Abstract
Beta blockers (BBs) play a crucial role in enhancing the quality of life and extending the survival of patients with heart failure and reduced ejection fraction (HFrEF). Initiating the therapy at low doses and gradually titrating the dose upwards is recommended to ensure therapeutic efficacy while mitigating potential adverse effects. Vigilant monitoring for signs of drug intolerance is necessary, with dose adjustments as required. The management of older HF patients requires a case-centered approach, taking into account individual comorbidities, functional status, and frailty. Older adults, however, are often underrepresented in randomized clinical trials, leading to some uncertainty in management strategies as patients with HF in clinical practice are older than those enrolled in trials. The present article performs a scoping review of the past 25 years of published literature on BBs in older HF patients, focusing on age, outcomes, and tolerability. Twelve studies (eight randomized-controlled and four observational) encompassing 26,426 patients were reviewed. The results indicate that BBs represent a viable treatment for older HFrEF patients, offering benefits in symptom management, cardiac function, and overall outcomes. Their role in HF with preserved EF, however, remains uncertain. Further research is warranted to refine treatment strategies and address specific aspects in older adults, including proper dosing, therapeutic adherence, and tolerability.
Keywords:
beta-blockers; heart failure; HFpEF; HFrEF; ventricular dysfunction; clinical trial; guidelines; aged; frailty; multimorbidity 1. Introduction
Heart failure (HF) is a global public health problem and a significant cause of morbidity and mortality in developed countries, affecting about 64.3 million people worldwide [1]. The prevalence of HF increases with age, particularly in patients over 75–80 years. Although improvements in preventative therapies and the management of comorbidities have reduced the incidence of HF, this condition remains the major cause of hospitalization among older adults [1]. In Europe, the prevalence of HF among individuals aged 80 or older ranges from 15% to 20% [1,2,3,4]. However, older adults have long been underrepresented in randomized controlled trials (RCTs) [5,6]. For this reason, treatment efficacy and the optimal management of HF in older patients remains unclear [7,8]. Older HF patients may exhibit age-related conditions, such as frailty, multimorbidity, reduced drug tolerance, and polypharmacy [9,10,11], which may reduce adherence to medical therapies, increase drug–drug interactions, and contribute to worsening HF [11].
According to the latest guidelines, HF is classified into three types based on the left ventricular ejection fraction (LVEF) [9,10,11,12,13,14], namely HF with reduced ejection fraction (HFrEF), i.e., <41%, HF with mildly reduced ejection fraction (HFmrEF), i.e., with between 41% and 49%, and HF with preserved ejection fraction (HFpEF), i.e., ≥50%. Regarding medical therapy, the current guidelines recommend using four “pillars”, regardless of age. The first three pillars are angiotensin-converting enzyme inhibitors (ACE-I)/angiotensin receptor blockers (ARB) or angiotensin receptor–neprilysin inhibitors (ARNI), mineralocorticoid receptor antagonists (MRA), and beta-receptor blocker agents (BBs) [12,13,14]. The EMPEROR [15] and DELIVER [16] trials demonstrated that, compared to placebo, sodium-glucose cotransporter-2 (SGLT2) inhibitors can reduce heart failure HF hospitalizations (HFH) and cardiovascular mortality by 21% and 18%, respectively. These findings support the idea of considering SGLT2 inhibition as the fourth pillar in HF management. However, older HF patients are reported to receive suboptimal therapy compared to their younger counterparts. This discrepancy arises from a reluctance to prescribe due to concerns over adverse events and lower adherence rates among older individuals. As a result, outcomes for these patients tend to be poorer [17]. It should be noted that guidelines address the crucial role of involving a multidisciplinary team in managing HF in older adults, especially considering the complex needs arising from multimorbidity, frailty, cognitive impairment, and polypharmacy. This team should include a specialized HF cardiologist, nurse, geriatrician, dietician, psychologist, physical therapist, and occupational therapist to ensure comprehensive care [13].
BBs have been shown to reduce both death and HFH [18,19,20,21,22]. However, RCTs provide only sparse evidence of their use in older adults. This review addresses the current evidence on the use of BBs in older patients with HF and explores the existing gaps in knowledge on the topic.
2. Materials and Methods
2.1. Search Strategy
A literature search was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. The search strategy, developed and agreed upon by two authors (I.P. and F.A.) and approved by a third (F.L.), utilized Boolean search terms: “beta blockers” AND “heart failure” OR “diastolic dysfunction” AND “elderly” OR “older”. The literature search was performed on PubMed and EMBASE databases independently by two investigators (I.P. and F.A.), aiming to identify studies on BB therapy in older adults with HF. Titles and abstracts of all articles published in the last 25 years were assessed. Reference lists of the papers obtained through the literature search were screened in order to include a larger number of relevant studies.
2.2. Selection Process
Article selection was based on the following inclusion criteria: (a) studies reporting BBs in HF, or diastolic dysfunction, in terms of efficacy, safety, or tolerability; (b) studies including older adults; (c) studies with cohorts of more than 100 patients; and (d) human studies. The exclusion criteria were: (a) non-comparative studies; (b) lack of usable data concerning efficacy, safety, and tolerability of BBs in HF; (c) studies with patient cohorts comprising 100 or fewer individuals; (d) reviews, meta-analyses, commentaries, and letters.
2.3. Quality Assessment
The quality of included studies was assessed using Down and Black’s Checklist for Measuring, which evaluates the quality of randomized and non-randomized studies in terms of reporting, external validity, internal validity, and power. Each checklist component is rated using a binary score (0/1) except for three items, rated on a scale from 0 to 2 and 0 to 5, respectively [24]. Two independent researchers (I.P. and F.L.) conducted the ratings. Divergences were resolved by quantification through Cohen’s kappa [25].
2.4. Endpoints and Definitions
The primary endpoints evaluated in this review focused on the safety, efficacy, or tolerability of outcomes related to BBs in HFpEF and HFrEF patients. Some studies defined safety as the composite outcome reduction in HFH and deaths during the follow-up. In other studies, efficacy was defined as composite or non-composite, specifically reducing the number of HFH and overall mortality rates. Tolerability refers to the extent to which patients can bear the adverse effects of BBs without significantly impacting their quality of life or leading to treatment discontinuation. A rigid cutoff to define older age was specifically avoided as the definition varied from study to study, ranging from >65 to >70 or 75, or even 80 years.
3. Results
The research produced a total of 7859 records. Of these, 7737 were excluded as unrelated to the topic, leaving 122 records to be screened by title and abstract. Following this initial screening, 60 articles were retrieved for further evaluation. Based on our eligibility criteria, 12 articles were selected for inclusion in our review (Figure 1).

Figure 1.
PRISMA flow diagram for literature selection and study process.
A summary of included studies is displayed in Table 1. The studies included in the present review comprise data from a total of 26.426 patients, with either HFrEF [25,26,27,28,29,30], HFpEF [31], or both [20,32,33,34,35].

Table 1.
Summary of studies addressing the use of beta blockers in heart failure included in the review.
Eight articles were randomized trials or trial subanalyses [21,26,27,28,29,30,31,32,33,34,35].
Two were prospective observational [29,33], one was cross-sectional [30], and one was a retrospective cohort study [36]. Data from the MERIT-HF trial were reported in two separate articles [27,28]. A single study investigated the effects of nebivolol [21], two investigated bisoprolol [26,33] and one investigated metoprolol controlled-release/extended-release carvedilol [29,31,32,33], and four studies did not specify the type of BB [30,34,35,36].
Follow-up duration ranged from 3 months to 3.3 years.
4. Discussion
4.1. Heart Failure in Older Adults
The aging population, alongside advancements in the treatment and prognosis of ischemic heart disease and the introduction of effective therapies that enhance survival, are key factors driving an increase in the global prevalence of HF [1,38]. In the USA, nearly the entire population of patients with HF is aged 60 years and older, with about half of those aged 80 years or older [39]. HF in older patients, compared to younger adults, exhibits distinct characteristics from those in younger patients. Older HF patients are predominantly female, with HFpEF being more common. Additionally, they typically carry a high burden of both cardiac and non-cardiac comorbidities, necessitating tailored and multicomponent treatments [39].
In older individuals, a series of age-related physiological changes significantly contribute to the development of HF. These changes encompass a reduction in myocytes, modifications within the extracellular matrix, enhanced collagen deposition and fibrosis, disturbances in calcium metabolism, and a decline in adenosine triphosphate functionality. Such alterations precipitate compensatory hypertrophy alongside modifications in myocardial contraction and relaxation mechanisms. Moreover, interstitial fibrotic remodeling, matrix degradation, and increased myocardial and vascular stiffness can lead to ventricular dilation, elevated left ventricular (LV) infilling pressures, enlargement of the left atrium, and diastolic dysfunction. The situation is further complicated by comorbid conditions common in this population, including diabetes mellitus (DM), obesity, chronic renal failure (CRF), AH, and AF [40,41]. These comorbidities can potentially activate pro-inflammatory and pro-fibrotic pathways, thereby intensifying cardiac damage through the inflammation of cardiac microvascular endothelial cells and an escalation in oxidative stress [42,43]. Acute or chronic myocardial injury in older patients results in sympathetic activation with chronic adrenergic receptor stimulation, reduced cardiac β-receptor density and responsiveness, and diminished cardiac inotropic reserve. The clinical outcomes of these processes include reduced systolic function, accelerated LV remodeling, and the emergence of life-threatening ventricular arrhythmias [44] (Figure 2).
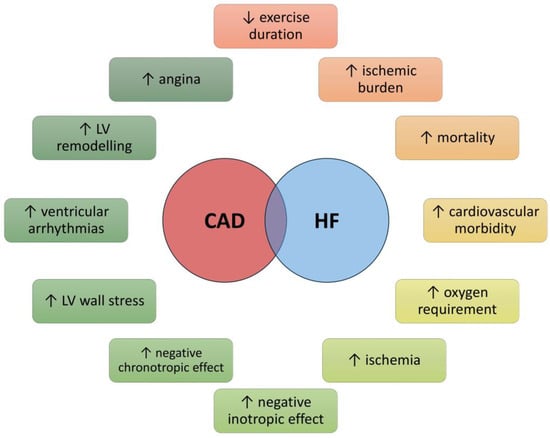
Figure 2.
Effects of the coexistence of coronary heart disease and heart failure on patients’ health. Abbreviations: CAD, coronary heart disease; HF, heart failure; LV, left ventricle.
Older patients have been shown to be less likely to receive an early diagnosis of HF [45]. A study by Oudejans et al. investigated the presence of signs and symptoms of HF in 206 geriatric patients suspected of HF (mean age 82 years). HF was diagnosed in approximately half of the patients, often presenting atypically with symptoms such as loss of appetite and low body mass index. Classic signs and symptoms of HF were absent in one-third of these patients [46].
In older patients, especially women, who were admitted to hospitals for HF, a high prevalence of HFpEF has been reported. The primary clinical manifestations include acute pulmonary edema and arterial hypertension. Older patients with HFpEF were more likely to present with nonspecific symptoms, such as weakness, weight loss, and loss of appetite. Additionally, about 65% of these patients reported respiratory fatigue despite the absence of pulmonary congestion upon physical examination [47,48]. In HFpEF, diastolic dysfunction and LV hypertrophy have been described [47,48]. Blood biomarkers, such as B-type natriuretic peptide (BNP), may not consistently exhibit elevated levels in older HFpEF patients, particularly those in sinus rhythm, with obesity, and/or normal kidney function [49,50]. However, echocardiography and BNP assessment are crucial for making a guideline-directed diagnosis of HF [51,52], and additional biomarkers, such as the soluble circulating form of the suppression of tumorigenicity two receptors (sST2), may guide prognostic stratification on admission [53,54].
In older adults, multimorbidity and polypharmacy, including non-steroidal anti-inflammatory drugs, antidepressants, antiarrhythmics, antibiotics, and anticoagulants, may directly exacerbate HF or amplify the risk of specific drug–drug interactions. Socioeconomic factors, such as limited access to caregivers and specialists, cognitive impairment, and financial constraints, can hinder adherence to medical therapies [55].
Notably, therapeutic options are considered more constrained for older patients due to the pharmacokinetic and pharmacodynamic changes associated with aging. The aging process leads to decreased lean mass and total body water and a relative increase in body fat, contributing to elevated plasma concentrations of hydrophilic drugs and reduced concentrations of lipophilic drugs. Diminished hepatic activity associated with aging disrupts first-pass metabolism, leading to the heightened activation of certain drugs and the diminished activation of others while declining renal function results in decreased clearance of several drugs [56].
4.2. Beta Blockers in Older Adults
BBs are a cornerstone in treating HF as they can improve symptoms, reduce hospitalizations, foster LV reverse remodeling, and enhance overall prognosis [57]. However, their utilization in older adults remains limited, largely due to concerns about potential adverse effects such as hypotension, bradycardia, and the risk of exacerbating HF, particularly HFpEF [40].
The previously described pathophysiological changes in the cardiovascular system in older age, coupled with heightened sympathetic nervous system activity, challenge the heart’s ability to function efficiently. BBs play a crucial role in this context by attenuating the adverse effects of sympathetic overdrive, improving myocardial relaxation, and reducing heart rate. This can be particularly beneficial given the altered cardiac physiology in older patients. However, the efficacy and tolerability of BBs in this demographic are influenced by age-related pharmacokinetic and pharmacodynamic variations and the high prevalence of comorbidities [58].
Some concerns have been raised regarding BB use in older patients. By altering the hemodynamic balance, β-blockers transition the patient’s profile from one characterized by normal cardiac output and elevated vascular resistance to one marked by reduced cardiac output and sustained high vascular resistance. This action, along with potential changes to DNA methylation profiles, may age the cardiovascular system of individuals, eventually accelerating the biological aging process [42,59]. Another relevant aspect is the potential role of BBs in increasing the risk of cognitive and functional impairment. According to Steinman et al., the prescription of BBs after acute myocardial infarction in nursing home residents reduced 90-day mortality by 26%. However, they determined an increased risk of functional decline, especially in individuals with significant cognitive or functional impairments [60]. The same study, however, showed that BBs did not negatively affect individuals with normal or mildly impaired cognition and those who maintain independence in activities of daily living [60]. More recently, Holm et al. addressed the association of BBs with an increased risk of developing vascular dementia (hazard ratio 1.72, 95% confidence interval 1.01–3.78; p = 0.048), but not all-cause, Alzheimer’s, or mixed dementia [61]. Conversely, data from the Danish national registers suggest that anti-hypertensive treatment with highly blood–brain barrier permeable BBs (e.g., carvedilol) may reduce the risk of Alzheimer’s disease by favoring the elimination of waste brain metabolites [62]. Regarding physical performance, Priel et al. demonstrated that BBs helped minimize exercise intolerance and symptoms by increasing oxygen pulse [63].
BBs are not recommended in HFpEF, which is a multifaceted clinical syndrome affecting various organ systems, accompanied by comorbidities such as AF, AH, DM, and obesity [64]. Conversely, in HFrEF, BBs are advised to manage heart rate, diminish LV hypertrophy, and mitigate ventricular arrhythmias, which are especially detrimental in this patient group [40]. The current review underscores a significant disconnection between clinical research and HF prevalence and demographic trends of incidence. A crucial point raised is the scarcity of recent evidence, which reveals a critical gap in our understanding. This deficiency highlights the urgent need to incorporate older age groups into large-scale international trials, reflecting their substantial presence within the real-world patient demographic. This inclusion is essential to bridge the gap between research and practice, ensuring that findings directly apply to the population most affected by HF, thereby enhancing patient care and outcomes.
4.3. Beta Blockers in Older Adults with Predominantly HFrEF
BBs are pivotal for managing patients with HFrEF, significantly enhancing prognosis. Nonetheless, their use among older adults, particularly those over 80, remains sparse. Research exploring BB prescription rates, adherence, tolerability, dose achievement, and their relationship with clinical outcomes has been undertaken, but findings are especially limited for this age group. The CHAMP-HF registry, capturing data from 3518 individuals with HFrEF between 2015 and 2017, reported BB prescriptions at 67% across the cohort, although only 28% received target doses at the 12-month mark. Older age, renal failure, lower blood pressure, and recent HFH were independent predictors of lower BB utilization and dosage [35].
A meta-analysis of 11 RCTs involving 13,833 patients with HFrEF in sinus rhythm, with a median age of 64, noted a decrease in mortality risk across all age groups for those treated with BBs. Drug discontinuation rates were comparable across age groups, with withdrawals primarily due to hypotension, bradycardia, worsening HF, and renal failure. Notably, the benefit of BBs in reducing hospitalization risk diminished with age [65]. The SENIORS trial, the only RCT targeting those aged ≥70, which evaluated BB effects on mortality and hospitalization regardless of LVEF, found a significant reduction in the combined risk of death and cardiovascular rehospitalization as the primary outcome, without a marked decrease in all-cause or cardiovascular mortality alone [21].
A secondary analysis from the TOPCAT trial indicated an association between BB use in patients with HFrEF and an increased risk of readmission for HF, although not for cardiovascular death [34]. Observational data, supported by subanalyses from the MERIT-HF, SENIORS, and MOCHA studies, suggest a reduction in mortality irrespective of BB dosage [26,27,64]. Moreover, an individual patient data meta-analysis of placebo-controlled RCTs by Kotecha et al. revealed an 18% mortality risk reduction for every five beats/min reduction in heart rate (HR) achieved through BB treatment, without a significant link between BB dosing and all-cause mortality [65].
Trials such as CIBIS-II [26], MERIT-HF [27,28], and COPERNICUS [32,66] demonstrated improved survival rates with bisoprolol, metoprolol CR/XL, and carvedilol for patients diagnosed with HFrEF. These studies typically involved younger participants compared to the SENIORS trial [21], with mean ages of 61, 64, and 63 years, respectively, against 76 years in the SENIORS trial [26,27,28,32,66]. Bisoprolol, carvedilol, and metoprolol have thus been endorsed in American and European guidelines as first-line BBs for HFrEF patients [12,13,14]. Nebivolol has also been recommended in European guidelines for HFrEF despite its uncertain effects on cardiovascular or all-cause mortality [12,13].
However, the potential poor tolerability of BBs in older patients may underlie their limited use. The CIBIS-ELD trial, comparing bisoprolol and carvedilol tolerability in individuals over 65 with HFrEF or HFpEF, evaluated target dose achievement (50–100 mg/day for carvedilol and 10 mg/day for bisoprolol) after 12 weeks [33]. Significant proportions, of 19% of patients with HFpEF and 27% with HFrEF, did not reach the target dose, with similar tolerability observed for both drugs. Compared to baseline, patients with HFrEF achieved greater clinical status and LVEF improvements, whereas those with HFpEF experienced more side effects for the same HR reduction [33]. In the CIBIS-II study, 63% of patients achieved the maximum bisoprolol maintenance dose (10 mg/day), and 37% reached 5 or 7.5 mg/day doses [26]. The tolerability was 85% (15% discontinuation rate) [26]. The percentage of patients who reached the target was not higher than in CIBIS-ELD (73%) [33], despite the ten-year-older average age in the latter compared to CIBIS-II patients [26]. The same consideration concerning CIBIS-ELD [33] can be made in relation to COPERNICUS [32,66], MERIT-HF [27,28], and SENIORS [21]. A meta-analysis of RCTs showed that withdrawal of therapy was not correlated with age [65].
The COLA II study assessed carvedilol tolerability in 1030 individuals older than 70 with HFrEF for 6 months. Approximately 80% tolerated the medication, although the maximum dose was seldom achieved, with mean doses varying by age group. The mean achieved dose was 33.3 mg/day and 29.3 mg/day in age groups 70–75 and >80 years, respectively. Advanced age and lower systolic or diastolic blood pressure, but not lower HR, were associated with lower tolerability [31]. A propensity score-matched analysis of the Swedish Heart Failure Registry highlighted that for individuals over 80, ACE-I and BB therapy correlated with enhanced survival without a rise in syncope-related hospitalizations [30]. Resting HR increases were linked to higher mortality in older outpatients with HFrEF [30], emphasizing HR achievement over BB dosing for survival benefits [37]. The STRONG-HF trial underscored that intensive guideline-directed medical therapy titration within two weeks post-discharge, with twice-monthly visits up to 2 months, could significantly enhance patient outcomes compared to usual care [67]. During hospitalization, the target BB dose was met at 55%. An intensive treatment strategy reduced symptoms, improved quality of life, and reduced the risk of 180-day all-cause death or HFH compared to usual care [67].
4.4. Beta Blockers in Older Adults with Predominantly HFpEF
In contrast to the well-established benefits of BBs therapy for patients with HFrEF, the evidence supporting their use in HFpEF is less conclusive, mainly due to the lack of specific data from RCTs. The TOPCAT trial, which included patients with preserved or mildly reduced LVEF, where around 80% were receiving BB treatment, highlights this evidence gap [34]. TOPCAT secondary analysis reported that BB therapy was associated with a higher risk of HHF in patients with preserved EF than those with HFmrEF [34]. An increase in resting HR compensates for decreased cardiac output, but significant elevations lead to increased oxygen consumption and coronary distress, predicting mortality, particularly in older patients. Patel et al. reported a 17% higher risk of HFH among HFpEF participants in the OPTIMIZE-HF registry [68]. Furthermore, the J-DHF study suggested that carvedilol might lower the rate of adverse clinical outcomes, notably in patients with advanced diastolic dysfunction [29].
Compared to conventional therapy, Takeda et al. demonstrated that carvedilol alleviated HF symptoms and neurohormonal activation in patients with HFpEF [69]. Data from three European and American registries—the EURObservational Research Program Long-Term Heart Failure (EORP LT-HF) [70,71], OPTIMIZE [36], and Get With The Guidelines (GWTG) [72]—indicate that older HF patients exhibit higher mortality, morbidity, and rates of HFH than younger patients. Conversely, other studies have indicated that HFpEF patients often experience chronotropic incompetence, with the addition of BBs potentially worsening the ability to increase HR and impair exercise capacity [73,74].
Notably, evidence addressing the use of BBs in HFpEF is predominantly derived from studies conducted in Japan. This concentration of studies in Japan may be attributed to the rising prevalence of HFpEF within the Japanese population, a trend closely linked to the aging demographic [75]. However, the applicability of findings from Japanese studies on BB therapy in HFpEF to other ethnic groups may be impacted by several factors, including genetic differences, variations in population health profiles, lifestyle and environmental factors, and the different prevalence of comorbid conditions. As observed by Obokata et al., Japanese people living with HFpEF show relevant phenotypic differences from Western HFpEF patients, including lower rates of obesity and higher rates of DM, AF, and CRF [76]. These considerations highlight the necessity for diverse, multinational research efforts to ensure the global relevance of treatment guidelines for HFpEF.
4.5. Adherence to Beta-Blocking Therapy and Multidisciplinary Approach in HF
Compared to other guideline-directed medical therapies, BBs, particularly at target doses, have significantly improved clinical outcomes in younger patients [77]. However, in older adults, the benefits of BBs are not as pronounced, which could be attributed to the low statistical power from arising from the relatively small subgroup analyses. Additionally, the challenges in both administering treatment and achieving target doses in older adults may be due to the higher prevalence of multimorbidity and frailty within this group. Historically, BBs have been utilized to address comorbidities associated with HFpEF, such as arterial hypertension (AH), coronary artery disease (CAD), and atrial fibrillation (AF). However, no evidence suggests BBs confer additional benefits for HFpEF patients without these comorbidities.
Adhering to medical therapy is crucial for managing multiple comorbidities in older patients, potentially improving outcomes. The use of cardio-selective BBs in a once-daily dosage and their gradual titration can enhance adherence and minimize adverse effects, particularly in patients prone to hypotension due to polypharmacy with diuretics, antihypertensives, and psychotropic medications. The role of a multidisciplinary team, including physicians, patients, and caregivers, in providing education and engagement with patients and their families is vital for successful management [78]. Collaboration between cardiologists and specialists across various disciplines is essential for delivering comprehensive, integrated care and follow-up (Figure 3). Deprescribing unnecessary medications to streamline regimens to only those essential should be performed in older adults [79,80]. Beyond pharmacotherapy, the recommendation of aerobic exercises, exercise training, and diet-induced weight loss are also beneficial for this patient group [81].

Figure 3.
A multidisciplinary approach to HF in older adults. Abbreviations: CABG, coronary artery bypass graft surgery; CR, cardiac rehabilitation; CV, cardiovascular; HF, heart failure.
5. Conclusions
RCTs on patients with HFrEF showed that BB therapy can reduce mortality and hospitalization by up to 10–40% at the 1-year follow-up. However, these participants are typically younger and have fewer comorbidities, which is not fully representing the broader HF population encountered in real-world settings. Achieving guideline-recommended target doses in older patients remains contentious, as clinical trials predominantly feature younger patients (mean age: 61–65 years). It raises questions about the clinical relevance of reaching these target doses in individuals aged 80 and above, especially considering the increased risk of hypotension and bradycardia, which could lead to falls and other complications in this age group. A practical approach for those over 80 might involve administering the maximum tolerated dose and/or aiming for a target HR to minimize the risk of drug-related adverse effects (Figure 4).
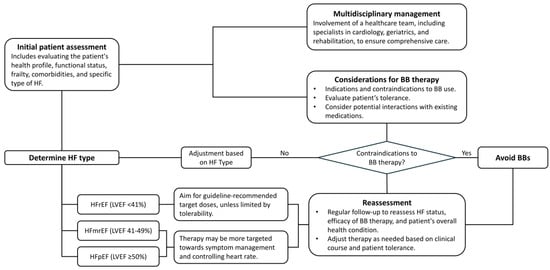
Figure 4.
Proposed algorithm for prescribing beta-blockers in older adults with heart failure. Abbreviations: BB: beta-blocker; HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HfpEF: heart failure with preserved ejection fraction; HfrEF: heart failure with reduced ejection fraction; LVEF: left ventricular ejection fraction.
Additionally, the debate over the underuse of beta blockers in older patients is complicated by the high prevalence of HF with preserved ejection fraction (HFpEF) within this demographic. For HFpEF, the prescription of beta blocker therapy is less evidence-based due to a scarcity of data from randomized controlled trials. Indeed, without specific clinical indications such as arrhythmias, AH, or CAD, the literature does not clearly support the use of beta blockers in patients with HFpEF [82], where they may impair exercise capacity and heighten the risk of adverse events. There is a pressing need for further studies that include a significant number of older patients to address these gaps.
Author Contributions
Conceptualization, I.P., F.L. and F.A.; methodology, C.M.R., C.R. and F.O.; validation, S.C. and F.O. investigation, I.P., F.L. and F.A.; writing—original draft preparation, I.P., F.L. and F.A.; writing—review and editing, C.M.R., S.C., C.R., M.G. and F.O.; visualization, S.C. and M.G.; supervision, I.P., M.M.G., M.G. and F.A.; funding acquisition, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Metra, M.; Dei Cas, L.; Massie, B.M. Treatment of heart failure in the elderly: Never say it’s too late. Eur. Heart J. 2009, 30, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Paren, P.; Schaufelberger, M.; Bjorck, L.; Lappas, G.; Fu, M.; Rosengren, A. Trends in prevalence from 1990 to 2007 of patients hospitalized with heart failure in Sweden. Eur. J. Heart Fail. 2014, 16, 737–742. [Google Scholar] [CrossRef]
- Daamen, M.A.; Hamers, J.P.; Gorgels, A.P.; Brunner-La Rocca, H.P.; Tan, F.E.; van Dieijen-Visser, M.P.; Schols, J.M. Heart failure in nursing home residents; a cross-sectional study to determine the prevalence and clinical characteristics. BMC Geriatr. 2015, 15, 167. [Google Scholar] [CrossRef]
- Nanna, M.G.; Chen, S.T.; Nelson, A.J.; Navar, A.M.; Peterson, E.D. Representation of Older Adults in Cardiovascular Disease Trials Since the Inclusion Across the Lifespan Policy. JAMA Intern. Med. 2020, 180, 1531–1533. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Spadafora, L.; Bernardi, M.; Galli, M.; Betti, M.; Perone, F.; Nicolaio, G.; Marzetti, E.; Martone, A.M.; Landi, F.; et al. Management of Coronary Artery Disease in Older Adults: Recent Advances and Gaps in Evidence. J. Clin. Med. 2023, 12, 5233. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lindenfeld, J.; Stolfo, D.; Adams, K.; Ahmad, T.; Desai, N.R.; Ammirati, E.; Gottlieb, S.S.; Psotka, M.A.; Rosano, G.M.C.; et al. Use of patient-reported outcomes in heart failure: From clinical trials to routine practice. Eur. J. Heart Fail. 2023, 25, 139–151. [Google Scholar] [CrossRef]
- Stolfo, D.; Sinagra, G.; Savarese, G. Evidence-based Therapy in Older Patients with Heart Failure with Reduced Ejection Fraction. Card. Fail. Rev. 2022, 8, e16. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Mah, D.; Aronow, W.S.; Lam, P.H.; Frishman, W.H.; Ahmed, A.; Deedwania, P. Considerations Regarding Management of Heart Failure in Older Adults. Cardiol. Rev. 2024. [Google Scholar] [CrossRef]
- Pandey, A.; Kitzman, D.; Reeves, G. Frailty Is Intertwined with Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019, 7, 1001–1011. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Aiello, A.; Colivicchi, F.; Lucà, F.; Fattirolli, F.; Gulizia, M.M.; Nardi, F.; Pino, P.G.; Gregorio, G. Cardiovascular prevention in the elderly: Limitations and opportunities. G. Ital. Cardiol. 2020, 21, 619–628. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Garred, C.H.; Zahir, D.; Butt, J.H.; Ravn, P.B.; Bruhn, J.; Gislason, G.H.; Fosbol, E.L.; Torp-Pedersen, C.; Petrie, M.C.; McMurray, J.J.V.; et al. Adherence and Discontinuation of Optimal Heart Failure Therapies According to Age: A Danish Nationwide Study. J. Am. Heart Assoc. 2022, 11, e026187. [Google Scholar] [CrossRef]
- Lainscak, M.; Milinkovic, I.; Polovina, M.; Crespo-Leiro, M.G.; Lund, L.H.; Anker, S.D.; Laroche, C.; Ferrari, R.; Coats, A.J.S.; McDonagh, T.; et al. Sex- and age-related differences in the management and outcomes of chronic heart failure: An analysis of patients from the ESC HFA EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 92–102. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.; Young, J.B.; et al. Age- and gender-related differences in quality of care and outcomes of patients hospitalized with heart failure (from OPTIMIZE-HF). Am. J. Cardiol. 2009, 104, 107–115. [Google Scholar] [CrossRef]
- Forman, D.E.; Cannon, C.P.; Hernandez, A.F.; Liang, L.; Yancy, C.; Fonarow, G.C.; for the Get With the Guidelines Steering Committee and Hospitals. Influence of age on the management of heart failure: Findings from Get with the Guidelines-Heart Failure (GWTG-HF). Am. Heart J. 2009, 157, 1010–1017. [Google Scholar] [CrossRef]
- Flather, M.D.; Shibata, M.C.; Coats, A.J.; Van Veldhuisen, D.J.; Parkhomenko, A.; Borbola, J.; Cohen-Solal, A.; Dumitrascu, D.; Ferrari, R.; Lechat, P.; et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2005, 26, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.; Austin, A.M.; O’Malley, A.J.; Gladders, B.; Barnato, A.E.; Tosteson, A.; Skinner, J. Association between Beta-Blockers and Mortality and Readmission in Older Patients with Heart Failure: An Instrumental Variable Analysis. J. Gen. Intern. Med. 2021, 36, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999, 353, 9–13. [Google Scholar] [CrossRef]
- Hjalmarson, A.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; El Allaf, D.; Vitovec, J.; Aldershvile, J.; et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000, 283, 1295–1302. [Google Scholar] [CrossRef]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- Yamamoto, K.; Origasa, H.; Hori, M.; Investigators, J.D. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur. J. Heart Fail. 2013, 15, 110–118. [Google Scholar] [CrossRef]
- Stolfo, D.; Uijl, A.; Benson, L.; Schrage, B.; Fudim, M.; Asselbergs, F.W.; Koudstaal, S.; Sinagra, G.; Dahlstrom, U.; Rosano, G.; et al. Association between beta-blocker use and mortality/morbidity in older patients with heart failure with reduced ejection fraction. A propensity score-matched analysis from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2020, 22, 103–112. [Google Scholar] [CrossRef]
- Macdonald, P.S.; Hill, J.; Krum, H.; Investigators, C.I. The impact of baseline HR and BP on the tolerability of carvedilol in the elderly: The COLA (Carvedilol Open Label Assessment) II Study. Am. J. Cardiovasc. Drugs 2006, 6, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, J.L.; Roecker, E.B.; Tendera, M.; Mohacsi, P.; Krum, H.; Katus, H.A.; Fowler, M.B.; Coats, A.J.; Castaigne, A.; Scherhag, A.; et al. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: The Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J. Am. Coll. Cardiol. 2004, 43, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Musial-Bright, L.; Gelbrich, G.; Trippel, T.; Radenovic, S.; Wachter, R.; Inkrot, S.; Loncar, G.; Tahirovic, E.; Celic, V.; et al. Tolerability and Feasibility of Beta-Blocker Titration in HFpEF Versus HFrEF: Insights From the CIBIS-ELD Trial. JACC Heart Fail. 2016, 4, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.N.; Plante, T.B.; Infeld, M.; Callas, P.W.; Juraschek, S.P.; Dougherty, G.B.; Meyer, M. Association of beta-Blocker Use with Heart Failure Hospitalizations and Cardiovascular Disease Mortality among Patients with Heart Failure with a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw. Open 2019, 2, e1916598. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure with Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Hammill, B.G.; O’Connor, C.M.; Schulman, K.A.; Curtis, L.H.; Fonarow, G.C. Clinical effectiveness of beta-blockers in heart failure: Findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J. Am. Coll. Cardiol. 2009, 53, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cullington, D.; Goode, K.M.; Clark, A.L.; Cleland, J.G. Heart rate achieved or beta-blocker dose in patients with chronic heart failure: Which is the better target? Eur. J. Heart Fail. 2012, 14, 737–747. [Google Scholar] [CrossRef]
- Forouzandeh, F.; Alexander, K.; Forman, D.; Kirkpatrick, J.N.; Rich, M.W.; Zieman, S.; Wenger, N.K. Cardiovascular Disease in the Older Adult. JACC: Adv. 2024, 3, 100820. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, A.; Kitzman, D.W.; Maurer, M.S. Heart failure in older adults: Embracing complexity. J. Geriatr. Cardiol. 2016, 13, 8–14. [Google Scholar] [CrossRef]
- Castiglione, V.; Gentile, F.; Ghionzoli, N.; Chiriaco, M.; Panichella, G.; Aimo, A.; Vergaro, G.; Giannoni, A.; Passino, C.; Emdin, M. Pathophysiological Rationale and Clinical Evidence for Neurohormonal Modulation in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2023, 9, e09. [Google Scholar] [CrossRef]
- Strait, J.B.; Lakatta, E.G. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Li, H.; Hastings, M.H.; Rhee, J.; Trager, L.E.; Roh, J.D.; Rosenzweig, A. Targeting Age-Related Pathways in Heart Failure. Circ. Res. 2020, 126, 533–551. [Google Scholar] [CrossRef]
- Ali, D.C.; Naveed, M.; Gordon, A.; Majeed, F.; Saeed, M.; Ogbuke, M.I.; Atif, M.; Zubair, H.M.; Changxing, L. Beta-Adrenergic receptor, an essential target in cardiovascular diseases. Heart Fail. Rev. 2020, 25, 343–354. [Google Scholar] [CrossRef]
- Parveen, S.; Zareini, B.; Arulmurugananthavadivel, A.; Kistorp, C.; Faber, J.; Kober, L.; Hassager, C.; Sorensen, T.B.; Andersson, C.; Zahir, D.; et al. Association between early detected heart failure stages and future cardiovascular and non-cardiovascular events in the elderly (Copenhagen Heart Failure Risk Study). BMC Geriatr. 2022, 22, 230. [Google Scholar] [CrossRef]
- Oudejans, I.; Mosterd, A.; Bloemen, J.A.; Valk, M.J.; van Velzen, E.; Wielders, J.P.; Zuithoff, N.P.; Rutten, F.H.; Hoes, A.W. Clinical evaluation of geriatric outpatients with suspected heart failure: Value of symptoms, signs, and additional tests. Eur. J. Heart Fail. 2011, 13, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Banach, M.; Jones, L.G.; Datta, S.; Ahmed, A.; Aronow, W.S. Update on diastolic heart failure or heart failure with preserved ejection fraction in the older adults. Ann. Med. 2013, 45, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: A Review. JAMA 2023, 329, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Farber-Eger, E.; Arora, P.; Collins, S.; Wells, Q.S.; Wang, T.J. Unexpectedly Low Natriuretic Peptide Levels in Patients with Heart Failure. JACC Heart Fail. 2021, 9, 192–200. [Google Scholar] [CrossRef]
- Patel, A.N.; Southern, W.N. BNP-Response to Acute Heart Failure Treatment Identifies High-Risk Population. Heart Lung Circ. 2020, 29, 354–360. [Google Scholar] [CrossRef]
- Vaishnav, J.; Sharma, K. A Stepwise Guide to the Diagnosis and Treatment of Heart Failure with Preserved Ejection Fraction. J. Card. Fail. 2022, 28, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Cediel, G.; Domingo, M.; Codina, P.; Santiago, E.; Lupon, J. Biomarkers in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2022, 8, e20. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L., Jr.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Andreotti, F.; Geisler, T.; Collet, J.P.; Gigante, B.; Gorog, D.A.; Halvorsen, S.; Lip, G.Y.H.; Morais, J.; Navarese, E.P.; Patrono, C.; et al. Acute, periprocedural and longterm antithrombotic therapy in older adults: 2022 Update by the ESC Working Group on Thrombosis. Eur. Heart J. 2023, 44, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Andres, T.M.; McGrane, T.; McEvoy, M.D.; Allen, B.F.S. Geriatric Pharmacology: An Update. Anesth. Clin. 2019, 37, 475–492. [Google Scholar] [CrossRef]
- Kotecha, D.; Flather, M.D.; Altman, D.G.; Holmes, J.; Rosano, G.; Wikstrand, J.; Packer, M.; Coats, A.J.S.; Manzano, L.; Bohm, M.; et al. Heart Rate and Rhythm and the Benefit of Beta-Blockers in Patients with Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Messerli, F.H. Why beta-blockers are not cardioprotective in elderly patients with hypertension. Curr. Cardiol. Rep. 2002, 4, 468–473. [Google Scholar] [CrossRef]
- Gao, X.; Colicino, E.; Shen, J.; Just, A.C.; Nwanaji-Enwerem, J.C.; Wang, C.; Coull, B.; Lin, X.; Vokonas, P.; Zheng, Y.; et al. Accelerated DNA methylation age and the use of antihypertensive medication among older adults. Aging 2018, 10, 3210–3228. [Google Scholar] [CrossRef]
- Steinman, M.A.; Zullo, A.R.; Lee, Y.; Daiello, L.A.; Boscardin, W.J.; Dore, D.D.; Gan, S.; Fung, K.; Lee, S.J.; Komaiko, K.D.; et al. Association of beta-Blockers with Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents after Acute Myocardial Infarction. JAMA Intern. Med. 2017, 177, 254–262. [Google Scholar] [CrossRef]
- Holm, H.; Ricci, F.; Di Martino, G.; Bachus, E.; Nilsson, E.D.; Ballerini, P.; Melander, O.; Hansson, O.; Nagga, K.; Magnusson, M.; et al. Beta-blocker therapy and risk of vascular dementia: A population-based prospective study. Vasc. Pharmacol. 2020, 125–126, 106649. [Google Scholar] [CrossRef] [PubMed]
- Beaman, E.E.; Bonde, A.N.; Larsen, S.M.U.; Ozenne, B.; Lohela, T.J.; Nedergaard, M.; Gislason, G.H.; Knudsen, G.M.; Holst, S.C. Blood-brain barrier permeable beta-blockers linked to lower risk of Alzheimer’s disease in hypertension. Brain 2023, 146, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Priel, E.; Wahab, M.; Mondal, T.; Freitag, A.; O’Byrne, P.M.; Killian, K.J.; Satia, I. The Impact of beta blockade on the cardio-respiratory system and symptoms during exercise. Curr. Res. Physiol. 2021, 4, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Teng, T.K.; Chandramouli, C.; Tromp, J.; Sakata, Y.; Lam, C.S. Epidemiology and Clinical Features of Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2022, 8, e27. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Manzano, L.; Krum, H.; Rosano, G.; Holmes, J.; Altman, D.G.; Collins, P.D.; Packer, M.; Wikstrand, J.; Coats, A.J.; et al. Effect of age and sex on efficacy and tolerability of beta blockers in patients with heart failure with reduced ejection fraction: Individual patient data meta-analysis. BMJ 2016, 353, i1855. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Fowler, M.B.; Roecker, E.B.; Coats, A.J.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Staiger, C.; et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002, 106, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Fonarow, G.C.; Ekundayo, O.J.; Aban, I.B.; Kilgore, M.L.; Love, T.E.; Kitzman, D.W.; Gheorghiade, M.; Allman, R.M.; Ahmed, A. Beta-blockers in older patients with heart failure and preserved ejection fraction: Class, dosage, and outcomes. Int. J. Cardiol. 2014, 173, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Fukutomi, T.; Suzuki, S.; Yamamoto, K.; Ogata, M.; Kondo, H.; Sugiura, M.; Shigeyama, J.; Itoh, M. Effects of carvedilol on plasma B-type natriuretic peptide concentration and symptoms in patients with heart failure and preserved ejection fraction. Am. J. Cardiol. 2004, 94, 448–453. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Dahlstrom, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Metra, M.; et al. EURObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2010, 12, 1076–1084. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Dahlstrom, U.; Filippatos, G.; Chioncel, O.; Crespo Leiro, M.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Fabbri, G.; et al. EURObservational Research Programme: Regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2013, 15, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Joynt Maddox, K.E.; Fox, D.K. Doing Our Part to Get with the Heart Failure Guidelines. JACC Heart Fail. 2023, 11, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Manoharan, K.; Davies, C.; Lumbers, R.T. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst. Rev. 2021, 5, CD012721. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; LeWinter, M.M. Heart Rate and Heart Failure with Preserved Ejection Fraction: Time to Slow beta-Blocker Use? Circ. Heart Fail. 2019, 12, e006213. [Google Scholar] [CrossRef] [PubMed]
- Isobe, M. The Heart Failure “Pandemic” in Japan: Reconstruction of Health Care System in the Highly Aged Society. JMA J. 2019, 2, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Sorimachi, H.; Harada, T.; Kagami, K.; Saito, Y.; Ishii, H. Epidemiology, Pathophysiology, Diagnosis, and Therapy of Heart Failure with Preserved Ejection Fraction in Japan. J. Card. Fail. 2023, 29, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Jhund, P.S.; McMurray, J.J.V. Drug therapy for heart failure with reduced ejection fraction: What is the ‘right’ dose? Eur. J. Heart Fail. 2022, 24, 421–430. [Google Scholar] [CrossRef]
- Sokos, G.; Kido, K.; Panjrath, G.; Benton, E.; Page, R., 2nd; Patel, J.; Smith, P.J.; Korous, S.; Guglin, M. Multidisciplinary Care in Heart Failure Services. J. Card. Fail. 2023, 29, 943–958. [Google Scholar] [CrossRef]
- Martone, A.M.; Parrini, I.; Ciciarello, F.; Galluzzo, V.; Cacciatore, S.; Massaro, C.; Giordano, R.; Giani, T.; Landi, G.; Gulizia, M.M.; et al. Recent Advances and Future Directions in Syncope Management: A Comprehensive Narrative Review. J. Clin. Med. 2024, 13, 727. [Google Scholar] [CrossRef]
- Sabouret, P.; Spadafora, L.; Fischman, D.; Ullah, W.; Zeitouni, M.; Gulati, M.; De Rosa, S.; Savage, M.P.; Costabel, J.P.; Banach, M.; et al. De-escalation of antiplatelet therapy in patients with coronary artery disease: Time to change our strategy? Eur. J. Intern. Med. 2023, 110, 1–9. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, F.; Fonarow, G.C.; Sreenivasan, J.; Greene, S.J.; Khan, S.U.; Usman, M.S.; Vaduganathan, M.; Fudim, M.; Anker, S.D.; et al. Dietary interventions and nutritional supplements for heart failure: A systematic appraisal and evidence map. Eur. J. Heart Fail. 2021, 23, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Oliva, F.; Abrignani, M.G.; Di Fusco, S.A.; Gori, M.; Giubilato, S.; Ceravolo, R.; Temporelli, P.L.; Cornara, S.; Rao, C.M. Heart Failure with Preserved Ejection Fraction: How to Deal with This Chameleon. J. Clin. Med. 2024, 13, 1375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).