Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation
Abstract
1. Introduction
1.1. The History of Dual Disorders
1.2. Real-World Settings
1.3. Lurasidone: A Potentially Effective Drug in Dual Disorder?
1.4. Aim of the Study
2. Materials and Methods
2.1. Participants and Recruitment Centers
2.2. Study Design and Treatment Information
2.3. Study Procedures and Psychometric Assessments
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Baseline Characteristics
3.2. Changes in Psychopathological Domains from Baseline to One-Month Follow-Up
3.3. Changes in Global Health Condition and Quality of Life from Baseline to One-Month Follow-Up
3.4. Safety and Tolerability of Lurasidone
4. Discussion
4.1. Challenges and Considerations
4.2. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adan, A.; Benaiges, I. Comorbidity between Substance Use Disorder and Severe Mental Illness. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 258–268. [Google Scholar] [CrossRef]
- Hryb, K.; Kirkhart, R.; Talbert, R. A Call for Standardized Definition of Dual Diagnosis. Psychiatry 2007, 4, 15. [Google Scholar]
- Martinotti, G.; Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Pettorruso, M.; Skryabin, V.; Sensi, S.L.; Giannantonio, M.D. Atypical Antipsychotic Drugs in Dual Disorders: Current Evidence for Clinical Practice. Curr. Pharm. Des. 2022, 28, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. European Drug Report 2023: Trends and Developments; EMCDDA: Lisbon, Portugal, 2023.
- Bahji, A. Navigating the Complex Intersection of Substance Use and Psychiatric Disorders: A Comprehensive Review. J. Clin. Med. 2024, 13, 999. [Google Scholar] [CrossRef]
- Bahji, A.; Mazhar, M.N.; Hudson, C.C.; Nadkarni, P.; MacNeil, B.A.; Hawken, E. Prevalence of Substance Use Disorder Comorbidity among Individuals with Eating Disorders: A Systematic Review and Meta-Analysis. Psychiatry Res. 2019, 273, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Danilewitz, M.; Bahji, A. Opioid Use Disorder Comorbidity in Individuals with Schizophrenia-Spectrum Disorders: A Systematic Review and Meta-Analysis. Can. J. Addict. 2021, 12, 26. [Google Scholar] [CrossRef]
- Bahji, A.; Danilewitz, M.; Vazquez, G.; Patten, S. The Prevalence of Cannabis Use Disorder Comorbidity in Individuals with Bipolar Disorder: A Systematic Review and Meta-Analysis. Can. J. Addict. 2021, 12, 22. [Google Scholar] [CrossRef]
- Jegede, O.; Rhee, T.G.; Stefanovics, E.A.; Zhou, B.; Rosenheck, R.A. Rates and Correlates of Dual Diagnosis among Adults with Psychiatric and Substance Use Disorders in a Nationally Representative U.S Sample. Psychiatry Res. 2022, 315, 114720. [Google Scholar] [CrossRef]
- Ricci, V.; Ceci, F.; Di Carlo, F.; Lalli, A.; Ciavoni, L.; Mosca, A.; Sepede, G.; Salone, A.; Quattrone, D.; Fraticelli, S.; et al. Cannabis use disorder and dissociation: A report from a prospective first-episode psychosis study. Drug Alcohol. Depend. 2021, 229 (Pt. A), 109118. [Google Scholar] [CrossRef]
- Martinotti, G.; De Risio, L.; Vannini, C.; Schifano, F.; Pettorruso, M.; Di Giannantonio, M. Substance-related exogenous psychosis: A postmodern syndrome. CNS Spectr. 2021, 26, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Chiappini, S.; Papanti, D.; De Berardis, D.; Corkery, J.M.; Schifano, F. The Bridge Between Classical and ‘Synthetic’/Chemical Psychoses: Towards a Clinical, Psychopathological, and Therapeutic Perspective. Front. Psychiatry 2019, 10, 851. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic And Statistical Manual of Mental Disorder (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Martinotti, G.; Merino Del Villar, C.; Garcia Cordoba, A.; Andrés Tubau, L.; Castro Sánchez, I.; Di Carlo, F.; Chiappini, S.; Pettorruso, M.; Schifano, F.; Di Giannantonio, M. Club Drugs and Psychiatric Sequelae: An Issue of Vulnerability and Previous Psychiatric History. Int. J. Environ. Res. Public Health 2021, 18, 6944. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, L.N.; Pfeiffer, P.; Ganoczy, D.; Lin, L.A. Quality of Outpatient Depression Treatment in Patients with Comorbid Substance Use Disorder. Am. J. Psychiatry 2021, 178, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Manwani, S.G.; Szilagyi, K.A.; Zablotsky, B.; Hennen, J.; Griffin, M.L.; Weiss, R.D. Adherence to pharmacotherapy in bipolar disorder patients with and without co-occurring substance use disorders. J. Clin. Psychiatry 2007, 68, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Gallegos, L.; Medina-Mora, M.E.; Benjet, C.; Ruiz-Velasco, S.; Magis-Rodriguez, C.; Marín-Navarrete, R. Multidimensional Patterns of Sexual Risk Behavior and Psychiatric Disorders in Men with Substance Use Disorders. Arch. Sex. Behav. 2019, 48, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Luba, R.; Carpenter, K.M.; Evans, S.M.; Slonim, J.; Foltin, R.W. Impulsivity and Treatment Outcomes in Individuals with Cocaine Use Disorder: Examining the Gap between Interest and Adherence. Subst. Use Misuse 2023, 58, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Miuli, A.; Mancusi, G.; Chiappini, S.; Stigliano, G.; De Pasquale, A.; Di Petta, G.; Bubbico, G.; Pasino, A.; Pettorruso, M.; et al. To bridge or not to bridge: Moral Judgement in Cocaine Use Disorders, a case-control study on human morality. Soc. Neurosci. 2023, 18, 271–281. [Google Scholar] [CrossRef]
- Chiappini, S.; d’Andrea, G.; De Filippis, S.; Di Nicola, M.; Andriola, I.; Bassetti, R.; Barlati, S.; Pettorruso, M.; Sensi, S.; Clerici, M.; et al. Esketamine in treatment-resistant depression patients comorbid with substance-use disorder: A viewpoint on its safety and effectiveness in a subsample of patients from the REAL-ESK study. Eur. Neuropsychopharmacol. 2023, 74, 15–21. [Google Scholar] [CrossRef]
- Machielsen, M.W.; Veltman, D.J.; van den Brink, W.; de Haan, L. The effect of clozapine and risperidone on attentional bias in patients with schizophrenia and a cannabis use disorder: An fMRI study. J. Psychopharmacol. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Desseilles, M.; Mathot, F.; Desseilles, M. Aripiprazole Diminishes Cannabis Use in Schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2008, 20, 117–118. [Google Scholar] [CrossRef]
- Feeley, R.J.; Arnaout, B.; Yoon, G. Effective Switch from Clozapine to Aripiprazole in Treatment-Resistant Schizophrenia and Comorbid Alcohol Use Disorder. J. Clin. Psychopharmacol. 2017, 37, 729–730. [Google Scholar] [CrossRef] [PubMed]
- van Nimwegen, L.J.; de Haan, L.; van Beveren, N.J.; van der Helm, M.; van den Brink, W.; Linszen, D. Effect of Olanzapine and Risperidone on Subjective Well-Being and Craving for Cannabis in Patients with Schizophrenia or Related Disorders: A Double-Blind Randomized Controlled Trial. Can. J. Psychiatry 2008, 53, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Rafizadeh, R.; Danilewitz, M.; Bousman, C.A.; Mathew, N.; White, R.F.; Bahji, A.; Honer, W.G.; Schütz, C.G. Effects of clozapine treatment on the improvement of substance use disorders other than nicotine in individuals with schizophrenia spectrum disorders: A systematic review and meta-analysis. J. Psychopharmacol. 2023, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rafizadeh, R.; Frankow, L.; Mahmood, H.; Poonia, S.; Mathew, N.; Danilewitz, M.; Bousman, C.A.; Honer, W.G.; Schütz, C.G. Association of clozapine treatment and rate of methamphetamine or amphetamine relapses and abstinence among individuals with concurrent schizophrenia spectrum and amphetamine use disorder: A retrospective cohort study. J. Psychopharmacol. 2023, 37, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.C.; McKinnon, R.A.; Miners, J.O.; Bastiampillai, T. Binding of clozapine to the GABAB receptor: Clinical and structural insights. Mol. Psychiatry 2020, 25, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- McQueen, G.; Sendt, K.V.; Gillespie, A.; Avila, A.; Lally, J.; Vallianatou, K.; Chang, N.; Ferreira, D.; Borgan, F.; Howes, O.D.; et al. Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophr. Bull. 2021, 47, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 4th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Stahl, S.M. Essential Psychopharmacology: Prescriber’s Guide, 6th ed.; Cambridge Univerisity Press: Cambridge, UK, 2021. [Google Scholar]
- Sadlonova, M.; Beach, S.R.; Funk, M.C.; Rosen, J.H.; Ramirez Gamero, A.F.; Karlson, R.A.; Huffman, J.C.; Celano, C.M. Risk Stratification of QTc Prolongation in Critically Ill Patients Receiving Antipsychotics for the Management of Delirium Symptoms. J. Intensive Care Med. 2023. [Google Scholar] [CrossRef]
- Miura, I.; Horikoshi, S.; Ichinose, M.; Suzuki, Y.; Watanabe, K. Lurasidone for the Treatment of Schizophrenia: Design, Development, and Place in Therapy. Drug Des. Devel. Ther. 2023, 17, 3023–3031. [Google Scholar] [CrossRef]
- Jaeschke, R.R.; Sowa-Kućma, M.; Pańczyszyn-Trzewik, P.; Misztak, P.; Styczeń, K.; Datka, W. Lurasidone: The 2016 update on the pharmacology, efficacy and safety profile. Pharmacol. Rep. 2016, 68, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Tarzian, M.; Soudan, M.; Alhajji, M.; Ndrio, M.; Fakoya, A.O. Lurasidone for Treating Schizophrenia and Bipolar Depression: A Review of Its Efficacy. Cureus 2023, 15, 38071. [Google Scholar] [CrossRef] [PubMed]
- Ricci, V.; Martinotti, G.; De Berardis, D.; Maina, G. Lurasidone use in Cannabis-Induced Psychosis: A Novel Therapeutic Strategy and Clinical Considerations in Four Cases Report. Int. J. Environ. Res. Public Health 2022, 19, 16057. [Google Scholar] [CrossRef]
- Zheng, W.; Cai, D.B.; Yang, X.H.; Li, L.; Zhang, Q.E.; Ng, C.H.; Ungvari, G.S.; Li, X.B.; Ning, Y.P.; Xiang, Y.T. Short-term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: A meta-analysis of randomized controlled trials. J. Psychiatr. Res. 2018, 103, 244–251. [Google Scholar] [CrossRef]
- Citrome, L.; Cucchiaro, J.; Sarma, K.; Phillips, D.; Silva, R.; Tsuchiya, S.; Loebel, A. Long-term safety and tolerability of lurasidone in schizophrenia: A 12-month, double-blind, active-controlled study. Int. Clin. Psychopharmacol. 2012, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Cucchiaro, J.; Simonelli, D.; Hsu, J.; Pikalov, A.; Loebel, A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: A 6-month, open-label, extension study. J. Clin. Psychiatry 2013, 74, 507–515. [Google Scholar] [CrossRef]
- Wei, Y.M.; Wang, X.J.; Yang, X.D.; Wang, C.S.; Wang, L.L.; Xu, X.Y.; Zhao, G.J.; Li, B.; Zhu, D.M.; Wu, Q.; et al. Safety and effectiveness of lurasidone in the treatment of Chinese schizophrenia patients: An interim analysis of post-marketing surveillance. World J. Psychiatry 2023, 13, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Cucchiaro, J.; Silva, R.; Hsu, J. Long-term safety and effectiveness of lurasidone in schizophrenia: A 22-month, open-label extension study. CNS Spectr. 2016, 21, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Ishigooka, J.; Iyo, M.; Hagi, K. Safety and effectiveness of lurasidone for the treatment of schizophrenia in Asian patients: Results of a 26-week open-label extension study. Asia Pac. Psychiatry 2020, 12, e12377. [Google Scholar] [CrossRef] [PubMed]
- Ricci, V.; De Berardis, D.; Maina, G. Third-Generation Antipsychotics and Lurasidone in the Treatment of Substance-Induced Psychoses: A Narrative Review. Healthcare 2024, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.A.; Albert, U.; de Filippis, S.; Vita, A.; De Berardis, D. Identification of clinical phenotypes in schizophrenia: The role of lurasidone. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211012250. [Google Scholar] [CrossRef]
- Mole, T.B.; Furlong, Y.; Clarke, R.J.; Rao, P.; Moore, J.K.; Pace, G.; Van Odyck, H.; Chen, W. Lurasidone for Adolescents with Complex Mental Disorders: A Case Series. J. Pharm. Pract. 2022, 35, 800–804. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Guy, W.; National Institute of Mental Health (U.S.). Psychopharmacology Research Branch, and Early Clinical Drug Evaluation Program. In ECDEU Assessment Manual for Pychopharmacology; DHEW ADM 76-338; National Institute of Mental Health (U.S.): Washington, DC, USA, 1976. [Google Scholar]
- Mottola, C.A. Measurement strategies: The visual analogue scale. Decubitus 1993, 6, 56–58. [Google Scholar] [PubMed]
- Rossi, A.; Rucci, P.; Mauri, M.; Maina, G.; Pieraccini, F.; Pallanti, S.; Endicott, J.; EQUIP Group. Validity and Reliability of the Italian Version of the Quality of Life, Enjoyment and Satisfaction Questionnaire. Qual. Life Res. 2005, 14, 2323–2328. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef]
- Margari, F.; Matarazzo, R.; Casacchia, M.; Roncone, R.; Dieci, M.; Safran, S.; Fiori, G.; Simoni, L. Italian validation of MOAS and NOSIE: A useful package for psychiatric assessment and monitoring of aggressive behaviours. Int. J. Methods Psychiatr. Res. 2005, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- WMA. Dichiarazione di Helsinki della World Medical Association. Evidence 2013, 5, 1–5. [Google Scholar]
- Smith, M.J.; Thirthalli, J.; Abdallah, A.B.; Murray, R.M.; Cottler, L.B. Prevalence of psychotic symptoms in substance users: A comparison across substances. Compr. Psychiatry 2009, 50, 245–250. [Google Scholar] [CrossRef]
- Messer, T.; Lammers, G.; Müller-Siecheneder, F.; Schmidt, R.-F.; Latifi, S. Substance abuse in patients with bipolar disorder: A systematic review and meta-analysis. Psychiatry Res. 2017, 253, 338–350. [Google Scholar] [CrossRef]
- Hunt, G.E.; Large, M.M.; Cleary, M.; Lai, H.M.X.; Saunders, J.B. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol. Depend. 2018, 191, 234–258. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services’ (HHS). SAMHSA Announces National Survey on Drug Use and Health (NSDUH) Results Detailing Mental Illness and Substance Use Levels in 2021; U.S. Department of Health and Human Services’ (HHS): Washington, DC, USA, 2021.
- National Collaborating Centre for Mental Health (UK). Psychosis with Coexisting Substance Misuse: Assessment and Management in Adults and Young People; National Collaborating Centre for Mental Health (UK): Leicester, UK, 2011. [Google Scholar]
- Fiorentini, A.; Cantù, F.; Crisanti, C.; Cereda, G.; Oldani, L.; Brambilla, P. Substance-Induced Psychoses: An Updated Literature Review. Front. Psychiatry 2021, 12, 694863. [Google Scholar] [CrossRef] [PubMed]
- Błachut, M.; Ścisło, P.; Jarząb, M.; Zając, M.; Piegza, M.; Badura-Brzoza, K.; Gorczyca, P. Impact of dual diagnosis in patients with schizophrenia and affective disorders during hospital treatment on the course of illness and outcomes of treatment—A preliminary report. Psychiatr. Pol. 2019, 53, 1237–1250, (In English, In Polish). [Google Scholar] [CrossRef]
- Harvey, P.D.; Ogasa, M.; Cucchiaro, J.; Loebel, A.; Keefe, R.S. Performance and interview-based assessments of cognitive change in a randomized, double- blind comparison of lurasidone vs. ziprasidone. Schizophr. Res. 2011, 127, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Osborne, I.J.; Mace, S.; Taylor, D. A prospective year-long follow-up of lurasidone use in clinical practice: Factors predicting treatment persistence. Ther. Adv. Psychopharmacol. 2018, 8, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Luo, D.; Wang, D.; Lai, J.; Li, Q.; Zhang, P.; Huang, H.; Wu, L.; Lu, S.; Hu, S. Lurasidone versus Quetiapine for Cognitive Impairments in Young Patients with Bipolar Depression: A Randomized, Controlled Study. Pharmaceuticals 2022, 15, 1403. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Mackala, S.; Basivireddy, J.; Ahn, S.; Walji, N.; Hu, C.; Lam, R.W.; Torres, I.J. Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: A randomised, open-label, pilot study. Lancet Psychiatry 2017, 4, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Caccia, S.; Pasina, L.; Nobili, A. Critical appraisal of lurasidone in the management of schizophrenia. Neuropsychiatr. Dis. Treat. 2012, 8, 155–168. [Google Scholar] [CrossRef]
- Martinotti, G.; Lupi, M.; Montemitro, C.; Miuli, A.; Di Natale, C.; Spano, M.C.; Mancini, V.; Lorusso, M.; Stigliano, G.; Tambelli, A.; et al. Transcranial Direct Current Stimulation Reduces Craving in Substance Use Disorders: A Double-blind, Placebo-Controlled Study. J. ECT 2019, 35, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Lappan, S.N.; Brown, A.W.; Hendricks, P.S. Dropout rates of in-person psychosocial substance use disorder treatments: A systematic review and meta-analysis. Addiction 2020, 115, 201–217. [Google Scholar] [CrossRef]
- Puértolas-Gracia, B.; Barbaglia, M.G.; Gotsens, M.; Parés-Badell, O.; Brugal, M.T.; Torrens, M.; Treviño, L.; Rodríguez-Díaz, C.; Vázquez-Vázquez, J.M.; Pascual, A.; et al. Lifetime Dual Disorder Screening and Treatment Retention: A Pilot Cohort Study. J. Clin. Med. 2022, 11, 3760. [Google Scholar] [CrossRef]
- Bouchard, M.; Lecomte, T.; Cloutier, B.; Herrera-Roberge, J.; Potvin, S. Dropout Rates in Psychosocial Interventions for People with Both Severe Mental Illness and Substance Misuse: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 842329. [Google Scholar] [CrossRef]
| Gender (male, %) | 17 (69.56) |
| Age (y) | 35.5 [18–54] |
| Substance abused | |
| Alcohol | 13 (56.52) |
| Cocaine | 8 (34.78) |
| Cannabis | 12 (52.17) |
| Benzodiazepines | 2 (8.7) |
| Methadone | 1 (4.34) |
| Oxycodone | 1 (4.34) |
| Amphetamines | 1 (4.34) |
| Polysubstance users | 10 (43.5) |
| Dual Diagnosis | |
| Schizoaffective Disorder | 10 (43.48) |
| Substance-induced psychosis | 13 (56.52) |
| Lurasidone dosages (mg) | 74 mg [18.5 mg–148 mg] |
| Other therapies | |
| Antipsychotics | Olanzapine 5 mg/die, 1 (4.34) Promazine 30–120 mg/die, 5 (21.74) Quetiapine 100–200 mg/die, 2 (8.7) Tiapride 100 mg/die, 1 (4.34) |
| Antidepressants | Trazodone 50–150 mg/die, 9 (39.13) Sertraline 100 mg/die, 1 (4.34) Clomipramine 75 mg/die, 1 (4.34) Mirtazapine 30 mg/die, 1 (4.34) |
| Antiepileptics | Gabapentin 900–1600 mg/die, 7 (30.43) Lithium Carbonate 300–900 mg/die, 2 (8.7) Valproate 1500 mg/die, 2 (8.7) Pregabalin 300–450 mg/die, 2 (8.7) Lamotrigine 50 mg/die, 1 (4.34) |
| Benzodiazepines and Z-drugs | Alprazolam 7 mg/die, 1 (4.34) Delorazepam 1–4 mg/die, 4 (17.4) Diazepam 4–10 mg/die. 5 (21.74) Lorazepam 1 mg/die, 1 (4.34) Clonazepam 5 mg/die, 1 (4.34) Flurazepam 30 mg/die, 1 (4.34) Zolpidem 10 mg/die, 1 (4.34) |
| Others | Naltrexone 50 mg/die, 1 (4.34) Levomethadone 5 mg/die, 1 (4.34) Methadone 10 mg/die, 1 (4.34) |
| Baseline (n = 23) | Follow-Up (n = 14) | Z | rrb | p | |
|---|---|---|---|---|---|
| PANSS | |||||
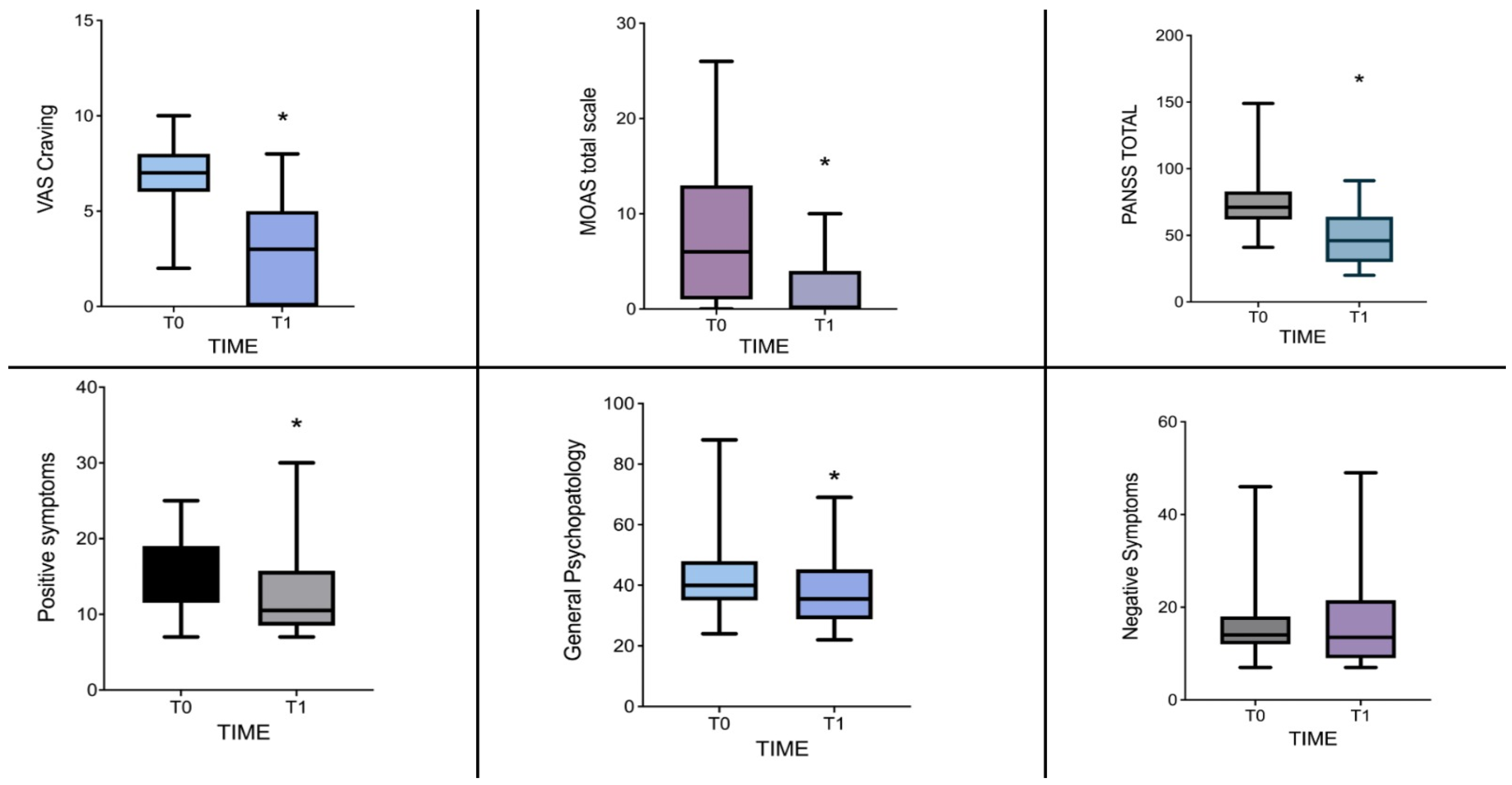
| Positive | 16 [7–25] | 10.5 [7–30] | 2.132 | 0.670 | 0.035 |
| Negative | 14 [7–46] | 13.5 [7–49] | 0.471 | 0.665 | |
| General | 40 [24–88] | 35.5 [22–69] | 2.605 | 0.790 | 0.010 |
| Total | 71 [41–149] | 60 [38–148] | 2.574 | 0.781 | 0.011 |
| CGI | 6 [5–7] | 3 [2–6] | 2.934 | 0.328 | 0.003 |
| MOAS | 6 [0–26] | 0 [0–10] | 2.000 | 0.654 | 0.050 |
| VAS craving | 8 [2–10] | 3.1 ± 2.5 | 3.202 | 0.971 | 0.001 |
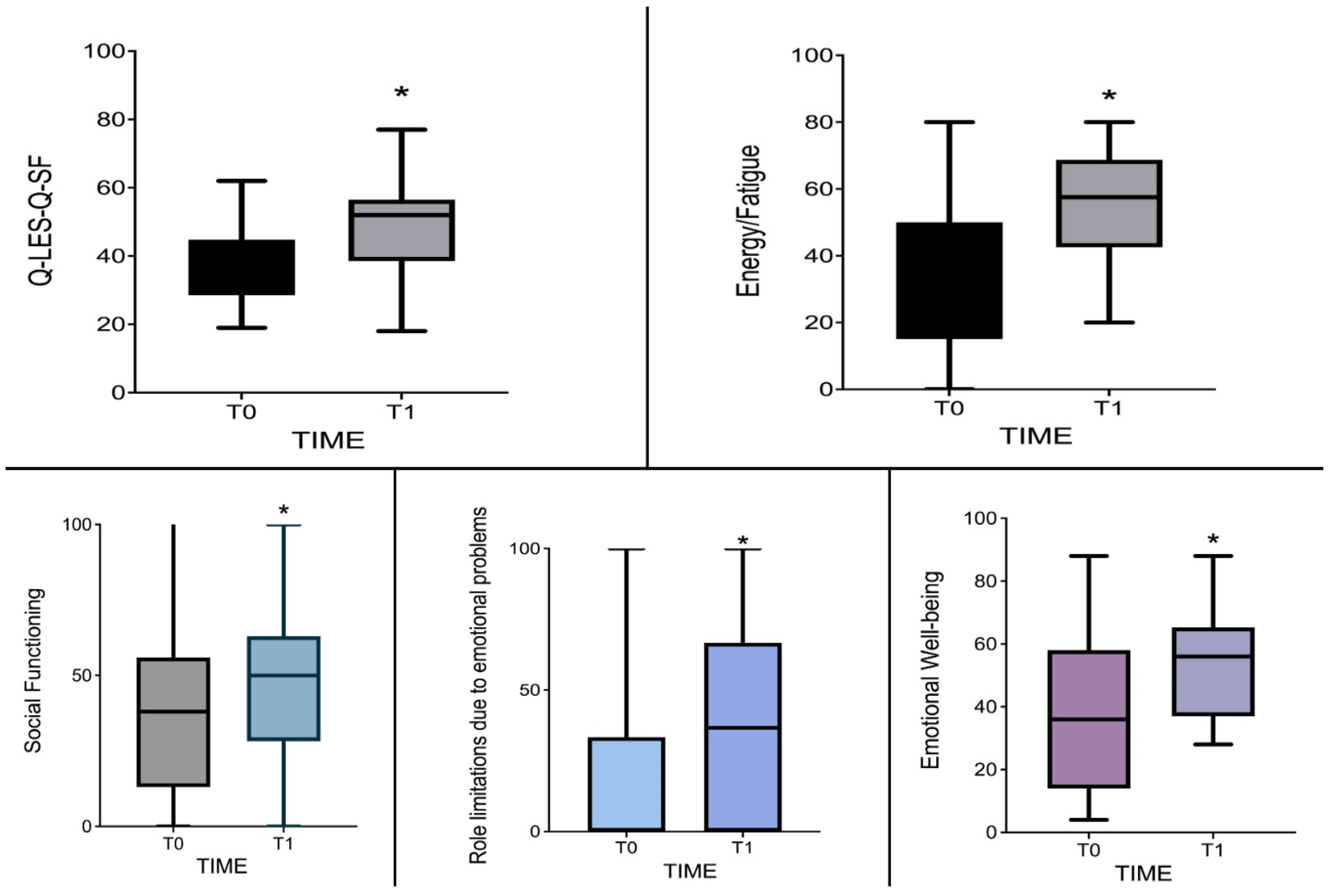
| Q-LES-Q-SF | 38 [19–62] | 2 [18–77] | −2.341 | 0.305 | 0.021 |
| SF-36 | |||||
| Physical functioning | 90 [45–100] | 95 [65–100] | −1.362 | 0.191 | |
| Limitations due to physical health | 25 [0–100] | 25 [0–100] | −0.770 | 0.482 | |
| Limitations due to emotional problems | 0 [0–100] | 36.67 [0–100] | −2.521 | −1.000 | 0.014 |
| Energy/fatigue | 30 [0–80] | 57.5 [20–80] | −2.903 | −0.949 | 0.004 |
| Emotional well-being | 36 [4–88] | 56 [28–88] | −2.510 | −0.821 | 0.013 |
| Social functioning | 25 [0–100] | 50 [0–100] | −2.432 | −0.795 | 0.016 |
| Pain | 77.5 [0–100] | 66.25 [20–100] | −0.118 | 0.953 | |
| General health | 45 [0–75] | 45 [5–85] | −1.117 | 0.255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallotto, C.; Chiappini, S.; Mosca, A.; d’Andrea, G.; Di Carlo, F.; Piro, T.; Susini, O.; Stefanelli, G.; Di Cesare, A.; Ricci, V.; et al. Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation. J. Clin. Med. 2024, 13, 2206. https://doi.org/10.3390/jcm13082206
Cavallotto C, Chiappini S, Mosca A, d’Andrea G, Di Carlo F, Piro T, Susini O, Stefanelli G, Di Cesare A, Ricci V, et al. Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation. Journal of Clinical Medicine. 2024; 13(8):2206. https://doi.org/10.3390/jcm13082206
Chicago/Turabian StyleCavallotto, Clara, Stefania Chiappini, Alessio Mosca, Giacomo d’Andrea, Francesco Di Carlo, Tommaso Piro, Ottavia Susini, Giulia Stefanelli, Andrea Di Cesare, Valerio Ricci, and et al. 2024. "Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation" Journal of Clinical Medicine 13, no. 8: 2206. https://doi.org/10.3390/jcm13082206
APA StyleCavallotto, C., Chiappini, S., Mosca, A., d’Andrea, G., Di Carlo, F., Piro, T., Susini, O., Stefanelli, G., Di Cesare, A., Ricci, V., Pepe, M., Dattoli, L., Di Nicola, M., Pettorruso, M., & Martinotti, G. (2024). Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation. Journal of Clinical Medicine, 13(8), 2206. https://doi.org/10.3390/jcm13082206








