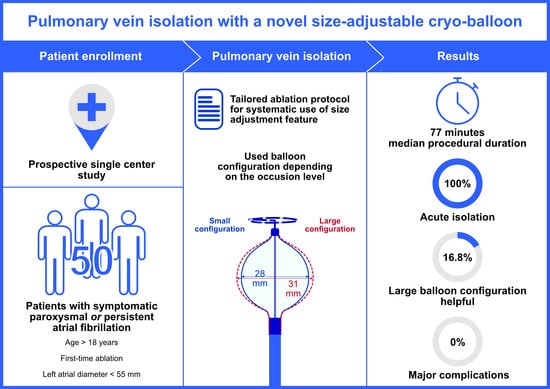
Pulmonary Vein Isolation with a Novel Size-Adjustable Cryo-Balloon Catheter: A Tailored Ablation Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
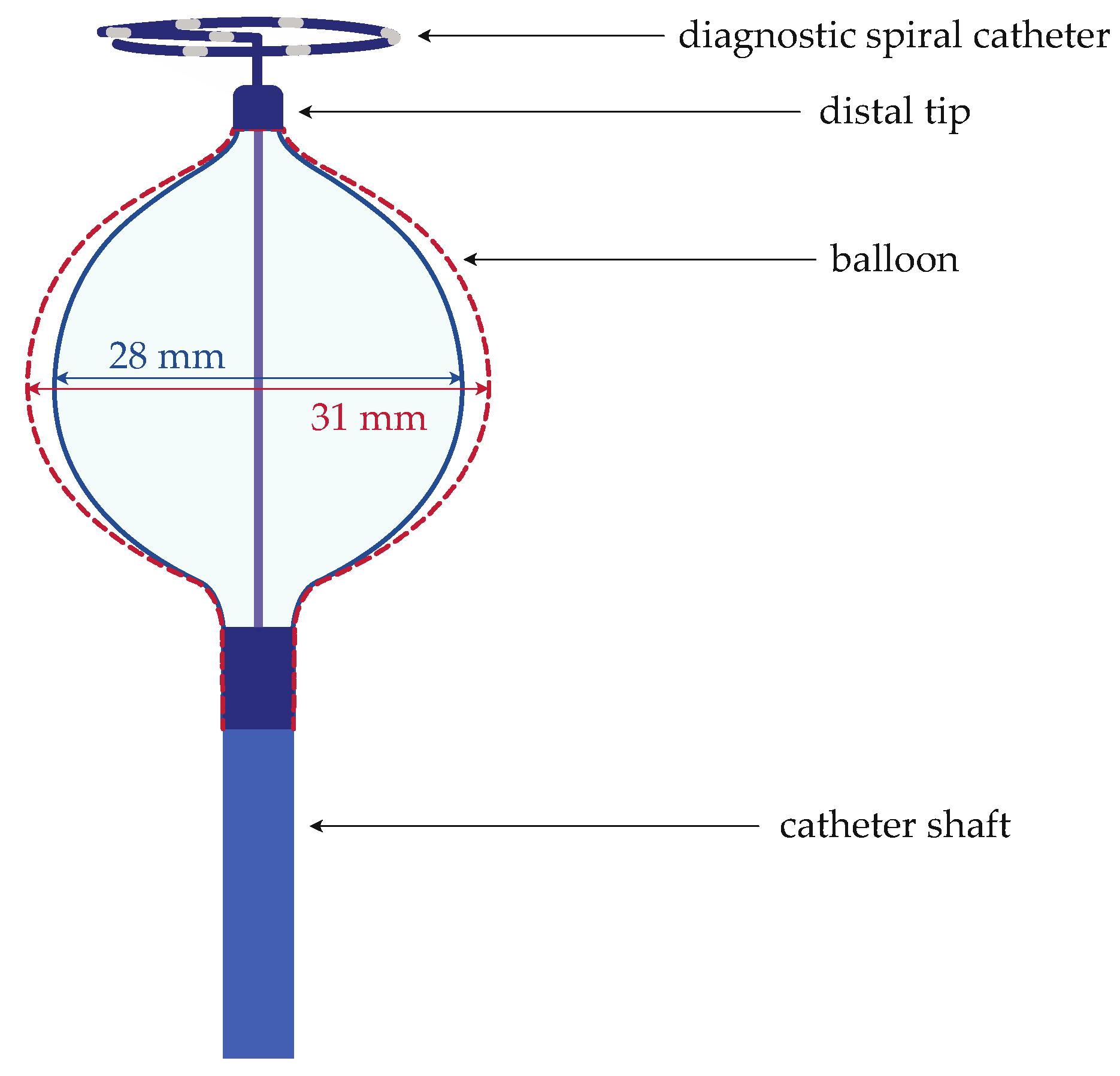
2.2. Components and Characteristics of the Ablation System
2.3. Periprocedural Management, Ablation Procedure, and Postprocedural Management
2.4. Tailored Ablation Protocol
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Procedural Characteristics and Ablation Data
3.3. Safety
4. Discussion
4.1. Efficacy
4.2. Safety
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, K.; Heudorfer, R.; Lenz, A.; Aktolga, D.; Rattka, M.; Bothner, C.; Pott, A.; Öchsner, W.; Rottbauer, W.; Dahme, T. Safety of Conscious Sedation in Electroanatomical Mapping Procedures and Cryoballoon Pulmonary Vein Isolation. Heart Vessels 2021, 36, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Teumer, Y.; Miesbichler, C.; Katov, L.; Mayer, B.; Rottbauer, W.; Bothner, C.; Weinmann, K. Comparison between a Novel Radiofrequency-Balloon and a Standard Cryo-Balloon in Pulmonary Vein Isolation: A Propensity-Score-Matched Analysis. J. Clin. Med. 2024, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Tilz, R.R.R.; Anic, A.; Defaye, P.; Luik, A.; De Asmundis, C.; Champ-Rigot, L.; Iacopino, S.; Sommer, P.; Albrecht, E.M.; et al. Acute Procedural Efficacy and Safety of a Novel Cryoballoon for the Treatment of Paroxysmal Atrial Fibrillation: Results from the POLAR ICE Study. J. Cardiovasc. Electrophysiol. 2023, 34, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Guckel, D.; Lucas, P.; Isgandarova, K.; El Hamriti, M.; Bergau, L.; Fink, T.; Sciacca, V.; Imnadze, G.; Braun, M.; Khalaph, M.; et al. News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation. J. Cardiovasc. Dev. Dis. 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Heeger, C.-H.; Popescu, S.S.; Inderhees, T.; Nussbickel, N.; Eitel, C.; Kirstein, B.; Phan, H.-L.; Hatahet, S.; Subin, B.; Traub, A.; et al. Novel or Established Cryoballoon Ablation System for Pulmonary Vein Isolation: The Prospective ICE-AGE-1 Study. Europace 2023, 25, euad248. [Google Scholar] [CrossRef] [PubMed]
- Moser, F.; Rottner, L.; Moser, J.; Schleberger, R.; Lemoine, M.; Münkler, P.; Dinshaw, L.; Kirchhof, P.; Reissmann, B.; Ouyang, F.; et al. The Established and the Challenger: A Direct Comparison of Current Cryoballoon Technologies for Pulmonary Vein Isolation. J. Cardiovasc. Electrophysiol. 2022, 33, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, K.A.; Mittal, S.; Varma, N.; Aryana, A.; Marrouche, N.; Anić, A.; Nair, D.; Champagne, J.; Iacopino, S.; De Asmundis, C.; et al. One-year Outcomes of Pulmonary Vein Isolation with a Novel Cryoballoon: Primary Results of the FROZEN AF Trial. J. Cardiovasc. Electrophysiol. 2024, 35, 832–842. [Google Scholar] [CrossRef]
- Földesi, C.; Misiková, S.; Ptaszyński, P.; Todd, D.; Herzet, J.; Braegelmann, K.M.; Kueffer, F.J.; Drephal, C.; Steinwender, C.; Zucchelli, G.; et al. Safety of Cryoballoon Ablation for the Treatment of Atrial Fibrillation: First European Results from the Cryo AF Global Registry. Pacing Clin. Electrophysiol. 2021, 44, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Heeger, C.-H.; Pott, A.; Sohns, C.; Riesinger, L.; Sommer, P.; Gasperetti, A.; Tondo, C.; Fassini, G.; Moser, F.; Lucas, P.; et al. Novel Cryoballoon Ablation System for Pulmonary Vein Isolation: Multicenter Assessment of Efficacy and Safety—ANTARCTICA Study. EP Eur. 2022, 24, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Reddy, V.Y.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Multi-National Survey on the Methods, Efficacy, and Safety on the Post-Approval Clinical Use of Pulsed Field Ablation (MANIFEST-PF). EP Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Heeger, C.-H.; Sohns, C.; Pott, A.; Metzner, A.; Inaba, O.; Straube, F.; Kuniss, M.; Aryana, A.; Miyazaki, S.; Cay, S.; et al. Phrenic Nerve Injury During Cryoballoon-Based Pulmonary Vein Isolation: Results of the Worldwide YETI Registry. Circ. Arrhythm. Electrophysiol. 2022, 15, e010516. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Poudyal, A.; Pulipati, P.; Larsen, T.; Krishnan, K.; Trohman, R.G.; Sharma, P.S.; Huang, H.D. A Systematic Review and Meta-analysis Comparing Second-generation Cryoballoon and Contact Force Radiofrequency Ablation for Initial Ablation of Paroxysmal and Persistent Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Straube, F.; Wegscheider, K.; Kuniss, M.; Andresen, D.; Wu, L.-Q.; Tebbenjohanns, J.; Noelker, G.; Tilz, R.R.; Chun, J.K.R.; et al. Outcomes of Cryoballoon or Radiofrequency Ablation in Symptomatic Paroxysmal or Persistent Atrial Fibrillation. EP Eur. 2019, 21, 1313–1324. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Total (n = 50) |
|---|---|
| Age [years], median (IQR) | 72 (65; 79) |
| Female, n (%) | 24 (48.0%) |
| BMI [kg/m2], median (IQR) | 26.7 (23.9; 30.8) |
| Paroxysmal AF, n (%) | 35 (70.0%) |
| CHA2DS2-VASc score, median (IQR) | 4 (3; 5) |
| LA diameter [mm], median (IQR) | 45 (42; 50) |
| LAVI [mL/m2], median (IQR) | 42 (35; 58) |
| LVEF (%), median (IQR) | 59 (46; 65) |
| Hypertension (n, %) | 37 (74.0%) |
| Diabetes mellitus (n, %) | 5 (10.0%) |
| Previous stroke/TIA (n, %) | 6 (12.0%) |
| Coronary artery disease (n, %) | 25 (50.0%) |
| Treated PVs (n = 196) | |
| Single-shot-isolation, n (%) | 171 (87.2%) |
| Freezes per patient (including bonus freezes), median (IQR) | 6 (6; 8) |
| Freezes per patient (without bonus freezes), median (IQR) | 4 (4; 6) |
| LSPV (n = 45) | |
| Single-shot-isolation, n (%) | 41 (91.1%) |
| TTI [s], median (IQR) | 43 (34; 60) |
| Freezes per vein, median (IQR) | 1 (1; 2) |
| LIPV (n = 45) | |
| Single-shot-isolation, n (%) | 40 (88.9%) |
| TTI [s], median (IQR) | 37 (27; 50) |
| Freezes per vein, median (IQR) | 2 (1; 2) |
| RIPV (n = 50) | |
| Single-shot-isolation, n (%) | 43 (86.0%) |
| TTI [s], median (IQR) | 54 (33; 69) |
| Freezes per vein, median (IQR) | 2 (1; 2) |
| RSPV (n = 50) | |
| Single-shot-isolation, n (%) | 43 (86.0%) |
| TTI [s], median (IQR) | 36 (30; 64) |
| Freezes per vein, median (IQR) | 2 (1; 2) |
| LCPV (n = 5) | |
| Single-shot-isolation, n (%) | 3 (60.0%) |
| TTI [s], median (IQR) | 30.0 (- 1) |
| Freezes per vein, median (IQR) | 3 (2; 4) |
| RMPV (n = 1) | |
| Single-shot-isolation, n (%) | 1 (100.0%) |
| TTI [s] | 20 |
| Freezes per vein | 1 |
| Small Balloon Only n (%) | Large Balloon Only n (%) | Mixed Balloon Size n (%) | |
|---|---|---|---|
| treated PVs (n = 196) | 157 (80.1%) | 27 (13.8%) | 12 (6.1%) |
| LSPV (n = 45) | 37 (82.2%) | 5 (11.1%) | 3 (6.7%) |
| LIPV (n = 45) | 38 (84.4%) | 3 (6.7%) | 4 (8.9%) |
| RIPV (n = 50) | 39 (78.0%) | 8 (16.0%) | 3 (6.0%) |
| RSPV (n = 50) | 42 (84.0%) | 6 (12.0%) | 2 (4.0%) |
| LCPV (n = 5) | 0 (0%) | 5 (100.0%) | 0 (0%) |
| RMPV (n = 1) | 1 (100.0%) | 0 (0%) | 0 (0%) |
| Small to Large Configuration | Large to Small Configuration | ||
|---|---|---|---|
| Not Isolated PV | Esophagus Temperature Drop | Not Isolated PV | |
| PVs overall, n = 12 (6.1%) | 5 | 1 | 6 |
| LSPV, n = 3 (1.5%) | 1 | 0 | 2 |
| LIPV, n = 4 (2.0%) | 2 | 1 | 1 |
| RIPV, n = 3 (1.5%) | 2 | 0 | 1 |
| RSPV, n = 2 (1.0%) | 0 | 0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teumer, Y.; Hilgarth, F.; Katov, L.; Melnic, R.; Rottbauer, W.; Bothner, C.; Weinmann, K. Pulmonary Vein Isolation with a Novel Size-Adjustable Cryo-Balloon Catheter: A Tailored Ablation Protocol. J. Clin. Med. 2024, 13, 2262. https://doi.org/10.3390/jcm13082262
Teumer Y, Hilgarth F, Katov L, Melnic R, Rottbauer W, Bothner C, Weinmann K. Pulmonary Vein Isolation with a Novel Size-Adjustable Cryo-Balloon Catheter: A Tailored Ablation Protocol. Journal of Clinical Medicine. 2024; 13(8):2262. https://doi.org/10.3390/jcm13082262
Chicago/Turabian StyleTeumer, Yannick, Franziska Hilgarth, Lyuboslav Katov, Rima Melnic, Wolfgang Rottbauer, Carlo Bothner, and Karolina Weinmann. 2024. "Pulmonary Vein Isolation with a Novel Size-Adjustable Cryo-Balloon Catheter: A Tailored Ablation Protocol" Journal of Clinical Medicine 13, no. 8: 2262. https://doi.org/10.3390/jcm13082262
APA StyleTeumer, Y., Hilgarth, F., Katov, L., Melnic, R., Rottbauer, W., Bothner, C., & Weinmann, K. (2024). Pulmonary Vein Isolation with a Novel Size-Adjustable Cryo-Balloon Catheter: A Tailored Ablation Protocol. Journal of Clinical Medicine, 13(8), 2262. https://doi.org/10.3390/jcm13082262