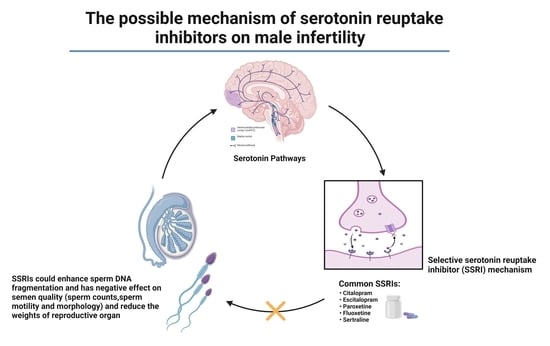
Evaluating the Use of Selective Serotonin Reuptake Inhibitors (SSRIs) and Male Infertility: A Critical Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables, Data Source, and Measurements
2.5. Macroscopic Examination
2.6. Microscopic Examination
2.7. Statical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Assisted Reproductive Technology |
| PR | Progressive motility |
| SSRIs | Selective serotonin reuptake inhibitors |
| SA | Semen analysis |
| WHO | World Health Organization |
References
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S.; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary of ART Terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Mirzaiinajmabadi, K.; Roudsari, R.L.; Rakhshkhorshid, M. The Factors Affecting Male Infertility: A Systematic Review. Int. J. Reprod. Biomed. 2021, 19, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Public Health Action Plan for the Detection, Prevention, and Management of Infertility; International Journal of Reproductive Biomedicine; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014. [Google Scholar]
- Ombelet, W. Global Access to Infertility Care in Developing Countries: A Case of Human Rights, Equity and Social Justice. Internet. Facts Views Vis. ObGyn 2011, 3, 257–266. Available online: http://www.thewalkingegg.com (accessed on 26 March 2024).
- Al-Turki, H.A. Prevalence of Primary and Secondary Infertility from Tertiary Centers in Eastern Saudi Arabia. Middle East Fertil. Soc. J. 2015, 20, 237–240. [Google Scholar] [CrossRef]
- Gurunath, S.; Pandian, Z.; Anderson, R.A.; Bhattacharya, S. Defining Infertility—A Systematic Review of Prevalence Studies. Hum. Reprod. Update 2011, 17, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Shah, A.; Gudi, A.; Homburg, R. Unexplained Infertility: An Update and Review of Practice. Reprod. Biomed. 2012, 24, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Pourmasumi, S.; Tabibnejad, N.; Reza Talebi, A.; Talebi, A.R. The Etiologies of Sperm DNA Abnormalities in Male Infertility: An Assessment and Review. Int. J. Reprod. BioMedicine 2017, 15, 331. [Google Scholar] [CrossRef]
- Pandruvada, S.; Royfman, R.; Shah, T.A.; Sindhwani, P.; Dupree, J.M.; Schon, S.; Avidor-Reiss, T. Lack of Trusted Diagnostic Tools for Undetermined Male Infertility. J. Assist. Reprod. Genet. 2021, 38, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Belladelli, F.; Fallara, G.; Pozzi, E.; Corsini, C.; Cilio, S.; Raffo, M.; Salonia, A. Effects of recreational cannabis on testicular function in primary infertile men. Andrology 2022, 10, 1172–1180. [Google Scholar] [CrossRef]
- Kolesnikova, L.I.; Kolesnikov, S.I.; Kurashova, N.A.; Bairova, T.A. Causes and Factors of Male Infertility. Vestn. Ross. Akad. Med. Nauk 2015, 70, 579–584. [Google Scholar] [CrossRef][Green Version]
- Brezina, P.R.; Yunus, F.N.; Zhao, Y. Effects of Pharmaceutical Medications on Male Fertility. J. Reprod. Infertil. 2012, 13, 3–11. [Google Scholar] [PubMed]
- O’Flaherty, C.; Hales, B.F.; Chan, P.; Robaire, B. Impact of Chemotherapeutics and Advanced Testicular Cancer or Hodgkin Lymphoma on Sperm Deoxyribonucleic Acid Integrity. Fertil. Steril. 2010, 94, 1374–1379. [Google Scholar] [CrossRef]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The Impact of Drugs on Male Fertility: A Review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Cilio, S.; Fallara, G.; Stanghellini, M.T.L.; Ciceri, F.; Montorsi, F.; Lunghi, F.; Salonia, A. Impact of Hydroxyurea to Treat Haematological Disorders on Male Fertility: Two Case Reports and a Systematic Review. World J. Men’s Health 2024, 42, e5. [Google Scholar] [CrossRef] [PubMed]
- Luis, G.R.; Alejandro, P.S.; Henry, D.; Nelson, A.G.; Jorge, A.C. Prevalence and Correlates of Major Depressive Disorder: A Systematic Review. Braz. J. Psychiatry 2020, 42, 657–672. [Google Scholar] [CrossRef]
- AlYousefi, N.A.; AlRukban, M.O.; AlMana, A.M.; AlTukhaim, T.H.; AlMeflh, B.A.; AlMutairi, Y.O.; AlMogheer, O.S. Exploring the Predictors of Depression Among Saudi Adolescents: Time for Urgent Firm Actions. Saudi Med. J. 2021, 42, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Alosaimi, F.D.; Alhabbad, A.; Abalhassan, M.F.; Fallata, E.O.; Alzain, N.M.; Alassiry, M.Z.; Haddad, B.A. Patterns of Psychotropic Medication Use in Inpatient and Outpatient Psychiatric Settings in Saudi Arabia. Neuropsychiatr. Dis. Treat. 2016, 12, 897–907. [Google Scholar] [CrossRef]
- Higgins, A.; Nash, M.; Lynch, A.M. Antidepressant-Associated Sexual Dysfunction: Impact, Effects, and Treatment. Drug Healthc. Patient Saf. 2010, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Beeder, L.A.; Samplaski, M.K. Effect of Antidepressant Medications on Semen Parameters and Male Fertility. Int. J. Urol. 2020, 27, 39–46. [Google Scholar] [CrossRef]
- Sylvester, C.; Menke, M.; Gopalan, P. Selective Serotonin Reuptake Inhibitors and Fertility: Considerations for Couples Trying to Conceive. Harv. Rev. Psychiatry 2019, 27, 108–118. [Google Scholar] [CrossRef]
- Della Corte, M.; Porpiglia, F.; Checcucci, E. The quality of life value in uro-oncological patients. Curr. Opin. Urol. 2023, 33, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Geretto, P.; Ammirati, E.; Falcone, M.; Manassero, A.; Agnello, M.; Della Corte, M.; Giammò, A. Comparison Study between Artificial Urinary Sphincter and Adjustable Male Sling: A Propensity-Score-Matched Analysis. J. Clin. Med. 2023, 12, 5489. [Google Scholar] [CrossRef] [PubMed]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Agarwal, A. The sixth edition of the WHO manual for human semen analysis: A critical review and SWOT analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Topiwala, A.; Chouliaras, L.; Ebmeier, K.P. Prescribing selective serotonin reuptake inhibitors in older age. Maturitas 2014, 77, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.; Ranjit, A.; Tuulio-Henriksson, A.; Kaprio, J. Smoking status as a predictor of antidepressant medication use. J. Affect Disord. 2017, 207, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. Sperm DNA Damage and Semen Quality Impairment After Treatment with Selective Serotonin Reuptake Inhibitors Detected Using Semen Analysis and Sperm Chromatin Structure Assay. J. Urol. 2008, 180, 2124–2128. [Google Scholar] [CrossRef]
- Xu, J.; He, K.; Zhou, Y.; Zhao, L.; Lin, Y.; Huang, Z.; Xie, N.; Yue, J.; Tang, Y. The Effect of SSRIs on Semen Quality: A Systematic Review and Meta-analysis. Front. Pharmacol. 2022, 13, 911489. [Google Scholar] [CrossRef]
- Tanrikut, C.; Feldman, A.S.; Altemus, M.; Paduch, D.A.; Schlegel, P.N. Adverse Effect of Paroxetine on Sperm. Fertil. Steril. 2010, 94, 1021–1026. [Google Scholar] [CrossRef]
- Martin-Tuite, P.; Shindel, A.W. Management Options for Premature Ejaculation and Delayed Ejaculation in Men. Sex. Med. Rev. 2020, 8, 473–485. [Google Scholar] [CrossRef]
| Using SSRIs N = 292 | %, SD, IQR | Non-SSRIs N = 270 | %, SD, IQR | Total N = 299 | %, SD, IQR | p Value | |
|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 41.52 | 3.28 | 34.20 | 6.93 | 34.91 | 7.00 | <0.001 * |
| Smoking, N (%) | 0.144 | ||||||
| No | 14 | 7.7 | 168 | 92.3 | 182 | 60.9 | |
| Yes | 15 | 12.8 | 102 | 87.2 | 117 | 39.1 | |
| Viscosity, n (%) | 0.197 | ||||||
| Normal | 15 | 12.4 | 106 | 87.6 | 121 | 40.5 | |
| Slightly viscous | 8 | 11.3 | 63 | 88.7 | 71 | 23.7 | |
| Highly viscous | 6 | 5.6 | 101 | 94.4 | 107 | 35.8 | |
| Liquefaction, N (%) | 0.028 * | ||||||
| Less than 30 min | 3 | 17.6 | 14 | 82.4 | 17 | 5.7 | |
| 30–60 min | 17 | 14.4 | 103 | 85.8 | 120 | 40.1 | |
| More than 60 min | 9 | 5.6 | 153 | 94.4 | <0.001 * 162 | 54.2 | |
| Motility, mean (SD) | 47.93 | 11.84 | 39.55 | 20.79 | 40.37 | 20.23 | 0.034 * |
| Sperm count, median (IQR) | 20.00 | 15.00–55.00 | 29.85 | 25.00–35.00 | 28.80 | 24.30–33.75 | 0.300 |
| Escitalopram N = 11 | %, SD, IQR | Paroxetine N = 7 | %, SD, IQR | Fluoxetine N = 11 | %, SD, IQR | Total N = 29 | %, SD, IQR | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 40.55 | 3.24 | 41.71 | 3.35 | 42.36 | 3.32 | 41.52 | 3.28 | 0.438 |
| Smoking, N (%) | 0.418 | ||||||||
| No | 4 | 28.6 | 3 | 21.4 | 7 | 50.0 | 14 | 48.3 | |
| Yes | 7 | 46.7 | 4 | 26.7 | 4 | 26.7 | 15 | 51.7 | |
| Dose, N (%) | 0.583 | ||||||||
| 10 mg | 2 | 40.0 | 0 | 0.0 | 3 | 60.0 | 5 | 17.2 | |
| 20 mg | 7 | 36.8 | 5 | 26.3 | 7 | 36.8 | 19 | 65.5 | |
| 40 mg | 2 | 40.0 | 2 | 40.0 | 1 | 20.0 | 5 | 17.2 | |
| Frequency, N (%) | 0.219 | ||||||||
| Once daily | 9 | 39.1 | 4 | 17.4 | 10 | 43.5 | 23 | 79.3 | |
| Twice daily | 2 | 33.3 | 3 | 50.0 | 1 | 16.7 | 6 | 20.7 | |
| Duration of use, N (%) | 0.101 | ||||||||
| Less than 2 years | 4 | 50.0 | 2 | 25.0 | 2 | 25.0 | 8 | 27.6 | |
| 2–5 years | 3 | 20.0 | 3 | 20.0 | 9 | 60.0 | 15 | 51.7 | |
| More than 5 years | 4 | 66.7 | 2 | 33.3 | 0 | 0.0 | 6 | 20.7 | |
| Viscosity, n (%) | 0.167 | ||||||||
| Normal | 7 | 46.7 | 4 | 26.7 | 4 | 26.7 | 15 | 51.7 | |
| Slightly viscous | 3 | 37.5 | 0 | 0.0 | 5 | 62.0 | 8 | 27.6 | |
| Highly viscous | 1 | 16.6 | 3 | 50.0 | 2 | 33.3 | 6 | 20.7 | |
| Liquefaction, N (%) | 0.100 | ||||||||
| Less than 30 min | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 | 3 | 10.3 | |
| 30–60 min | 6 | 35.3 | 3 | 17.6 | 8 | 47.1 | 17 | 58.6 | |
| More than 60 min | 2 | 22.2 | 4 | 44.4 | 3 | 33.3 | 9 | 31.0 | |
| Motility, mean (SD) | 44.55 | 11.93 | 45.00 | 12.25 | 53.18 | 10.55 | 47.93 | 11.84 | 0.177 |
| Sperm count, median (IQR) | 20.00 | 15.00–45.00 | 10.00 | 4.00–60.00 | 44.00 | 13.00–90.00 | 20.00 | 15.00–55.00 | 0.069 |
| B | S.E | p-Value | Odds Ratio | |
|---|---|---|---|---|
| Age | 0.119 | 0.027 | <0.001 * | 1.127 |
| Motility | 0.021 | 0.010 | <0.036 * | 1.022 |
| Liquification 30–60 min | −0.261 | 0.010 | 0.074 | 0.770 |
| Liquification > 60 min | −1.293 | 0.723 | 0.074 | 0.275 |
| B | S.E | p-value | Adjusted £ Odds ratio | |
| Motility | 0.024 | 0.011 | 0.029 * | 1.025 |
| Liquification 30–60 min | 0.072 | 0.725 | 0.921 | 1.075 |
| Liquification > 60 min | −1.102 | 0.761 | 0.148 | 0.332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsabhan, J.F.; Almalag, H.M.; Alnuaim, L.A.; Albaker, A.B.; Alaseem, M.M. Evaluating the Use of Selective Serotonin Reuptake Inhibitors (SSRIs) and Male Infertility: A Critical Retrospective Study. J. Clin. Med. 2024, 13, 2129. https://doi.org/10.3390/jcm13072129
Alsabhan JF, Almalag HM, Alnuaim LA, Albaker AB, Alaseem MM. Evaluating the Use of Selective Serotonin Reuptake Inhibitors (SSRIs) and Male Infertility: A Critical Retrospective Study. Journal of Clinical Medicine. 2024; 13(7):2129. https://doi.org/10.3390/jcm13072129
Chicago/Turabian StyleAlsabhan, Jawza F., Haya M. Almalag, Lulu A. Alnuaim, Awatif B. Albaker, and Maryam M. Alaseem. 2024. "Evaluating the Use of Selective Serotonin Reuptake Inhibitors (SSRIs) and Male Infertility: A Critical Retrospective Study" Journal of Clinical Medicine 13, no. 7: 2129. https://doi.org/10.3390/jcm13072129
APA StyleAlsabhan, J. F., Almalag, H. M., Alnuaim, L. A., Albaker, A. B., & Alaseem, M. M. (2024). Evaluating the Use of Selective Serotonin Reuptake Inhibitors (SSRIs) and Male Infertility: A Critical Retrospective Study. Journal of Clinical Medicine, 13(7), 2129. https://doi.org/10.3390/jcm13072129