The Factors Influencing Chronic Kidney Disease Incidence: Database from the Korean National Health Insurance Sharing Service (NHISS)
Abstract
1. Introduction
2. Methods
2.1. Data
2.2. Study Population
- Patients with insurance claim with diagnosis code N18.x (International Classification of Diseases, 10th Revision [ICD-10]; N181, N182, N183, N184, N185, and N189)
- Patients prescribed treatment codes (O7071, O7072, O7073, O7074, O7076, and O7077) for peritoneal dialysis
- Patients who had undergone kidney transplantation (patients with an insurance claim with diagnosis code Z940 (kidney transplantation status) and those prescribed with surgery code R3280 (renal transplantation)
- Calculation exception codes: Patients with insurance claims because of hemodialysis (V001; hemodialysis maintained for >90 d), peritoneal dialysis (V003), and kidney transplantation (V005).
- Hypertension: Patients with insurance claims with diagnostic codes (I10, I11, I12, I13, and I15);
- Diabetes: Patients with insurance claims with diagnostic codes (E10, E11, E12, E13, and E14);
- Dyslipidemia: Patients with insurance claims with diagnostic codes (E780, E781, E782, E783, E784, and E785);
- Stroke: Patients with insurance claim with diagnostic codes (G45, G46, I63, and I64);
- Heart failure: Patients with insurance claim with diagnostic codes (I50);
- Atrial flutter and fibrillation: Patients with an insurance claim with diagnostic code (I48); and
- Myocardial infarction: Patients with insurance claims with diagnostic codes (I21, I22, and I252).
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Baseline Characteristics
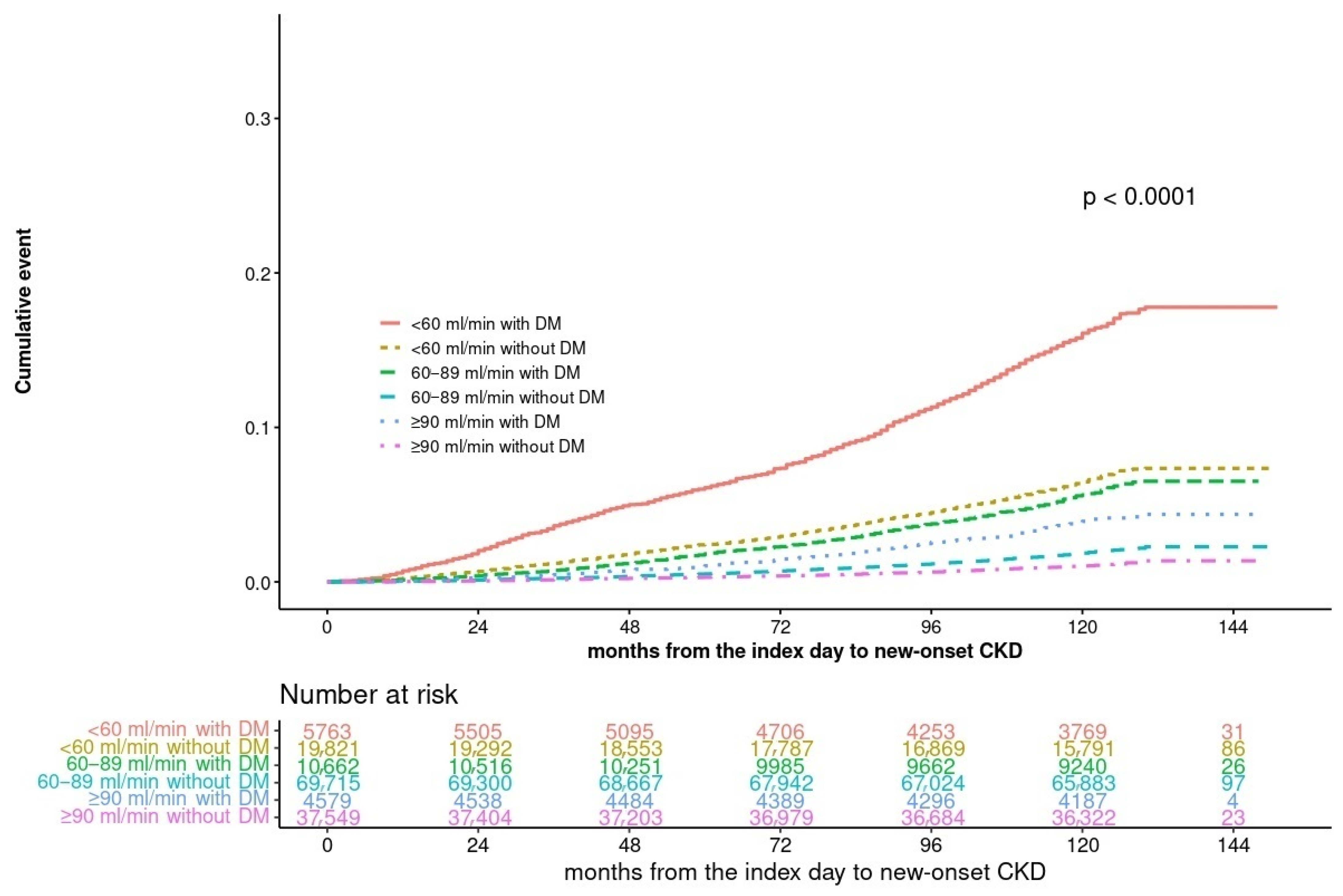
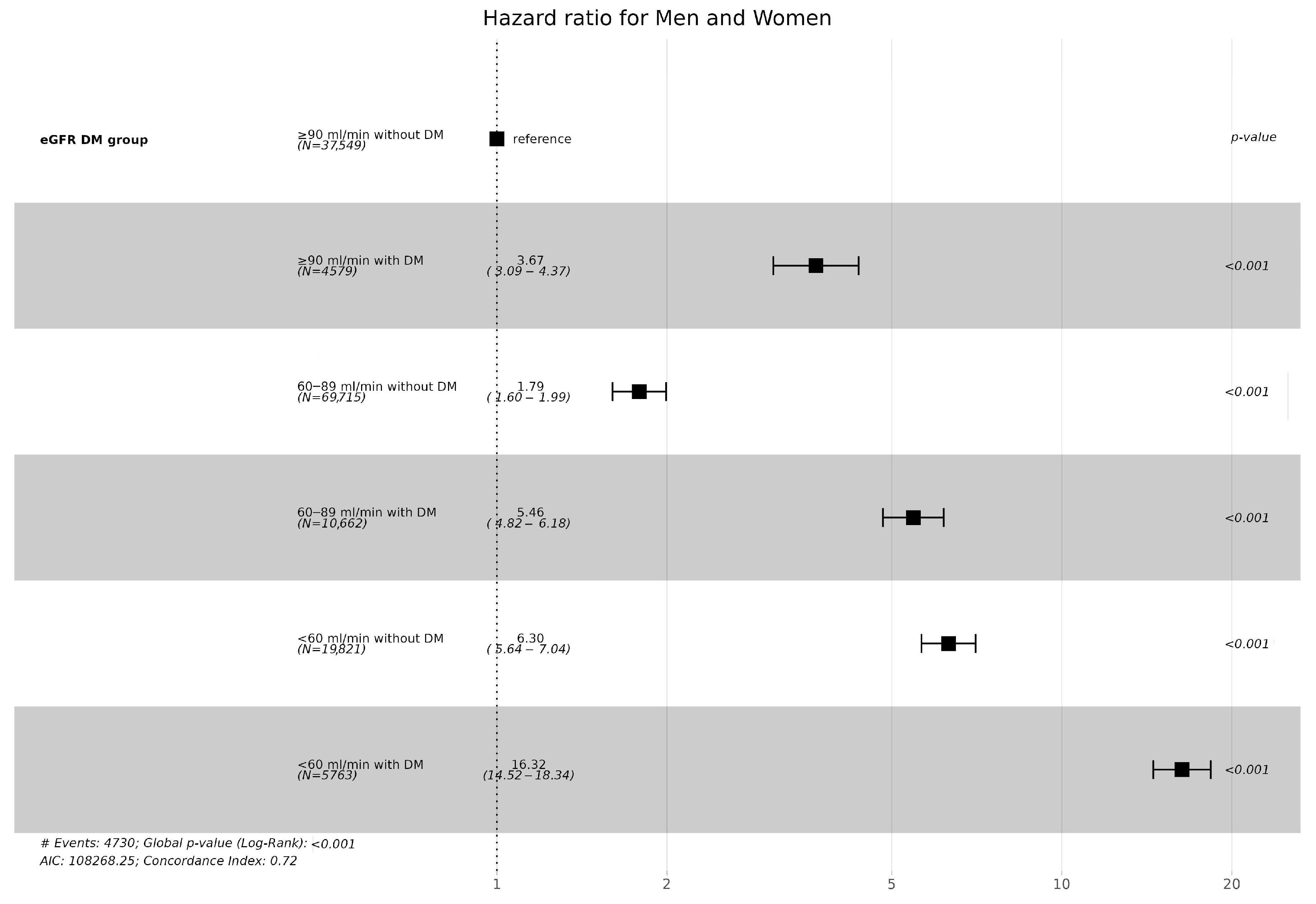
3.2. CKD Incidence According to Diabetes and eGFR Levels
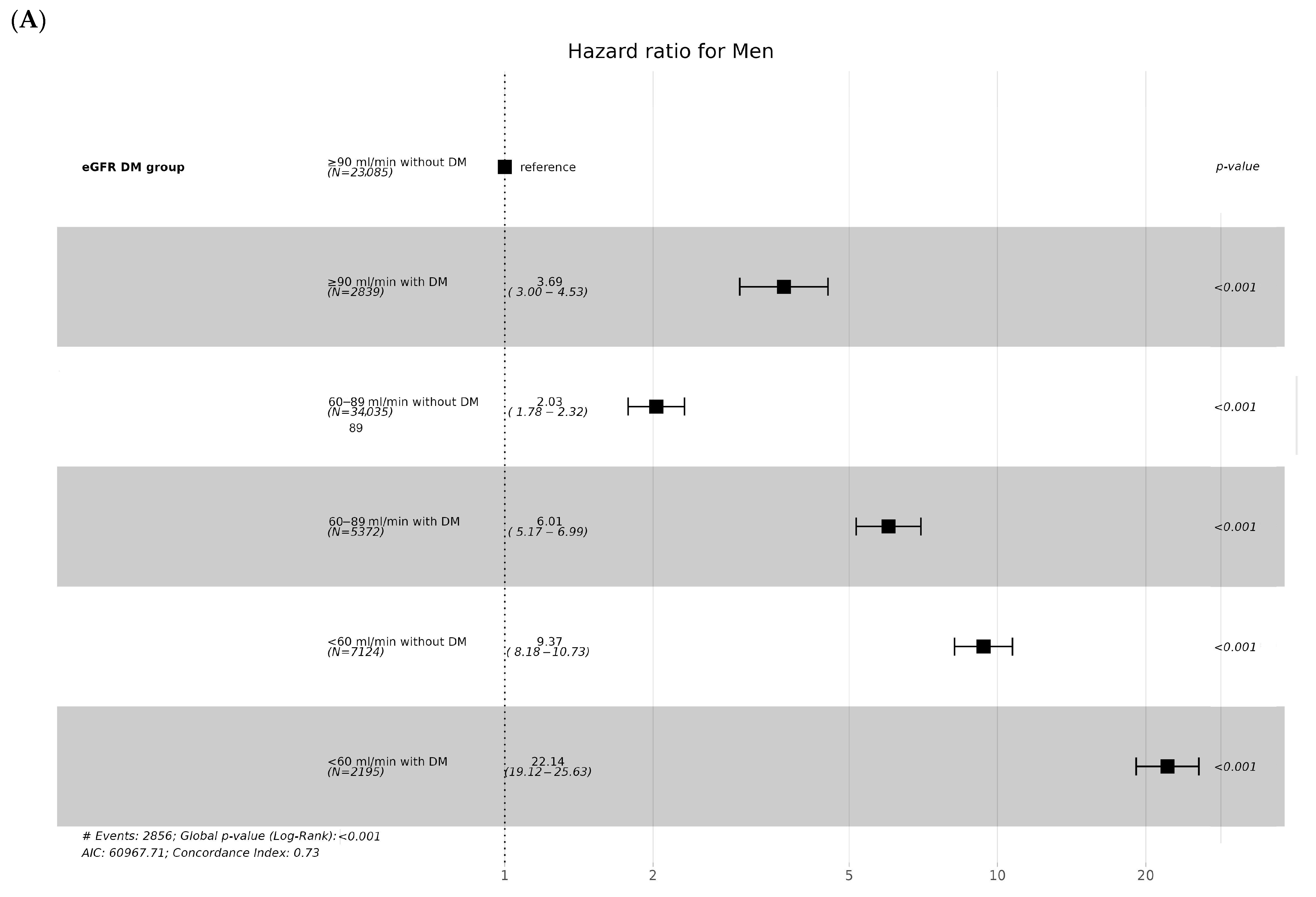
3.3. CKD Incidence according to Age and Sex
3.4. CKD Incidence according to Cardiovascular Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Baek, H.; Jung, H.H. Prevalence of Chronic Kidney Disease in Korea: The Korean National Health and Nutritional Examination Survey 2011–2013. J. Korean Med. Sci. 2016, 31, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, C.S.; Han, D.C.; Kim, G.S.; Chin, H.J.; Kim, S.J.; Cho, W.Y.; Kim, Y.H.; Kim, Y.S. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: A population-based cross-sectional epidemiologic study. J. Korean Med. Sci. 2009, 24, S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Hemmelgarn, B.R.; Zhang, J.; Manns, B.J.; Tonelli, M.; Larsen, E.; Ghali, W.A.; Southern, D.A.; McLaughlin, K.; Mortis, G.; Culleton, B.F. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006, 69, 2155–2161. [Google Scholar] [CrossRef]
- Ryan, T.P.; Sloand, J.A.; Winters, P.C.; Corsetti, J.P.; Fisher, S.G. Chronic kidney disease prevalence and rate of diagnosis. Am. J. Med. 2007, 120, 981–986. [Google Scholar] [CrossRef]
- Jaques, D.A.; Vollenweider, P.; Bochud, M.; Ponte, B. Aging and hypertension in kidney function decline: A 10 year population-based study. Front. Cardiovasc. Med. 2022, 9, 1035313. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.W.; Andres, R.; Tobin, J.D.; Norris, A.H.; Shock, N.W. The effect of age on creatinine clearance in men: A cross-sectional and longitudinal study. J. Gerontol. 1976, 31, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K. Factors influencing the development of end-stage renal disease. Clin. Exp. Nephrol. 2005, 9, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Jankowska, M.; Soler, M.J.; Stevens, K.I.; Torra, R. Why do we keep ignoring sex in kidney disease? Clin. Kidney J. 2023, 16, 2327–2335. [Google Scholar] [CrossRef]
- Mills, K.T.; Xu, Y.; Zhang, W.; Bundy, J.D.; Chen, C.S.; Kelly, T.N.; Chen, J.; He, J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015, 88, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Orth, S.R.; Hallan, S.I. Smoking: A risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients--absence of evidence or evidence of absence? Clin. J. Am. Soc. Nephrol. 2008, 3, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, L.; Ma, Z.; Zhong, L.; Wang, Y.; Gao, Y.; He, L.; Su, X. Cigarette smoking and chronic kidney disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Nephrol. Dial. Transplant. 2017, 32, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A.; Stevens, P.; Bilous, R.; Lamb, E.; Coresh, J. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013, 3, 46. [Google Scholar]
- Al Kibria, G.M.; Hasan, M.Z. Income disparities in prevalence and trends of chronic kidney disease among US adults, 2003–2018. J. Public Health 2022, 30, 2181–2189. [Google Scholar] [CrossRef]
- Chang, T.I.; Lim, H.; Park, C.H.; Rhee, C.M.; Kalantar-Zadeh, K.; Kang, E.W.; Kang, S.W.; Han, S.H. Association Between Income Disparities and Risk of Chronic Kidney Disease: A Nationwide Cohort Study of Seven Million Adults in Korea. Mayo Clin. Proc. 2020, 95, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Smith, D.H.; Thorp, M.L.; Yang, X.; Juhaeri, J. Predicting the risk of end-stage renal disease in the population-based setting: A retrospective case-control study. BMC Nephrol. 2011, 12, 17. [Google Scholar] [CrossRef]
- Anavekar, N.S.; McMurray, J.J.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef]
- Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Krljanac, G.; Lasica, R. Gender differences in the prognostic impact of chronic kidney disease in patients with left ventricular systolic dysfunction following ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Hellenic J. Cardiol. 2016, 57, 109–115. [Google Scholar] [CrossRef][Green Version]
- McAlister, F.A.; Ezekowitz, J.; Tonelli, M.; Armstrong, P.W. Renal insufficiency and heart failure: Prognostic and therapeutic implications from a prospective cohort study. Circulation 2004, 109, 1004–1009. [Google Scholar] [CrossRef]
- Smith, G.L.; Lichtman, J.H.; Bracken, M.B.; Shlipak, M.G.; Phillips, C.O.; DiCapua, P.; Krumholz, H.M. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J. Am. Coll. Cardiol. 2006, 47, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Katz, R.; Kestenbaum, B.; Fried, L.F.; Siscovick, D.; Sarnak, M.J. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis 2009, 204, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.K.; Banerjee, D.; Jouhra, F. Management of Heart Failure in Patients with Chronic Kidney Disease. Eur. Cardiol. 2022, 17, e17. [Google Scholar] [CrossRef] [PubMed]
- Hsich, E.M.; Piña, I.L. Heart failure in women: A need for prospective data. J. Am. Coll. Cardiol. 2009, 54, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.; Mazumdar, M.; Newman, G.; Bomback, A.S.; Ballantyne, C.M.; Jaffe, A.S.; August, P.A.; Kshirsagar, A.V. Screening for kidney disease in vascular patients: SCreening for Occult REnal Disease (SCORED) experience. Nephrol. Dial. Transplant. 2009, 24, 2452–2457. [Google Scholar] [CrossRef]
- Chwojnicki, K.; Król, E.; Wierucki, Ł.; Kozera, G.; Sobolewski, P.; Nyka, W.M.; Zdrojewski, T. Renal Dysfunction in Post-Stroke Patients. PLoS ONE 2016, 11, e0159775. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Kamouchi, M.; Hata, J.; Ago, T.; Kitayama, J.; Nakane, H.; Sugimori, H.; Kitazono, T. Proteinuria and clinical outcomes after ischemic stroke. Neurology 2012, 78, 1909–1915. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, T.; Chopp, M.; Venkat, P.; Chen, J. Brain-kidney interaction: Renal dysfunction following ischemic stroke. J. Cereb. Blood Flow Metab. 2020, 40, 246–262. [Google Scholar] [CrossRef]
- Hung, P.H.; Huang, Y.T.; Hsiao, C.Y.; Sung, P.S.; Guo, H.R.; Tsai, K.J. Young stroke patients are at high risk for subsequent end-stage renal disease: A population-based observational study. Nephrol. Dial. Transplant. 2014, 29, 873–878. [Google Scholar] [CrossRef]
- Ding, W.Y.; Gupta, D.; Wong, C.F.; Lip, G.Y.H. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc. Res. 2021, 117, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
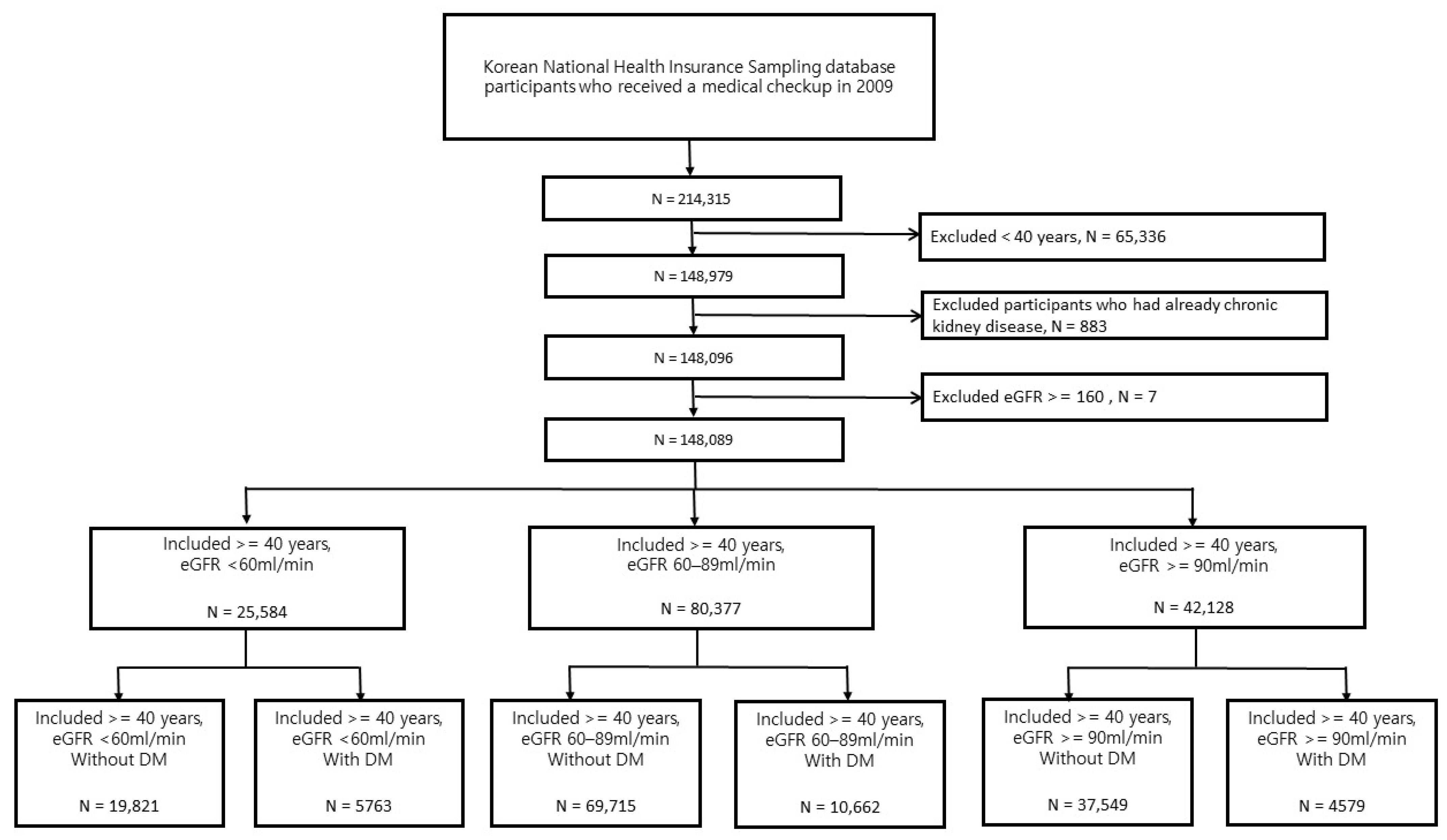
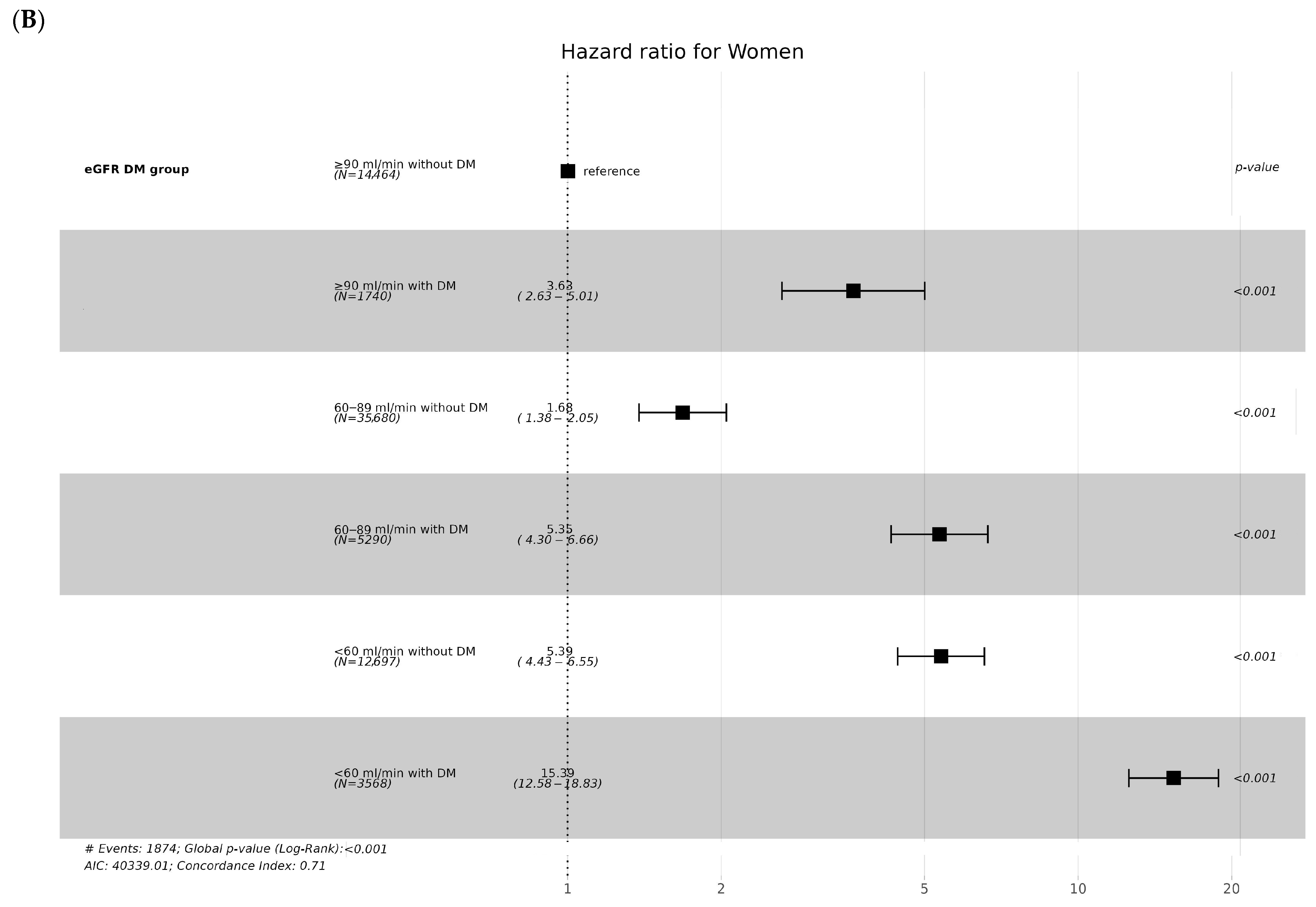
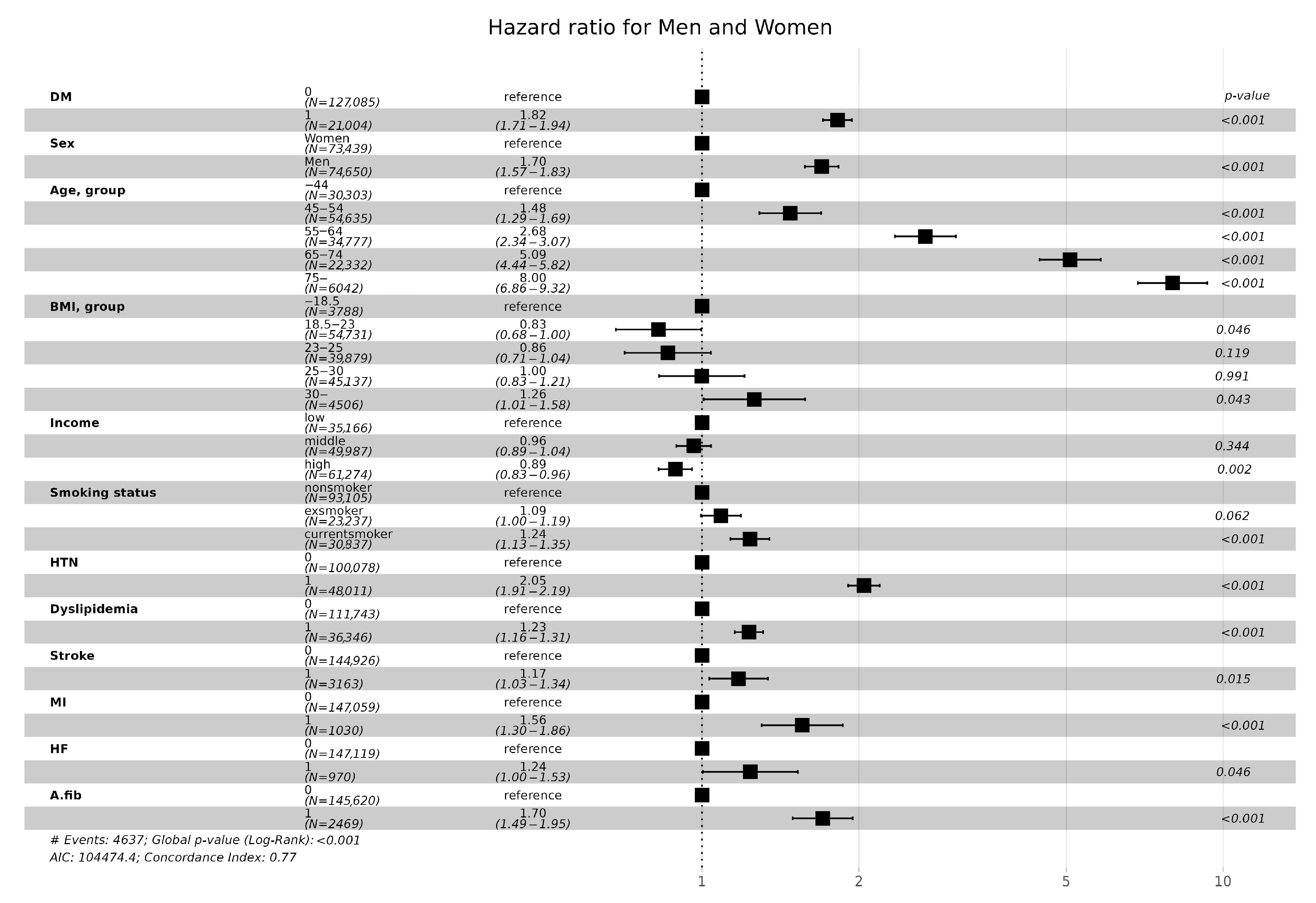
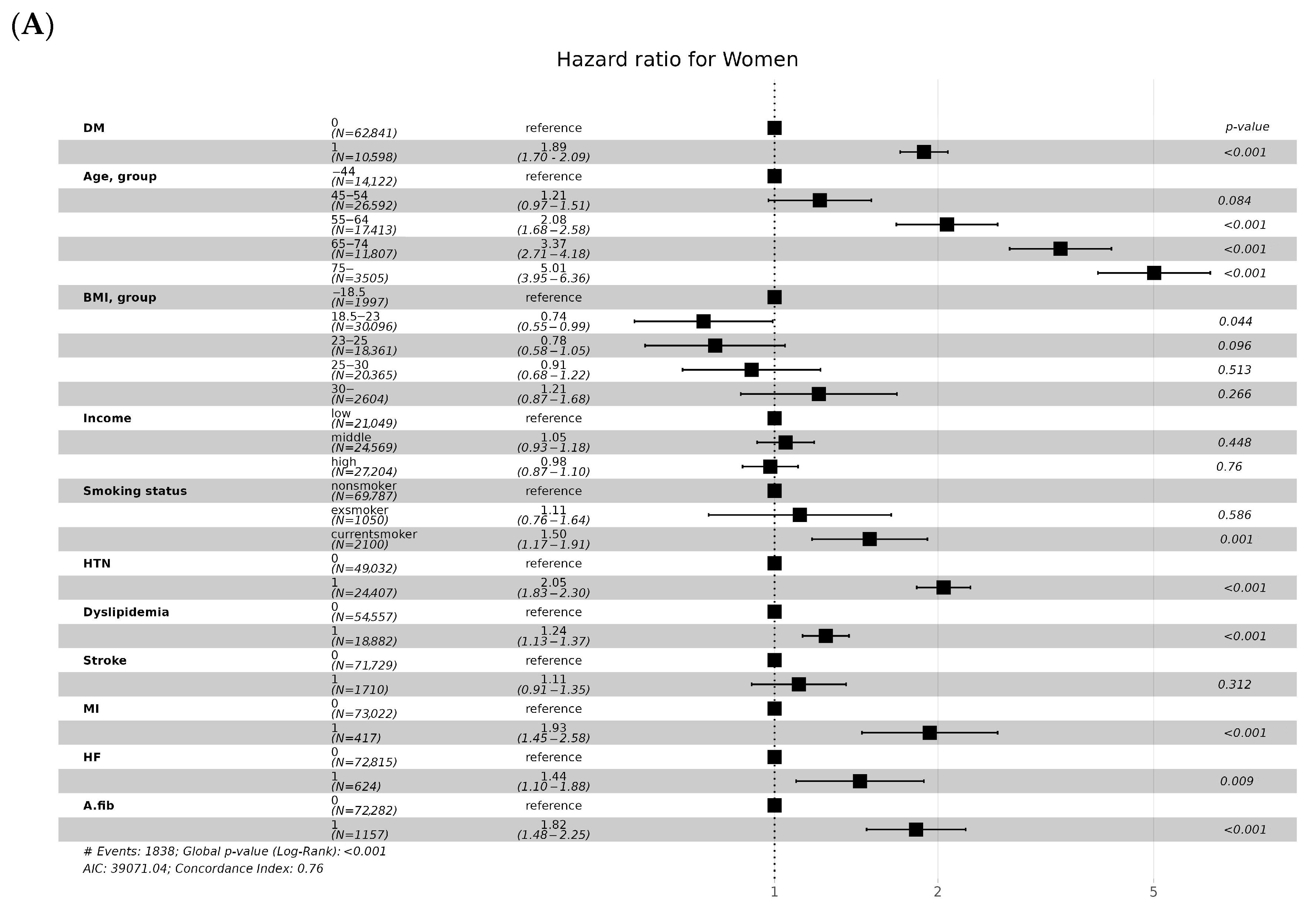

| Variables | eGFR and DM | ||||||
|---|---|---|---|---|---|---|---|
| ≥90 mL/min/1.73 m2 w/o DM | ≥90 mL/min/1.7 3 m2 with DM | 60–89 mL/min/1.73 m2 w/o DM | 60–89 mL/min/1.73 m2 with DM | <60 mL/min/1.73 m2 w/o DM | <60 mL/min/1.73 m2 with DM | p-Value | |
| Total (n) | 37,549 | 4579 | 69715 | 10,662 | 19,821 | 5763 | |
| CKD (n) | 419 (1.1%) | 183 (4.0%) | 1380 (2.0%) | 620 (5.9%) | 1268 (6.4%) | 860 (14.9%) | <0.001 |
| Sex | <0.001 | ||||||
| Women | 14,464 (38.5%) | 1740 (38.0%) | 35,680 (51.2%) | 5290 (49.6%) | 12,697 (64.1%) | 3568 (61.9%) | |
| Men | 23,085 (61.5%) | 2839 (62.0%) | 34,035 (48.8%) | 5372 (50.4%) | 7124 (35.9%) | 2195 (38.1%) | |
| Age, group (years) | <0.001 | ||||||
| 40–44 | 14,580 (38.8%) | 804 (17.6%) | 13,617 (19.5%) | 523 (4.91%) | 740 (3.73%) | 39 (0.68%) | |
| 45–54 | 17,168 (45.7%) | 2047 (44.7%) | 29,263 (42.0%) | 2749 (25.8%) | 3134 (15.8%) | 274 (4.75%) | |
| 55–64 | 4807 (12.8%) | 1295 (28.3%) | 18,277 (26.2%) | 4190 (39.3%) | 5037 (25.4%) | 1171 (20.3%) | |
| 65–74 | 946 (2.52%) | 408 (8.91%) | 7812 (11.2%) | 2883 (27.0%) | 7461 (37.6%) | 2822 (49.0%) | |
| ≥75 | 48 (0.13%) | 25 (0.55%) | 746 (1.07%) | 317 (2.97%) | 3449 (17.4%) | 1457 (25.3%) | |
| BMI, group (kg/m2) | <0.001 | ||||||
| <18.5 | 163 (0.43%) | 13 (0.28%) | 1474 (2.12%) | 98 (0.92%) | 1741 (8.79%) | 299 (5.19%) | |
| 18.5–23 | 8010 (21.3%) | 636 (13.9%) | 29,647 (42.5%) | 3152 (29.6%) | 10,679 (53.9%) | 2607 (45.3%) | |
| 23–25 | 9982 (26.6%) | 1043 (22.8%) | 19,974 (28.7%) | 3160 (29.6%) | 4275 (21.6%) | 1445 (25.1%) | |
| 25–30 | 17,000 (45.3%) | 2347 (51.3%) | 17,610 (25.3%) | 3869 (36.3%) | 2983 (15.1%) | 1328 (23.1%) | |
| ≥30 | 2381 (6.34%) | 539 (11.8%) | 984 (1.41%) | 382 (3.58%) | 138 (0.70%) | 82 (1.42%) | |
| Income, group | <0.001 | ||||||
| Low | 7923 (21.4%) | 1038 (22.9%) | 17,220 (25.0%) | 2613 (24.8%) | 5057 (25.8%) | 1315 (23.0%) | |
| Middle | 12,810 (34.6%) | 1645 (36.3%) | 23,728 (34.4%) | 3647 (34.6%) | 6418 (32.7%) | 1739 (30.4%) | |
| High | 16,338 (44.1%) | 1848 (40.8%) | 27,976 (40.6%) | 4290 (40.7%) | 8163 (41.6%) | 2659 (46.5%) | |
| Smoking, group | <0.001 | ||||||
| Non-smoker | 19,777 (52.9%) | 2490 (54.7%) | 44,768 (64.6%) | 6979 (65.9%) | 14,808 (75.2%) | 4283 (74.8%) | |
| Ex-smoker | 7054 (18.9%) | 900 (19.8%) | 10,583 (15.3%) | 1744 (16.5%) | 2230 (11.3%) | 726 (12.7%) | |
| Current-smoker | 10,527 (28.2%) | 1162 (25.5%) | 13,897 (20.1%) | 1870 (17.7%) | 2662 (13.5%) | 719 (12.6%) | |
| HTN | 8064 (21.5%) | 2429 (53.0%) | 18,022 (25.9%) | 6388 (59.9%) | 8892 (44.9%) | 4216 (73.2%) | <0.001 |
| Dyslipidemia | 6934 (18.5%) | 2194 (47.9%) | 13,841 (19.9%) | 5199 (48.8%) | 5301 (26.7%) | 2877 (49.9%) | <0.001 |
| Stroke | 342 (0.91%) | 130 (2.84%) | 1013 (1.45%) | 503 (4.72%) | 708 (3.57%) | 467 (8.10%) | <0.001 |
| MI | 125 (0.33%) | 48 (1.05%) | 300 (0.43%) | 161 (1.51%) | 228 (1.15%) | 168 (2.92%) | <0.001 |
| HF | 87 (0.23%) | 44 (0.96%) | 265 (0.38%) | 143 (1.34%) | 246 (1.24%) | 185 (3.21%) | <0.001 |
| A. fib | 387 (1.03%) | 93 (2.03%) | 828 (1.19%) | 331 (3.10%) | 565 (2.85%) | 265 (4.60%) | <0.001 |
| Death (n) | 1195 (3.18%) | 329 (7.18%) | 3771 (5.41%) | 1351 (12.7%) | 4146 (20.9%) | 1868 (32.4%) | <0.001 |
| Creatinine (mg/dL) | 1.0 ± 1.2 | 0.9 ± 1.0 | 1.1 ± 1.3 | 1.0 ± 1.1 | 1.2 ± 1.4 | 1.2 ± 1.2 | <0.001 |
| SBP (mmHg) | 124.2 ± 14.7 | 126.9 ± 14.8 | 122.9 ± 15.3 | 127.0 ± 15.7 | 125.7 ± 16.7 | 129.3 ± 16.8 | <0.001 |
| DBP (mmHg) | 78.0 ± 10.3 | 79.1 ± 9.9 | 76.6 ± 10.1 | 78.0 ± 10.0 | 76.9 ± 10.3 | 77.5 ± 10.3 | <0.001 |
| Variables | eGFR and DM | ||||||
|---|---|---|---|---|---|---|---|
| ≥90 mL/min/1.73 m2 w/o DM | ≥90 mL/min/1.73 m2 with DM | 60–89 mL/min/1.73 m2 w/o DM | 60–89 mL/min/1.73 m2 with DM | <60 mL/min/1.73 m2 w/o DM | <60 mL/min/1.73 m2 with DM | p-Value | |
| Total (n) | 37,549 | 4579 | 69,715 | 10,662 | 19,821 | 5763 | |
| CKD | 419 (1.1%) | 183 (4.0%) | 1380 (2.0%) | 620 (5.9%) | 1268 (6.4%) | 860 (14.9%) | <0.001 |
| Sex | |||||||
| Women (n) | 14,464 | 1740 | 35,680 | 5290 | 12,697 | 3568 | |
| CKD | 123 (0.9%) | 53 (3.0%) | 509 (1.4%) | 235 (4.4%) | 548 (4.3%) | 406 (11.4%) | <0.001 |
| Men (n) | 23,085 | 2839 | 34,035 | 5372 | 7124 | 2195 | |
| CKD | 296 (1.3%) | 130 (4.6%) | 871 (2.6%) | 385 (7.2%) | 720 (10.1%) | 454 (20.7%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, H.-J.; Ahn, S.-K.; Han, S.; Kim, M.-J.; Na, K.R.; Park, H.; Choi, D.E. The Factors Influencing Chronic Kidney Disease Incidence: Database from the Korean National Health Insurance Sharing Service (NHISS). J. Clin. Med. 2024, 13, 2164. https://doi.org/10.3390/jcm13082164
Ko H-J, Ahn S-K, Han S, Kim M-J, Na KR, Park H, Choi DE. The Factors Influencing Chronic Kidney Disease Incidence: Database from the Korean National Health Insurance Sharing Service (NHISS). Journal of Clinical Medicine. 2024; 13(8):2164. https://doi.org/10.3390/jcm13082164
Chicago/Turabian StyleKo, Ho-Joon, Soon-Ki Ahn, Suyeon Han, Moo-Jun Kim, Ki Ryang Na, Hyerim Park, and Dae Eun Choi. 2024. "The Factors Influencing Chronic Kidney Disease Incidence: Database from the Korean National Health Insurance Sharing Service (NHISS)" Journal of Clinical Medicine 13, no. 8: 2164. https://doi.org/10.3390/jcm13082164
APA StyleKo, H.-J., Ahn, S.-K., Han, S., Kim, M.-J., Na, K. R., Park, H., & Choi, D. E. (2024). The Factors Influencing Chronic Kidney Disease Incidence: Database from the Korean National Health Insurance Sharing Service (NHISS). Journal of Clinical Medicine, 13(8), 2164. https://doi.org/10.3390/jcm13082164






