Improvement in Pain, Quality of Life, and Urinary Dysfunction following Correction of Lumbar Lordosis and Reduction in Lumbar Spondylolistheses Using Chiropractic BioPhysics® Structural Spinal Rehabilitation: A Case Series with >1-Year Long-Term Follow-Up Exams
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusion and Exclusion Criteria
- Health histories revealed disorder of the urinary system (International Classification of Diseases, Tenth Revision (ICD-10) N39.9) and urgency of urination (ICD R39.15).
- Physical examination revealed low back pain (ICD-10 M54.50) and lumbar radiculopathy (ICD-10 M54.16).
- Patient-reported outcomes (PROs) and measures (PROMs) using short-form 36-question health-related quality of life (HRQOL) questionnaire (SF-36) revealed decreased quality of life, and numeric rating scale (NRS) revealed moderate-to-severe low back pain and severe urinary urgency.
- Sagittal radiographic exams revealed lumbar hyperlordosis (ICD-10 M40.56) and spondylolistheses of the lumbar spine (ICD-10 M43.16).
2.2. Patient-Reported Outcomes and Measures
2.2.1. Numeric Rating Scale
2.2.2. Short-Form 36-Question Health-Related Quality-of-Life Questionnaire
2.3. Radiographic Analysis
2.4. Patients’ Presentations
2.4.1. Patient 1
2.4.2. Patient 2
2.4.3. Patient 3
2.4.4. Summary of Patients’ Presentations
2.5. Chiropractic BioPhysics® Structural Spinal Rehabilitation
2.5.1. Mirror Image® Spinal Traction
2.5.2. Mirror Image® Spinal Exercises
2.5.3. Mirror Image® Adjustments
3. Results
3.1. Post-Treatment and Long-Term Follow-Up Exam Results
3.1.1. Patient 1
3.1.2. Patient 2
3.1.3. Patient 3
3.1.4. Summary of Patients’ Post-Treatment and Long-Term Follow-Up Exam Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, S.; Horton, R. Low back pain: A major global challenge. Lancet 2018, 391, 2302. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Budgell, B. Somato-Autonomic Reflex. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3767–3770. ISBN 978-3-540-29678-2. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Q.S.; Xu, L.; Chen, Z.H.; Zhu, Z.Z.; Li, S.; Qiu, Y.; Sun, X. Does kyphotic configuration on upright lateral radiograph correlate with instability in patients with degenerative lumbar spondylolisthesis? Clin. Neurol. Neurosurg. 2018, 173, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.H.; Stone, K.H. The Etiology of Spondylolisthesis. J. Bone Surg. 1963, 45, 39–59. [Google Scholar] [CrossRef]
- Kent, C. Models of Vertebral Subluxation: A Review. J. Vert. Subluxation Res. 1996, 1, 1–7. [Google Scholar]
- He, L.-C.; Wang, Y.-X.J.; Gong, J.-S.; Griffith, J.F.; Zeng, X.-J.; Kwok, A.W.; Leung, J.C.; Kwok, T.; Ahuja, A.T.; Leung, P.C. Prevalence and Risk Factors of Lumbar Spondylolisthesis in Elderly Chinese Men and Women. Eur. Radiol. 2014, 24, 441–448. [Google Scholar] [CrossRef]
- Jacobsen, S.; Sonne-Holm, S.; Rovsing, H.; Monrad, H.; Gebuhr, P. Degenerative Lumbar Spondylolisthesis: An Epidemiological Perspective: The Copenhagen Osteoarthritis Study. Spine 2007, 32, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Kim, D.H.; Li, L.; Guermazi, A.; Berkin, V.; Hunter, D.J. Spondylolysis and Spondylolisthesis: Prevalence and Association with Low Back Pain in the Adult Community-Based Population. Spine 2009, 34, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Love, T.W.; Fagan, A.B.; Fraser, R.D. Degenerative Spondylolisthesis: Developmental or Acquired? J. Bone Jt. Surg. Br. 1999, 81, 670–674. [Google Scholar] [CrossRef]
- DeVine, J.G.; Schenk-Kisser, J.M.; Skelly, A.C. Risk Factors for Degenerative Spondylolisthesis: A Systematic Review. Evid. Based Spine Care J. 2012, 3, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Hanscom, B.; Tosteson, A.N.A.; Blood, E.A.; Birkmeyer, N.J.O.; Hilibrand, A.S.; Herkowitz, H.; Cammisa, F.P.; et al. Surgical versus Nonsurgical Treatment for Lumbar Degenerative Spondylolisthesis. N. Engl. J. Med. 2007, 356, 2257–2270. [Google Scholar] [CrossRef]
- Chan, A.K.; Sharma, V.; Robinson, L.C.; Mummaneni, P.V. Summary of Guidelines for the Treatment of Lumbar Spondylolisthesis. Neurosurg. Clin. N. Am. 2019, 30, 353–364. [Google Scholar] [CrossRef]
- Wong, L.C. Rehabilitation of a Patient with a Rare Multi-Level Isthmic Spondylolisthesis: A Case Report. J. Can. Chiropr. Assoc. 2004, 48, 142–151. [Google Scholar]
- Vibert, B.T.; Sliva, C.D.; Herkowitz, H.N. Treatment of Instability and Spondylolisthesis: Surgical versus Nonsurgical Treatment. Clin. Orthop. Relat. Res. 2006, 443, 222–227. [Google Scholar] [CrossRef]
- Excoffon, S.G.; Wallace, H. Chiropractic and Rehabilitative Management of a Patient with Progressive Lumbar Disk Injury, Spondylolisthesis, and Spondyloptosis. J. Manip. Physiol. Ther. 2006, 29, 66–71. [Google Scholar] [CrossRef]
- Dunn, A.S.; Baylis, S.; Ryan, D. Chiropractic Management of Mechanical Low Back Pain Secondary to Multiple-Level Lumbar Spondylolysis with Spondylolisthesis in a United States Marine Corps Veteran: A Case Report. J. Chiropr. Med. 2009, 8, 125–130. [Google Scholar] [CrossRef]
- Fedorchuk, C.; Himel, B.; Lightstone, D.F. Improved Pain and Quality of Life with Corrected Thoracic and Lumbosacral Spondylolisthesis Subluxations Using CBP®: A Case Study and 1-Year Follow-Up. J. Radiol. Case Rep. 2022, 16, 21–38. [Google Scholar] [CrossRef]
- Fedorchuk, C.; Lightstone, D.F.; McRae, C.; Kaczor, D. Correction of Grade 2 Spondylolisthesis Following a Non-Surgical Structural Spinal Rehabilitation Protocol Using Lumbar Traction: A Case Study and Selective Review of Literature. J. Radiol. Case Rep. 2017, 11, 13–26. [Google Scholar] [CrossRef]
- Fedorchuk, C.A.; Lightstone, D.F.; Oakley, P.A.; Harrison, D.E. Correction of a Double Spondylolisthesis of the Lumbar Spine Utilizing Chiropractic BioPhysics® Technique: A Case Report with 1 Year Follow-Up. J. Phys. Ther. Sci. 2021, 33, 89–93. [Google Scholar] [CrossRef]
- Weldring, T.; Smith, S.M. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, 61–68. [Google Scholar] [CrossRef]
- de Williams, A.C.; Davies, H.T.O.; Chadury, Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain 2000, 85, 457–463. [Google Scholar] [CrossRef]
- Hanley, M.A.; Jensen, M.P.; Ehde, D.M.; Robinson, L.R.; Cardenas, D.D.; Turner, J.A.; Smith, D.G. Clinically significant change in pain intensity ratings in persons with spinal cord injury or amputation. Clin. J. Pain 2006, 22, 25–31. [Google Scholar] [CrossRef]
- Gousse, A.; Vetter, J.; Lai, H.H. Assessment of bladder pressure and discomfort symptoms: How do overactive bladder differ from interstitial cystitis/bladder pain syndrome patients? BMC Urol. 2023, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Notte, S.M.; Marshall, T.S.; Lee, M.; Hakimi, Z.; Odeyemi, I.; Chen, W.H.; Revicki, D.A. Content validity and test-retest reliability of Patient Perception of Intensity of Urgency Scale (PPIUS) for overactive bladder. BMC Urol. 2012, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.D.; Piva, S.R.; Fritz, J.M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005, 30, 1331–1334. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef]
- Syddall, H.E.; Martin, H.J.; Harwood, R.H.; Cooper, C.; Aihie Sayer, A. The SF-36: A simple, effective measure of mobility-disability for epidemiological studies. J. Nutr. Health Aging 2009, 13, 57–62. [Google Scholar] [CrossRef]
- Walters, S.J.; Munro, J.F.; Brazier, J.E. Using the SF-36 with older adults: A cross-sectional community-based survey. Age Ageing 2001, 30, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Yarlas, A.; Bayliss, M.; Cappelleri, J.C.; Maher, S.; Bushmakin, A.G.; Chen, L.A.; Manuchehri, A.; Healey, P. Psychometric validation of the SF-36® Health Survey in ulcerative colitis: Results from a systematic literature review. Qual. Life Res. 2018, 27, 273–290. [Google Scholar] [CrossRef]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in Orthopaedics: A Brief Guide. J. Bone Jt. Surg. Am. 2015, 97, 1628–1634. [Google Scholar] [CrossRef]
- Carreon, L.Y.; Bratcher, K.R.; Canan, C.E.; Burke, L.O.; Djurasovic, M.; Glassman, S.D. Differentiating minimum clinically important difference for primary and revision lumbar fusion surgeries. J. Neurosurg. Spine 2013, 18, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Oakley, P.A.; Harrison, D.D.; Harrison, D.E.; Haas, J.W. Evidence-Based Protocol for Structural Rehabilitation of the Spine and Posture: Review of Clinical Biomechanics of Posture (CBP®) Publications. J. Can. Chiropr. Assoc. 2005, 49, 270–296. [Google Scholar]
- Harrison, D.E.; Harrison, D.D.; Colloca, C.J.; Betz, J.; Janik, T.J.; Holland, B. Repeatability over Time of Posture, Radiograph Positioning, and Radiograph Line Drawing: An Analysis of Six Control Groups. J. Manip. Physiol. Ther. 2003, 26, 87–98. [Google Scholar] [CrossRef]
- Fedorchuk, C.; Comer, R.D.; McRae, C.; Bak, D.; Lightstone, D.F. Validity of Radiographic Analyses Between Hand-Drawn and Computer- Aided Measurements: A Double-Blinded Test-Retest Trial. Curr. Med. Imaging 2023, 19, 1071–1078. [Google Scholar] [CrossRef]
- Harrison, D.E.; Janik, T.J.; Harrison, D.D.; Cailliet, R.; Harmon, S.F. Can the Thoracic Kyphosis Be Modeled With a Simple Geometric Shape?: The Results of Circular and Elliptical Modeling in 80 Asymptomatic Patients. J. Spinal Disord. Tech. 2002, 15, 213–220. [Google Scholar] [CrossRef]
- Janik, T.J.; Harrison, D.D.; Cailliet, R.; Troyanovich, S.J.; Harrison, D.E. Can the Sagittal Lumbar Curvature Be Closely Approximated by an Ellipse? J. Orthop. Res. 1998, 16, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Troyanovich, S.J.; Robertson, G.A.; Harrison, D.D.; Holland, B. Intra- and Interexaminer Reliability of the Chiropractic Biophysics Lateral Lumbar Radiographic Mensuration Procedure. J. Manip. Physiol. Ther. 1995, 18, 519–524. [Google Scholar]
- Harrison, D.D.; Janik, T.J.; Troyanovich, S.J.; Holland, B. Comparisons of Lordotic Cervical Spine Curvatures to a Theoretical Ideal Model of the Static Sagittal Cervical Spine. Spine 1996, 21, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Troyanovich, S.; Cailliet, R.; Janik, T.; Harrison, D.; Harrison, D. Radiographic Mensuration Characteristics of the Sagittal Lumbar Spine from a Normal Population with a Method to Synthesize Prior Studies of Lordosis. J. Spinal Disord. Tech. 1997, 10, 380–386. [Google Scholar] [CrossRef]
- Harrison, D.D.; Cailliet, R.; Janik, T.J.; Troyanovich, S.J.; Harrison, D.E.; Holland, C.B. Elliptical Modeling of the Sagittal Lumbar Lordosis and Segmental Rotation Angles as a Method to Discriminate Between Normal and Low Back Pain Subjects. J. Spinal Disord. 1998, 11, 430–439. [Google Scholar] [CrossRef]
- Fedorchuk, C.; Opitz, K. Improvement in Quality of Life and Improved Cervical Curve in an 11-Year-Old Child with Asthma Following Chiropractic Intervention: A Case Study. J. Pediatr. Matern. Fam. Health-Chiropr. 2014, 2014, 37–46. [Google Scholar]
- Bastecki, A.V.; Harrison, D.E.; Haas, J.W. Cervical Kyphosis Is a Possible Link to Attention-Deficit/Hyperactivity Disorder. J. Manip. Physiol. Ther. 2004, 27, e14. [Google Scholar] [CrossRef]
- Labelle, H.; Roussouly, P.; Berthonnaud, E.; Transfeldt, E.; O’Brien, M.; Chopin, D.; Hresko, T.; Dimnet, J. Spondylolisthesis, pelvic incidence, and spinopelvic balance: A correlation study. Spine 2004, 15, 2049–2054. [Google Scholar] [CrossRef]
- Rajnics, P.; Templier, A.; Skalli, W.; Lavaste, F.; Illés, T. The association of sagittal spinal and pelvic parameters in asymptomatic persons and patients with isthmic spondylolisthesis. J. Spinal Disord. Tech. 2002, 15, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Schuller, S.; Charles, Y.P.; Steib, J.P. Sagittal spinopelvic alignment and body mass index in patients with degenerative spondylolisthesis. Eur. Spine J. 2011, 20, 713–719. [Google Scholar] [CrossRef]
- Chu, E.C.-P. Reducing Cervical Retrolisthesis With Long-Term Monthly Chiropractic Maintenance Care: A Case Report. J. Med. Cases 2022, 13, 359–364. [Google Scholar] [CrossRef]
- Oakley, P.A.; Ehsani, N.N.; Moustafa, I.M.; Harrison, D.E. Restoring Lumbar Lordosis: A Systematic Review of Controlled Trials Utilizing Chiropractic Bio Physics® (CBP®) Non-Surgical Approach to Increasing Lumbar Lordosis in the Treatment of Low Back Disorders. J. Phys. Ther. Sci. 2020, 32, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Oakley, P.A. An Introduction to Chiropractic BioPhysics® (CBP®) Technique: A Full Spine Rehabilitation Approach to Reducing Spine Deformities. In Complementary Therapies; Bernardo-Filho, M., Taiar, R., da Cunha de Sa-Caputo, D., Seixas, A., Eds.; IntechOpen: London, UK, 2022; pp. 1–34. ISBN 978-1-83969-012-9. [Google Scholar] [CrossRef]
- Oakley, P.A.; Kallan, S.; Harrison, D.E. Structural rehabilitation of the lumbar lordosis: A selective review of CBP® case reports. J. Contemp. Chiropr. 2022, 5, 206–211. [Google Scholar]




| Assessment | Normal Value | Pre-Treatment Exam 9/2019 | Post-Treatment Exam 12/2019 | 4-Year Follow-Up Exam 1/2024 | |
|---|---|---|---|---|---|
| Back Pain NRS | 0 | 7 | 1 | 2 | |
| SF-36 HRQOL Scales | PF | 72.0 | 45.0 | 85.0 | 80.0 |
| RP | 81.0 | 25.0 | 100.0 | 100.0 | |
| RE | 81.0 | 66.7 | 100.0 | 100.0 | |
| VT | 61.0 | 40.0 | 60.0 | 55.0 | |
| MH | 81.0 | 76.0 | 92.0 | 96.0 | |
| SF | 83.0 | 87.5 | 100.0 | 100.0 | |
| BP | 75.0 | 45.0 | 90.0 | 87.5 | |
| GH | 72.0 | 67.5 | 80.0 | 80.0 | |
| ΔH | 84.0 | 25.0 | 75.0 | 75.0 | |
| PCS | 46.8 | 32.6 | 51.7 | 49.9 | |
| MCS | 52.8 | 53.8 | 57.5 | 58.7 | |
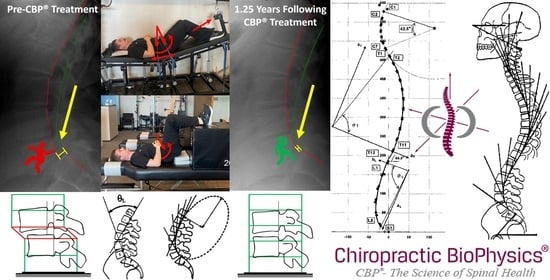
| ARA L1-L5 (°) | −40 | −47.8 | −41.1 | −43.4 | |
| Tz L5-S1 (mm) | 0 | 15.8 | 4.2 | 4.3 | |
| Urination Frequency (times/24 h) | 0 | 6 | 6 | 6 | |
| Urinary Urgency NRS | 0 | 8 | 2 | 2 | |
| Assessment | Normal Value | Pre-Treatment Exam 2/2022 | Post-Treatment Exam 5/2022 | 1.25-Year Follow-Up Exam 8/2023 | |
|---|---|---|---|---|---|
| Back Pain NRS | 0 | 8 | 1 | 2 | |
| SF-36 HRQOL Scales | PF | 72.0 | 0.0 | 55.0 | 55.0 |
| RP | 81.0 | 0.0 | 100.0 | 100.0 | |
| RE | 81.0 | 0.0 | 100.0 | 100.0 | |
| VT | 61.0 | 15.0 | 80.0 | 75.0 | |
| MH | 81.0 | 80.0 | 100.0 | 80.0 | |
| SF | 83.0 | 25.0 | 75.0 | 100.0 | |
| BP | 75.0 | 22.5 | 70.0 | 62.5 | |
| GH | 72.0 | 67.5 | 90.0 | 90.0 | |
| ΔH | 84.0 | 25.0 | 100.0 | 75.0 | |
| PCS | 46.8 | 21.8 | 44.1 | 45.4 | |
| MCS | 52.8 | 42.2 | 62.7 | 60.1 | |
| ARA L1-L5 (°) | −40 | −49.9 | −42.2 | −43.6 | |
| Tz L5-S1 (mm) | 0 | 13.8 | 4.2 | 4.3 | |
| Urination Frequency (times/24 h) | 0 | 12 | 6 | 6 | |
| Urinary Urgency NRS | 0 | 9 | 3 | 3 | |
| Assessment | Normal Value | Pre-Treatment Exam 3/2015 | Post-Treatment Exam 1/2016 | 3.75-Year Follow-Up Exam 8/2019 | |
|---|---|---|---|---|---|
| Back Pain NRS | 0 | 7 | 1 | 2 | |
| SF-36 HRQOL Scales | PF | 72.0 | 50.0 | 95.0 | 85.0 |
| RP | 81.0 | 25.0 | 80.0 | 75.0 | |
| RE | 81.0 | 66.7 | 90.0 | 100.0 | |
| VT | 61.0 | 40.0 | 70.0 | 70.0 | |
| MH | 81.0 | 52.0 | 76.0 | 72.0 | |
| SF | 83.0 | 60.0 | 100.0 | 87.5 | |
| BP | 75.0 | 50.0 | 90.0 | 87.5 | |
| GH | 72.0 | 50.0 | 85.0 | 80.0 | |
| ΔH | 84.0 | 25.0 | 75.0 | 75.0 | |
| PCS | 46.8 | 35.0 | 54.8 | 51.4 | |
| MCS | 52.8 | 43.5 | 52.7 | 52.8 | |
| ARA L1-L5 (°) | −40 | −51.2 | −45.2 | −45.4 | |
| Tz L5-S1 (mm) | 0 | 13.8 | 4.2 | 4.3 | |
| Urination Frequency (times/24 h) | 0 | 6 | 6 | 6 | |
| Urinary Urgency NRS | 0 | 7 | 2 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorchuk, C.A.; Fedorchuk, C.G.; Lightstone, D.F. Improvement in Pain, Quality of Life, and Urinary Dysfunction following Correction of Lumbar Lordosis and Reduction in Lumbar Spondylolistheses Using Chiropractic BioPhysics® Structural Spinal Rehabilitation: A Case Series with >1-Year Long-Term Follow-Up Exams. J. Clin. Med. 2024, 13, 2024. https://doi.org/10.3390/jcm13072024
Fedorchuk CA, Fedorchuk CG, Lightstone DF. Improvement in Pain, Quality of Life, and Urinary Dysfunction following Correction of Lumbar Lordosis and Reduction in Lumbar Spondylolistheses Using Chiropractic BioPhysics® Structural Spinal Rehabilitation: A Case Series with >1-Year Long-Term Follow-Up Exams. Journal of Clinical Medicine. 2024; 13(7):2024. https://doi.org/10.3390/jcm13072024
Chicago/Turabian StyleFedorchuk, Curtis A., Cole G. Fedorchuk, and Douglas F. Lightstone. 2024. "Improvement in Pain, Quality of Life, and Urinary Dysfunction following Correction of Lumbar Lordosis and Reduction in Lumbar Spondylolistheses Using Chiropractic BioPhysics® Structural Spinal Rehabilitation: A Case Series with >1-Year Long-Term Follow-Up Exams" Journal of Clinical Medicine 13, no. 7: 2024. https://doi.org/10.3390/jcm13072024
APA StyleFedorchuk, C. A., Fedorchuk, C. G., & Lightstone, D. F. (2024). Improvement in Pain, Quality of Life, and Urinary Dysfunction following Correction of Lumbar Lordosis and Reduction in Lumbar Spondylolistheses Using Chiropractic BioPhysics® Structural Spinal Rehabilitation: A Case Series with >1-Year Long-Term Follow-Up Exams. Journal of Clinical Medicine, 13(7), 2024. https://doi.org/10.3390/jcm13072024