Muscle tension and spasticity status weretested using the Modified Ashworth Scale (MAS), Ashworth Scale (AS), Tardieu Scale, and Modified Tardieu Scale (MTS) [29,32,34,53,80,81,82,83,84,85,87,88,89,90,92]. Yuan et al. (2023) did not check their patients’ muscle tension status using the above scales [91]. Tabra et al. (2021) used an electrophysiological assessment of spasticity based on the Hmax/Mmax amplitude ratio as a reliable measure of α motor neuron excitability and spasticity [92]. The study’s authors did not use common measures of muscle tone: AS, MAS, TS, or MTS.On a case-by-case basis, the researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess the severity of selected symptoms and monitor the condition of stroke patients [82], voluntary control grading (VCG) [88] to assess the voluntary control of the affected hand, the Motricity Index to assess motor impairment [92], the Modified Barthel Index (MBI) [91] and Barthel Index (BI) [32] to assess daily activities, the Disability Assessment Scale (DAS) [89] to assess the functional disability of the upper limb, and the Mini Mental State Examiation-Korea (MMSE-K) to assess cognitive function [85]. Manganotti et al.(2005) used video monitoring with a digital goniometer to assess patients’ range of motion [80].
The number of strokes applied by shockwave cartridges ranged from 1000 to 6000 strokes [34,82]. However, clinicians most often used values in the range of 1000–3000 strokes [29,32,53,83,87,90,91,92]. The shockwave pressure parameters used ranged from 1.2 to 2.5 Ba [34,84], a frequency of 4–18 Hz, and energy pulses of 0.03–0.93 mJ/mm2 [29,32,81,82,84]. None of the authors, except for Savevska et al. (2016), specified the parameters required for shockwave treatment. Savevska et al. (2016) were the only authorsto specify the size of the shockwave probe: 15 mm [89].
Reductions in spasticity and muscle tension were noted in every study analyzed. Three studies noted an immediate effect after oneshockwavesession [32,81,88]. There is no clear consensus on the durability of ESWT treatment. Manganotti et al. (2005) [80] points out that thiscan be up to 12 weeks after treatment, while someauthors indicate the spasticity reduction is maintained4 weeks after the intervention [82,84,88]. ESWT interventions for spastic muscles can have a positive effect as early as 1 week after starting [82]. However, Bae at al. (2010) [81] showed that this effect disappears after one week and does not persist after treatment (this is also true 4 weeks after the ESWT treatment in their study). Other cited positive effects of ESWT interventions include the following: the improvement of the functional motility of the affected limb [83], the improvement of hand function and control [34], the prevention of spasticity progression, the reduction in the use of oral antispasmodic medications [83], the improvement of active elevation of the hemiplegic upper limb [82], and pain relief [84]. The use of shockwaves in post-stroke patients may be effective in controlling the peripheral component of spasticity in terms of changes in muscle mechanical properties [29].Yoon et al. (2017) indicates that the intervention will be effective when the treatment is performed on the muscle bellies or muscle–tendon junctions of spastic muscles [87]. It should be mentioned that according to Li et al. (2020), shockwave interventions do not affect the swelling of limbs [84]. In their study, Senatarah et al. (2023) showed better results after ESTW interventions in patients after a hemorrhagic stroke compared to those of patients after a ischemic stroke after 4 weeks [88]. There are no clear recommendations on how many rESTW interventions should be performed to maintain the effect of reducing muscle tone and improving upper-limb function. The total number of rESWT interventions may affect clinical recovery during the treatment phase [90]. None of the studies reviewed found side effects after the ESTW treatments. The first research study on the use of shockwaves in the treatment of spasticity dates back to 2005 [80]. According to the National Library of Medicine’s PMC database, there are 427 articles (research articles, reviews, and meta-analyses) on the subject of the use ofshockwavesfor spasticity. As many as 305 papers deal with spasticity in post-stroke patients. In the future, we can expect great interest in shockwave therapy for spasticity.
The treatment of spasticity after a stroke is an interesting topic, but the area is dominated by relatively small studies without a control group. Studies should be conducted on a larger number of participants to estimate the treatment’s effect. Our study could be improved by listing whether the articles were systematic reviews.Additionally, we could have included a more detailed discussion of the limitations of the current study, including some suggestions for the future.
6. Conclusions
ESTW therapy can lead to positive effects in post-stroke patients: the improvement of muscle tone, reduction in spasticity, improvement of hand function and control, reduction in pain sensations, and improvement of upper-limb motor function. Radial shockwave interventions can complement rehabilitation in post-stroke patients with upper-limb spasticity. ESWT treatment is safe, with no long-term side effects. This review has provided a comprehensive overview of the benefits of ESWT forupper-limb rehabilitation of patients after a stroke. This may facilitate future treatment decisions for patients and initiate studies evaluating the use of ESWT at different times after a stroke. While therapy based on traditional neurophysiological methods is still useful, noninvasive ESWT is a promising additionthat can be applied to patients with spasticity. The degree of spasticity influences the final treatment result. Taking this into account, other therapeutic approaches require further clinical trials. Despite limited scientific evidence, our review shows that ESWT is mostly beneficial for patients. A protocol for the selection of treatment parameters should be standardized, and recommendations for the use of ESTW in post-stroke patients with spasticity should be made. Furthermore, patients’ level of spasticity should be taken into account in future clinical trials. In our review, we used the PEDroscale, which is a comprehensive tool to assess the methodological quality of the neuro-rehabilitation literature.
Author Contributions
Conceptualization, M.S. and E.M.; methodology, K.M. and E.M.; software, M.S. and K.M.; formal analysis, K.M. and E.M.; investigation, M.S., K.M. and E.M.; resources, M.S., K.M. and E.M.; data curation, M.S.; writing—original draft preparation, M.S., K.M. and E.M.; writing—review and editing, M.S., K.M., J.R. and E.M.; visualization, M.S.; supervision, E.M.; project administration, E.M.; funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Bonita, R.; Mendis, S.; Truelsen, T.; Bogousslavsky, J.; Toole, J.; Yatsu, F. The global stroke initiative. Lancet Neurol. 2004, 3, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Wafa, H.A.; Wolfe, C.D.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of stroke in Europe: Thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and risk factors for spasticity after stroke: A systematic review and meta-analysis. Front. Neurol. 2021, 11, 616097. [Google Scholar] [CrossRef]
- Lance, J.W. Pathophysiology of spasticity and clinical experience with baclofen. In Spasticity: Disordered Motor Control; Lance, J.W., Feldman, R.G., Young, R.R., Koella, W.P., Eds.; Year Book: Chicago, IL, USA, 1980; pp. 185–204. [Google Scholar]
- Cheng, H.; Fang, X.; Liao, L.; Tao, Y.; Gao, C. Prevalence and factors influencing the occurrence of spasticity in stroke patients: A retrospective study. Neurol. Res. 2023, 45, 166–172. [Google Scholar] [CrossRef]
- Mihai, E.E.; Popescu, M.N.; Iliescu, A.N.; Berteanu, M. A systematic review on extracorporeal shock wave therapy and botulinum toxin for spasticity treatment: A comparison on efficacy. Eur. J. Phys. Rehabil. Med. 2022, 58, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Chulian, R.; Heredia-Rizo, A.M.; Moral-Munoz, J.A.; Lucena-Anton, D.; Luque-Moreno, C. Dry needling for the management of spasticity, pain, and range of movement in adults after stroke: A systematic review. Complement. Ther. Med. 2020, 52, 102515. [Google Scholar] [CrossRef]
- Pundik, S.; McCabe, J.; Skelly, M.; Tatsuoka, C.; Daly, J.J. Association of spasticity and motor dysfunction in chronic stroke. Ann. Phys. Rehabil. Med. 2019, 62, 397–402. [Google Scholar] [CrossRef]
- Wissel, J.; Manack, A.; Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef]
- Malhotra, S.; Rosewilliam, S.; Hermens, H.; Roffe, C.; Jones, P.; Pandyan, A.D. A randomized controlled trial of surface neuromuscular electrical stimulation applied early after acute stroke: Effects on wrist pain, spasticity and contractures. Clin. Rehabil. 2013, 27, 579–590. [Google Scholar] [CrossRef]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef]
- Ren, Y.; Park, H.-S.; Zhang, L.-Q. Developing a whole-arm exoskeleton robot with hand opening and closing mechanism for upper limb stroke rehabilitation. In Proceedings of the 2009 IEEE International Conference on Rehabilitation Robotics, Kyoto, Japan, 23–26 June 2009; pp. 761–765. [Google Scholar]
- Doussoulin, A.; Rivas, C.; Bacco, J.; Sepúlveda, P.; Carvallo, G.; Gajardo, C.; Soto, A.; Rivas, R. Prevalence of spasticity and postural patterns in the upper extremity post stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105253. [Google Scholar] [CrossRef]
- Li, S.; Francisco, G.E.; Rymer, W.Z. A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabilit. Neural Repair 2021, 35, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.T.; Rymer, W.Z. Spastic hypertonia: Mechanisms and measurement. Arch. Phys. Med. Rehabil. 1989, 70, 144–155. [Google Scholar]
- Pandyan, A.D.; Gregoric, M.; Barnes, M.P.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G.R. Spasticity: Clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.A.; Laureys, S.; Gosseries, O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdes, R.; Serra-Llobet, P.; Rodriguez-Rubio, P.R.; Lopez-de-Celis, C.; Llauro-Fores, M.; Calvo-Sanz, J. The effectiveness of extracorporeal shock wave therapy for improving upper limb spasticity and functionality in stroke patients: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1141–1156. [Google Scholar] [CrossRef]
- Chang, E.Y.; Ghosh, N.; Yanni, D.; Lee, S.; Alexandru, D.; Mozaffar, T. A review of spasticity treatments: Pharmacological and interventional approaches. Crit. Rev. Phys. Rehabil. Med. 2013, 25, 11–22. [Google Scholar] [CrossRef]
- Deltombe, T.; Lejeune, T.; Gustin, T. Botulinum toxin type A or selective neurotomy for treating focal spastic muscle overactivity? Ann. Phys. Rehabil. Med. 2019, 62, 220–224. [Google Scholar] [CrossRef]
- Santamato, A.; Micello, M.F.; Ranieri, M.; Valeno, G.; Albano, A.; Baricich, A.; Cisari, C.; Intiso, D.; Pilotto, A.; Logroscino, G.; et al. Employment of higher doses of botulinum toxin type A to reduce spasticity after stroke. J. Neurol. Sci. 2015, 350, 1–6. [Google Scholar] [CrossRef]
- Gracies, J.-M.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: A double-blind randomised controlled trial. Lancet Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: The TOWER study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Bensmail, D.; Yelnik, A. Non-pharmacological interventions for spasticity in adults: An overview of systematic reviews. Ann. Phys. Rehabil. Med. 2019, 62, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Lew, H.L.; Ozcakar, L.; Wu, C.H. Recent Advances in the Treatment of Spasticity: Extracorporeal Shock Wave Therapy. J. Clin. Med. 2021, 10, 4723. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.M.; Bluman, E.; Tenforde, A.S. Effect of Shockwave Treatment for Management of Upper and Lower Extremity Musculoskeletal Conditions: A Narrative Review. PM R 2018, 10, 1385–1403. [Google Scholar] [CrossRef] [PubMed]
- Dymarek, R.; Halski, T.; Ptaszkowski, K.; Slupska, L.; Rosinczuk, J.; Taradaj, J. Extracorporeal shock wave therapy as an adjunct wound treatment: A systematic review of the literature. Ostomy Wound Manag. 2014, 60, 26–39. [Google Scholar]
- Leng, Y.; Lo, W.L.A.; Hu, C.; Bian, R.; Xu, Z.; Shan, X.; Huang, D.; Li, L. The Effects of Extracorporeal Shock Wave Therapy on Spastic Muscle of the Wrist Joint in Stroke Survivors: Evidence from Neuromechanical Analysis. Front. Neurosci. 2020, 14, 580762. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Csaszar, N.B.; Milz, S.; Schieker, M.; Maffulli, N.; Rompe, J.D.; Furia, J.P. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: A systematic review on studies listed in the PEDro database. Br. Med. Bull. 2015, 116, 115–138. [Google Scholar] [CrossRef]
- Wu, Y.T.; Chang, C.N.; Chen, Y.M.; Hu, G.C. Comparison of the effect of focused and radial extracorporeal shock waves on spastic equinus in patients with stroke: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 518–525. [Google Scholar] [CrossRef]
- Dymarek, R.; Taradaj, J.; Rosinczuk, J. The Effect of Radial Extracorporeal Shock Wave Stimulation on Upper Limb Spasticity in Chronic Stroke Patients: A Single-Blind, Randomized, Placebo-Controlled Study. Ultrasound Med. Biol. 2016, 42, 1862–1875. [Google Scholar] [CrossRef]
- Guo, P.; Gao, F.; Zhao, T.; Sun, W.; Wang, B.; Li, Z. Positive Effects of Extracorporeal Shock Wave Therapy on Spasticity in Poststroke Patients: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Chang, C.Y.; Chou, Y.C.; Chen, L.C.; Chu, H.Y.; Chiang, S.L.; Chang, S.T.; Wu, Y.T. Effect of Radial Shock Wave Therapy on Spasticity of the Upper Limb in Patients with Chronic Stroke: A Prospective, Randomized, Single Blind, Controlled Trial. Medicine 2016, 95, e3544. [Google Scholar] [CrossRef] [PubMed]
- Chaussy, C.; Brendel, W.; Schmiedt, E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet 1980, 2, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, W.; Jiang, W.; Qian, Q. Effects of extracorporeal shock wave therapy on spasticity in post-stroke patients: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2018, 50, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Blottner, D.; Lück, G. Just in time and place: NOS/NO system assembly in neuromuscular junction formation. Microsc. Res. Tech. 2001, 55, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Cavalieri, E.; Amelio, E.; Ciampa, A.R.; de Prati, A.C.; Marlinghaus, E.; Russo, S.; Suzuki, H. Extracorporeal shock waves: From lithotripsy to anti-inflammatory action by NO production. Nitric Oxide 2005, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, T.; Nemoto, N.; Iwakura, N.; Ochiai, N.; Uchida, K.; Saisu, T.; Ohtori, S.; Nakagawa, K.; Sasho, T.; Takaso, M. Extracorporeal shock wave treatment can selectively destroy end plates in neuromuscular junctions. Muscle Nerve 2018, 57, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Ma, J.; Wang, S.; Wu, D.; Tan, B.; Yin, Y.; Jia, L.; Cheng, L. Long-term Effects of Extracorporeal Shock Wave Therapy on Poststroke Spasticity: A Meta-Analysis of Randomized Controlled Trials. J. Stroke Cerebrovasc. Dis. 2020, 29, 104591. [Google Scholar] [CrossRef] [PubMed]
- Daliri, S.S.; Forogh, B.; Emami Razavi, S.Z.; Ahadi, T.; Madjlesi, F.; Ansari, N.N. A single blind, clinical trial to investigate the effects of a single session extracorporeal shock wave therapy on wrist flexor spasticity after stroke. NeuroRehabilitation 2015, 36, 67–72. [Google Scholar] [CrossRef]
- Leone, J.A.; Kukulka, C.G. Effects of tendon pressure on alpha motoneuron excitability in patients with stroke. Phys. Ther. 1988, 68, 475–480. [Google Scholar] [CrossRef]
- Goertz, O.; Lauer, H.; Hirsch, T.; Ring, A.; Lehnhardt, M.; Langer, S.; Steinau, H.; Hauser, J. Extracorporeal shock waves improve angiogenesis after full thickness burn. Burns 2012, 38, 1010–1018. [Google Scholar] [CrossRef]
- Kuo, Y.-R.; Wu, W.-S.; Hsieh, Y.-L.; Wang, F.-S.; Wang, C.-T.; Chiang, Y.-C.; Wang, C.-J. Extracorporeal shock wave enhanced extended skin flap tissue survival via increase of topical blood perfusion and associated with suppression of tissue pro-inflammation. J. Surg. Res. 2007, 143, 385–392. [Google Scholar] [CrossRef]
- Link, K.A.; Koenig, J.B.; Silveira, A.; Plattner, B.L.; Lillie, B.N. Effect of unfocused extracorporeal shock wave therapy on growth factor gene expression in wounds and intact skin of horses. Am. J. Vet. Res. 2013, 74, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, T.; Ochiai, N.; Ohtori, S.; Saisu, T.; Sasho, T.; Nakagawa, K.; Iwakura, N.; Miyagi, M.; Ishikawa, T.; Tatsuoka, H. Degeneration and recovery of the neuromuscular junction after application of extracorporeal shock wave therapy. J. Orthop. Res. 2012, 30, 1660–1665. [Google Scholar] [CrossRef]
- Duan, H.; Lian, Y.; Jing, Y.; Xing, J.; Li, Z. Research progress in extracorporeal shock wave therapy for upper limb spasticity after stroke. Front. Neurol. 2023, 14, 1121026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.G. Effects of extracorporeal shock wave therapy on functional recovery and neurotrophin-3 expression in the spinal cord after crushed sciatic nerve injury in rats. Ultrasound Med. Biol. 2015, 41, 790–796. [Google Scholar] [CrossRef]
- Speed, C.A.; Nichols, D.; Richards, C.; Humphreys, H.; Wies, J.T.; Burnet, S.; Hazleman, B.L. Extracorporeal shock wave therapy for lateral epicondylitis—A double blind randomised controlled trial. J. Orthop. Res. 2002, 20, 895–898. [Google Scholar] [CrossRef]
- Munver, R.; Delvecchio, F.C.; Kuo, R.L.; Brown, S.A.; Zhong, P.; Preminger, G.M. In vivo assessment of free radical activity during shock wave lithotripsy using a microdialysis system: The renoprotective action of allopurinol. J. Urol. 2002, 167, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, K.W.; Kim, Y.H.; Seol, P.H.; Kwak, H.J.; Park, S.W.; Ryu, B.J. Effect of Radial Extracorporeal Shock Wave Therapy on Hemiplegic Shoulder Pain Syndrome. Ann. Rehabil. Med. 2016, 40, 509–519. [Google Scholar] [CrossRef]
- Park, K.D.; Lee, W.Y.; Park, M.H.; Ahn, J.K.; Park, Y. High- versus low-energy extracorporeal shock-wave therapy for myofascial pain syndrome of upper trapezius: A prospective randomized single blinded pilot study. Medicine 2018, 97, e11432. [Google Scholar] [CrossRef]
- Guo, J.; Qian, S.; Wang, Y.; Xu, A. Clinical study of combined mirror and extracorporeal shock wave therapy on upper limb spasticity in poststroke patients. Int. J. Rehabil. Res. 2019, 42, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.U.; Bellomo, R.G.; Saggini, A. Treatment of Chronic Wounds and Ulcers with Focused and Defocused Shock Waves. In Pearls and Pitfalls in Skin Ulcer Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 175–180. [Google Scholar]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Astur, D.C.; Santos, B.; Moraes, E.R.d.; Arliani, G.G.; Santos, P.R.D.d.; Pochini, A.d.C. Extracorporeal shockwave TERAPY to treat chronic muscle injury. Acta Ortop. Bras. 2015, 23, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Mattyasovszky, S.G.; Langendorf, E.K.; Ritz, U.; Schmitz, C.; Schmidtmann, I.; Nowak, T.E.; Wagner, D.; Hofmann, A.; Rommens, P.M.; Drees, P. Exposure to radial extracorporeal shock waves modulates viability and gene expression of human skeletal muscle cells: A controlled in vitro study. J. Orthop. Surg. Res. 2018, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Langendorf, E.K.; Klein, A.; Drees, P.; Rommens, P.M.; Mattyasovszky, S.G.; Ritz, U. Exposure to radial extracorporeal shockwaves induces muscle regeneration after muscle injury in a surgical rat model. J. Orthop. Res. 2020, 38, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, J.; Banie, L.; Reed-Maldonado, A.B.; Ning, H.; Lu, Z.; Ruan, Y.; Zhou, T.; Wang, H.S.; Oh, B.S. Low-intensity extracorporeal shock wave therapy promotes myogenesis through PERK/ATF4 pathway. Neurourol. Urodyn. 2018, 37, 699–707. [Google Scholar] [CrossRef]
- Zissler, A.; Steinbacher, P.; Zimmermann, R.; Pittner, S.; Stoiber, W.; Bathke, A.C.; Sänger, A.M. Extracorporeal shock wave therapy accelerates regeneration after acute skeletal muscle injury. Am. J. Sports Med. 2017, 45, 676–684. [Google Scholar] [CrossRef]
- Wuerfel, T.; Schmitz, C.; Jokinen, L.L. The effects of the exposure of musculoskeletal tissue to extracorporeal shock waves. Biomedicines 2022, 10, 1084. [Google Scholar] [CrossRef]
- Wu, K.-T.; Chou, W.-Y.; Wang, C.-J.; Chen, C.-Y.; Ko, J.-Y.; Chen, P.-C.; Cheng, J.-H.; Yang, Y.-J. Efficacy of extracorporeal shockwave therapy on calcified and noncalcified shoulder tendinosis: A propensity score matched analysis. BioMed Res. Int. 2019, 2019, 2958251. [Google Scholar] [CrossRef]
- Simplicio, C.L.; Purita, J.; Murrell, W.; Santos, G.S.; Dos Santos, R.G.; Lana, J.F.S.D. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J. Clin. Orthop. Trauma. 2020, 11, S309–S318. [Google Scholar] [CrossRef] [PubMed]
- Alshihri, A.; Niu, W.; Kämmerer, P.W.; Al-Askar, M.; Yamashita, A.; Kurisawa, M.; Spector, M. The effects of shock wave stimulation of mesenchymal stem cells on proliferation, migration, and differentiation in an injectable gelatin matrix for osteogenic regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1630–1640. [Google Scholar] [CrossRef]
- Notarnicola, A.; Vicenti, G.; Maccagnano, G.; Silvestris, F.; Cafforio, P.; Moretti, B. Extracorporeal shock waves induce osteogenic differentiation of human bone-marrow stromal cells. J. Biol. Regul. Homeost. Agents 2016, 30, 139–144. [Google Scholar] [PubMed]
- Leone, L.; Raffa, S.; Vetrano, M.; Ranieri, D.; Malisan, F.; Scrofani, C.; Vulpiani, M.C.; Ferretti, A.; Torrisi, M.R.; Visco, V. Extracorporeal Shock Wave Treatment (ESWT) enhances the in vitro-induced differentiation of human tendon-derived stem/progenitor cells (hTSPCs). Oncotarget 2016, 7, 6410. [Google Scholar] [CrossRef] [PubMed]
- Modena, D.A.O.; da Silva, C.N.; Grecco, C.; Guidi, R.M.; Moreira, R.G.; Coelho, A.A.; Sant’Ana, E.; de Souza, J.R. Extracorporeal shockwave: Mechanisms of action and physiological aspects for cellulite, body shaping, and localized fat—Systematic review. J. Cosmet. Laser Ther. 2017, 19, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.A.; Tóth-Kischkat, A.; Schultheiss, R. Principles of shock wave therapy. Clin. Orthop. Relat. Res. 2001, 387, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Sáez, J.; Muñoz, P.; Serracanta, J.; Monte, A.; Barret, J.P. Extracorporeal shock wave therapy role in the treatment of burn patients. A systematic literature review. Burns 2020, 46, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Hong, A.R.; Kim, J.-B.; Yu, J.H.; Cho, Y.S.; Joo, S.Y.; Seo, C.H. Extracorporeal shock wave therapy alters the expression of fibrosis-related molecules in fibroblast derived from human hypertrophic scar. Int. J. Mol. Sci. 2018, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Zhang, B.R.; Shi, K.; Wang, J.; Yu, Q.-H.; Yu, J.A. Lower energy radial shock wave therapy improves characteristics of hypertrophic scar in a rabbit ear model. Exp. Ther. Med. 2018, 15, 933–939. [Google Scholar] [CrossRef]
- Lee, S.Y.; Joo, S.Y.; Cho, Y.S.; Hur, G.Y.; Seo, C.H. Effect of extracorporeal shock wave therapy for burn scar regeneration: A prospective, randomized, double-blinded study. Burns 2021, 47, 821–827. [Google Scholar] [CrossRef]
- Kołodziejczak, A.; Wieczorek, A.; Rotsztejn, H. The assessment of the effects of the combination of microdermabrasion and cavitation peeling in the therapy of seborrhoeic skin with visible symptoms of acne punctata. J. Cosmet. Laser Ther. 2019, 21, 286–290. [Google Scholar] [CrossRef]
- Djedovic, G.; Kamelger, F.S.; Jeschke, J.; Piza-Katzer, H. Effect of extracorporeal shock wave treatment on deep partial-thickness burn injury in rats: A pilot study. Plast. Surg. Int. 2014, 2014, 495967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-C.; Zhang, B.-R.; Hong, L.; Shi, K.; Wu, W.-W.; Yu, J.-A. Extracorporeal shock wave therapy with low-energy flux density inhibits hypertrophic scar formation in an animal model. Int. J. Mol. Med. 2018, 41, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Aschermann, I.; Noor, S.; Venturelli, S.; Sinnberg, T.; Busch, C.; Mnich, C.D. Extracorporal shock waves activate migration, proliferation and inflammatory pathways in fibroblasts and keratinocytes, and improve wound healing in an open-label, single-arm study in patients with therapy-refractory chronic leg ulcers. Cell. Physiol. Biochem. 2017, 41, 890–906. [Google Scholar] [CrossRef]
- Swayne, O.B.; Rothwell, J.C.; Ward, N.S.; Greenwood, R.J. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb. Cortex 2008, 18, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cheng, A.; Zhang, Z.; Sun, Y.; Liu, Y. Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol. 2021, 21, 369. [Google Scholar] [CrossRef]
- Manganotti, P.; Amelio, E. Long-term effect of shock wave therapy on upper limb hypertonia in patients affected by stroke. Stroke 2005, 36, 1967–1971. [Google Scholar] [CrossRef]
- Bae, H.; Lee, J.M.; Lee, K.H. The effects of extracorporeal shock wave therapy on spasticity in chronic stroke patients. J. Korean Acad. Rehabil. Med. 2010, 34, 663–669. [Google Scholar]
- Yoo, S.D.; Kim, H.S.; Jung, P.K. The Effect of Shock Wave Therapy on Upper Limb Spasticityin the Patients with Stroke. J. Korean Acad. Rehabil. Med. 2008, 32, 406–410. [Google Scholar]
- Brunelli, S.; Gentileschi, N.; Spanò, B.; Pratesi, L.; Calvani, A.; Mucci, R.; Foti, C. Effect of Early Radial Shock Wave Treatment on Spasticity in Subacute Stroke Patients: A Pilot Study. BioMed Res. Int. 2022, 2022, 8064548. [Google Scholar] [CrossRef]
- Li, G.; Yuan, W.; Liu, G.; Qiao, L.; Zhang, Y.; Wang, Y.; Wang, W.; Zhao, M.; Wang, Y.; Wang, J. Effects of radial extracorporeal shockwave therapy on spasticity of upper-limb agonist/antagonist muscles in patients affected by stroke: A randomized, single-blind clinical trial. Age Ageing 2020, 49, 246–252. [Google Scholar] [CrossRef]
- Park, S.K.; Yang, D.J.; Uhm, Y.H.; Yoon, J.H.; Kim, J.H. Effects of extracorporeal shock wave therapy on upper extremity muscle tone in chronic stroke patients. J. Phys. Ther. Sci. 2018, 30, 361–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dymarek, R.; Taradaj, J.; Rosińczuk, J. Extracorporeal shock wave stimulation as alternative treatment modality for wrist and fingers spasticity in poststroke patients: A prospective, open-label, preliminary clinical trial. Evid. Based Complement. Altern. Med. 2016, 2016, 4648101. [Google Scholar] [CrossRef]
- Yoon, S.H.; Shin, M.K.; Choi, E.J.; Kang, H.J. Effective site for the application of extracorporeal shock-wave therapy on spasticity in chronic stroke: Muscle belly or myotendinous junction. Ann. Rehabil. Med. 2017, 41, 547–555. [Google Scholar] [CrossRef]
- Senarath, I.D.; Thalwathte, R.D.; Pathirage, M.; Kularatne, S.A. The effectiveness of radial extracorporeal shock wave therapy vs transcutaneous electrical nerve stimulation in the management of upper limb spasticity in chronic-post stroke hemiplegia—A randomized controlled trial. PLoS ONE 2023, 18, e0283321. [Google Scholar] [CrossRef]
- Savevska, C.G.; Dimitrova, E.N.; Gocevska, M. Effects of radial extracorporeal shock wave therapy on hand spasticity in poststroke patient. Hippokratia 2016, 20, 309. [Google Scholar]
- Fan, T.; Zhou, X.; He, P.; Zhan, X.; Zheng, P.; Chen, R.; Li, R.; Li, R.; Wei, M.; Zhang, X. Effects of radial extracorporeal shock wave therapy on flexor spasticity of the upper limb in post-stroke patients: Study protocol for a randomized controlled trial. Front. Neurol. 2021, 12, 712512. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, J.; Cheng, Q.-F.; Zhang, Q. Clinical efficacy of ultrasound-guided stellate ganglion block combined with extracorporeal shock wave therapy on limb spasticity in patients with ischemic stroke. BMC Neurol. 2023, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Tabra, S.A.A.; Zaghloul, M.I.; Alashkar, D.S. Extracorporeal shock wave as adjuvant therapy for wrist and hand spasticity in post-stroke patients: A randomized controlled trial. Egypt. Rheumatol. Rehabil. 2021, 48, 21. [Google Scholar] [CrossRef]
- Kwon, S.; Park, J.-H.; Kim, W.-S.; Han, K.; Lee, Y.; Paik, N.-J. Health-related quality of life and related factors in stroke survivors: Data from Korea National Health and Nutrition Examination Survey (KNHANES) 2008 to 2014. PLoS ONE 2018, 13, e0195713. [Google Scholar] [CrossRef]
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-stroke spasticity: Predictors of early development and considerations for therapeutic intervention. PM R 2015, 7, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ge, L.; Hu, H.; Yan, L.; Li, L. Effects of non-invasive brain stimulation on post-stroke spasticity: A systematic review and meta-analysis of randomized controlled trials. Brain Sci. 2022, 12, 836. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).