Hospital Incidence and Treatment Outcomes of Patients with Aneurysms and Dissections of the Iliac Artery in Switzerland—A Secondary Analysis of Swiss DRG Statistics Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analysis
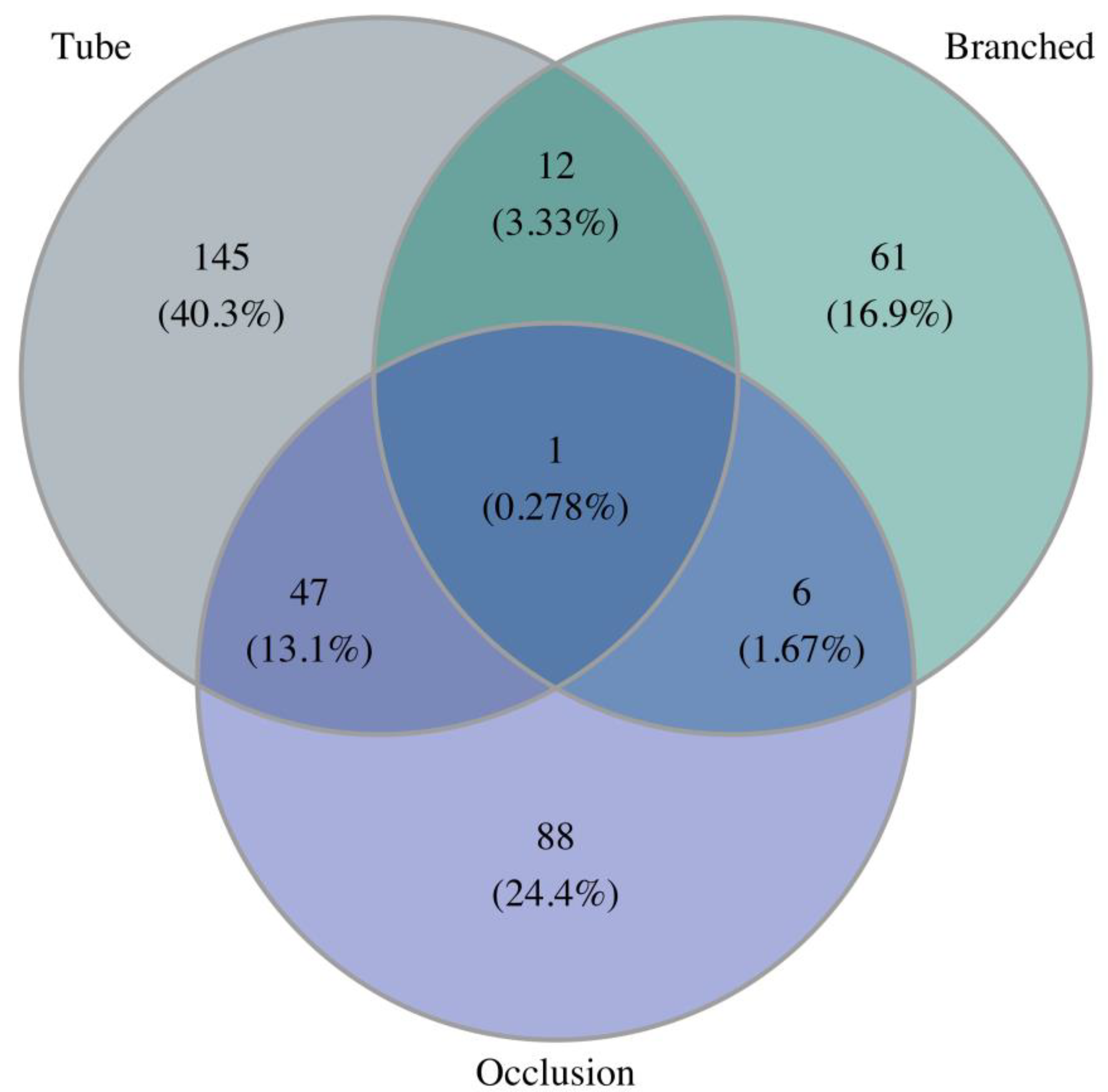
3. Results
3.1. Epidemiology
3.2. Treatment Modality
3.3. Treatment Outcomes—Elective
3.4. Treatment Outcomes—Emergency
3.5. Hospital Mortality
4. Discussion
4.1. Epidemiology
4.2. Treatment Outcomes
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice--European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef]
- Brunkwall, J.; Hauksson, H.; Bengtsson, H.; Bergqvist, D.; Takolander, R.; Bergentz, S.-E. Solitary Aneurysms of the Iliac Arterial System: An Estimate of Their Frequency of Occurrence. J. Vasc. Surg. 1989, 10, a13733. [Google Scholar] [CrossRef]
- Reber, P.U.; Brunner, K.; Hakki, H.; Stirnemann, P.; Kniemeyer, H.W. Häufigkeit, Klassifikation Und Therapie Der Isolierten Beckenarterienaneurysmen. Der Chir. 2001, 72, 419–424. [Google Scholar] [CrossRef]
- Perini, P.; Mariani, E.; Fanelli, M.; Ucci, A.; Rossi, G.; Massoni, C.B.; Freyrie, A. Surgical and Endovascular Management of Isolated Internal Iliac Artery Aneurysms: A Systematic Review and Meta-Analysis. Vasc. Endovasc. Surg. 2021, 55, 254–264. [Google Scholar] [CrossRef]
- Sandhu, R.S.; Pipinos, I.I. Isolated Iliac Artery Aneurysms. Semin. Vasc. Surg. 2005, 18, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Steenberge, S.P.; Caputo, F.J.; Rowse, J.W.; Lyden, S.P.; Quatromoni, J.G.; Kirksey, L.; Smolock, C.J. Natural History and Growth Rates of Isolated Common Iliac Artery Aneurysms. J. Vasc. Surg. 2022, 76, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Charisis, N.; Bouris, V.; Rakic, A.; Landau, D.; Labropoulos, N. A Systematic Review on Endovascular Repair of Isolated Common Iliac Artery Aneurysms and Suggestions Regarding Diameter Thresholds for Intervention. J. Vasc. Surg. 2021, 74, 1752–1762.e1. [Google Scholar] [CrossRef] [PubMed]
- Honjo, O.; Yamada, Y.; Kuroko, Y.; Kushida, Y.; Une, D.; Hioki, K. Spontaneous Dissection and Rupture of Common Iliac Artery in a Patient with Fibromuscular Dysplasia: A Case Report and Review of the Literature on Iliac Artery Dissections Secondary to Fibromuscular Dysplasia. J. Vasc. Surg. 2004, 40, 1032–1036. [Google Scholar] [CrossRef]
- Hayman, E.; Abayazeed, A.; Moghadamfalahi, M.; Cain, D. Vascular Type Ehlers-Danlos Syndrome with Fatal Spontaneous Rupture of a Right Common Iliac Artery Dissection: Case Report and Review of Literature. J. Radiol. Case Rep. 2014, 8, 63. [Google Scholar] [CrossRef]
- Sakaue, Y.; Nomura, T.; Ono, K.; Wada, N.; Keira, N.; Tatsumi, T. Enlarged False Lumen Following Spontaneous External Iliac Artery Dissection-Induced Chronic Limb Threatening Ischemia. Cardiovasc. Interv. Ther. 2022, 37, 583–584. [Google Scholar] [CrossRef]
- Chaer, R.A.; Barbato, J.E.; Lin, S.C.; Zenati, M.; Kent, K.C.; McKinsey, J.F. Isolated Iliac Artery Aneurysms: A Contemporary Comparison of Endovascular and Open Repair. J. Vasc. Surg. 2008, 47, 708–713.e1. [Google Scholar] [CrossRef] [PubMed]
- Statistik Der Stationären Betriebe Des. Gesundheitswesens. Krankenhaustypologie. [Internet]. 2013. Volume 9 p. 2006. Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/erhebungen/ks.assetdetail.23546402.html (accessed on 11 April 2024).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A Modification of the Elixhauser Comorbidity Measures Into a Point System for Hospital Death Using Administrative Data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, A. Comorbidity: An R Package for Computing Comorbidity Scores. J. Open Source Softw. 2018, 3, 648. [Google Scholar] [CrossRef]
- Meuli, L.; Menges, A.-L.; Steigmiller, K.; Kuehnl, A.; Reutersberg, B.; Held, U.; Zimmermann, A. Hospital Incidence and Mortality of Patients Treated for Abdominal Aortic Aneurysms in Switzerland—A Secondary Analysis of Swiss DRG Statistics Data. Swiss Med. Wkly. 2022, 152, w30191. [Google Scholar] [CrossRef] [PubMed]
- Demografische Bilanz nach Alter. Bundesamt für Statistik [Internet]. Available online: https://www.pxweb.bfs.admin.ch/pxweb/de/px-x-0102020000_103/-/px-x-0102020000_103.px/ (accessed on 11 April 2024).
- Altman, D.; Machin, D.; Bryant, T.; Gardner, M. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: http://www.R-Project.Org/ (accessed on 11 April 2024).
- Meuli, L.; Menges, A.L.; Stoklasa, K.; Steigmiller, K.; Reutersberg, B.; Zimmermann, A. Inter-Hospital Transfer of Patients With Ruptured Abdominal Aortic Aneurysm in Switzerland. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 484–492. [Google Scholar] [CrossRef]
- Nachbur, B.H.; Inderbitzi, R.G.C.; Bär, W. Isolated Iliac Aneurysms. Eur. J. Vasc. Surg. 1991, 5, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gouveia e Melo, R.; Fenelli, C.; Fernández Prendes, C.; Öz, T.; Stavroulakis, K.; Rantner, B.; Stana, J.; Tsilimparis, N. A Cross-Sectional Study on the Anatomic Feasibility of Iliac Side Branch Grafts in a Real-World Setting. J. Vasc. Surg. 2022, 76, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Oussoren, F.K.; Maldonado, T.S.; Reijnen, M.M.P.J.; Heyligers, J.M.M.; Akkersdijk, G.; Attisani, L.; Bellosta, R.; Heyligers, J.M.M.; Hoencamp, R.; Garrard, L.; et al. Solitary Iliac Branch Endoprosthesis Placement for Iliac Artery Aneurysms. J. Vasc. Surg. 2022, 75, 1268–1275.e1. [Google Scholar] [CrossRef]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Di Eusanio, M.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights From the International Registry of Acute Aortic Dissection. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef]
- Kritpracha, B.; Pigott, J.P.; Price, C.I.; Russell, T.E.; Corbey, M.J.; Beebe, H.G. Distal Internal Iliac Artery Embolization: A Procedure to Avoid. J. Vasc. Surg. 2003, 37, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ko, R.-E.; Yoo, K.; Gil, E.; Choi, K.-J.; Park, C.-M. Non-Occlusive Mesenteric Ischemia in Critically Ill Patients. PLoS ONE 2022, 17, e0279196. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, K.; Beck-Esmay, J.; Koyfman, A.; Long, B. High Risk and Low Prevalence Diseases: Mesenteric Ischemia. Am. J. Emerg. Med. 2023, 65, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhorzel, S.; Busch, A.; Trenner, M.; Reutersberg, B.; Salvermoser, M.; Eckstein, H.-H.; Zimmermann, A. Open Versus Endovascular Repair of Isolated Iliac Artery Aneurysms. Vasc. Endovasc. Surg. 2019, 53, 12–20. [Google Scholar] [CrossRef]
- Trenner, M.; Eckstein, H.-H.; Kallmayer, M.A.; Reutersberg, B.; Kühnl, A. Secondary Analysis of Statutorily Collected Routine Data. Gefässchirurgie 2019, 24, 220–227. [Google Scholar] [CrossRef]




| Variable | Endovascular Repair (n = 492) | Open Repair (n = 295) | Total (n = 787) | p Value |
|---|---|---|---|---|
| Male sex | 455 (92) | 269 (91) | 724 (92) | 0.518 |
| Age, years | 75 (68, 80) | 69 (63, 75) | 73 (66, 79) | <0.001 |
| van Walraven score | 2 (0, 6) | 3 (0.5, 10) | 2 (0, 8) | <0.001 |
| Coronary artery disease | 114 (23) | 78 (26) | 192 (24) | 0.301 |
| Chronic heart failure | 19 (3.9) | 15 (5.1) | 34 (4.3) | 0.414 |
| Cerebrovascular disease | 30 (6.1) | 18 (6.1) | 48 (6.1) | 0.998 |
| Arterial hypertension | 246 (50) | 144 (49) | 390 (50) | 0.747 |
| Chronic pulmonary disease | 41 (8.3) | 29 (9.8) | 70 (8.9) | 0.475 |
| Diabetes mellitus | 61 (12) | 32 (11) | 93 (12) | 0.514 |
| Chronic kidney disease | 85 (17) | 50 (17) | 135 (17) | 0.906 |
| Cancer | 7 (1.4) | 7 (2.4) | 14 (1.8) | 0.329 |
| Obesity | 16 (3.3) | 8 (2.7) | 24 (3.0) | 0.670 |
| Type of hospital | 0.045 | |||
| University hospital (Level 1) | 181 (37) | 135 (45.8) | 316 (40) | |
| Major hospital (Level 2) | 267 (54) | 138 (46.8) | 405 (51) | |
| Other (Level 3 to 5) | 44 (8.9) | 22 (7.5) | 66 (8.4) | |
| Location before admission | 0.182 | |||
| Home | 455 (92) | 272 (92) | 727 (92) | |
| Acute care hospital | 34 (6.9) | 19 (6.4) | 53 (6.7) | |
| Nursing home | 2 (0.4) | 0 (0.0) | 2 (0.3) | |
| Other | 1 (0.2) | 4 (1.4) | 5 (0.6) | |
| Treatment period | <0.001 | |||
| 2011–2014 | 115 (29.3) | 162 (54.9) | 277 (40.3) | |
| 2015–2018 | 278 (70.7) | 133 (45.1) | 411 (59.7) |
| Variable | Endovascular Repair (n = 91) | Open Repair (n = 73) | Total (n = 164) | p Value |
|---|---|---|---|---|
| Male sex | 74 (81) | 66 (90) | 140 (85) | 0.102 |
| Age, years | 74 (65, 81) | 73 (60, 78) | 73 (62, 80) | 0.154 |
| van Walraven score | 10 (2, 19) | 10 (3, 17) | 10 (3, 18) | 0.984 |
| Coronary artery disease | 22 (24) | 17 (23) | 39 (24) | 0.894 |
| Chronic heart failure | 10 (11) | 6 (8.2) | 16 (9.8) | 0.552 |
| Cerebrovascular disease | 5 (5.5) | 1 (1.4) | 6 (3.7) | 0.227 |
| Arterial hypertension | 35 (38) | 27 (37) | 62 (38) | 0.846 |
| Chronic pulmonary disease | 14 (15) | 12 (16) | 26 (16) | 0.854 |
| Diabetes mellitus | 7 (7.7) | 9 (12) | 16 (9.8) | 0.320 |
| Chronic kidney disease | 26 (29) | 20 (27) | 46 (28) | 0.868 |
| Cancer | 3 (3.3) | 0 (0) | 3 (1.8) | 0.254 |
| Obesity | 1 (1.1) | 1 (1.4) | 2 (1.2) | 0.999 |
| Type of hospital | 0.668 | |||
| University hospital (Level 1) | 42 (46) | 28 (38) | 70 (43) | |
| Major hospital (Level 2) | 45 (49) | 41 (56) | 86 (52) | |
| Other (Level 3 to 5) | 4 (4.4) | 4 (5.5) | 8 (4.9) | |
| Location before admission | 0.196 | |||
| Home | 69 (76) | 59 (81) | 128 (78) | |
| Acute care hospital | 16 (18) | 14 (19) | 30 (18) | |
| Nursing home | 2 (2.2) | 0 (0) | 2 (1.2) | |
| Other | 4 (4.4) | 0 (0) | 4 (2.4) | |
| Treatment period | 0.487 | |||
| 2011–2014 | 35 (38) | 32 (44) | 67 (41) | |
| 2015–2018 | 56 (62) | 41 (56) | 97 (59) |
| Variable | Endovascular Repair (n = 492) | Open Repair (n = 295) | Total (n = 787) | p Value |
|---|---|---|---|---|
| Length of stay ICU, hours | 0 (0, 0) | 16 (0, 26) | 0 (0, 19) | <0.001 |
| Length of hospital stay, days | 4 (3, 6) | 9 (7, 14) | 6 (3, 10) | <0.001 |
| Packed red blood cells | <0.001 | |||
| 0 | 430 (87) | 201 (68.1) | 631 (80) | |
| 1–5 | 46 (9.3) | 66 (22.4) | 112 (14) | |
| >5 | 16 (3.3) | 28 (9.5) | 44 (5.6) | |
| Fresh frozen plasma | 0.010 | |||
| 0 | 487 (99) | 283 (95.9) | 770 (98) | |
| 1–5 | 4 (0.8) | 10 (3.4) | 14 (1.8) | |
| >5 | 1 (0.2) | 2 (0.7) | 3 (0.4) | |
| Platelet transfusion | 0.609 | |||
| 0 | 491 (100) | 294 (99.7) | 785 (100) | |
| 1–5 | 1 (0.2) | 0 (0.0) | 1 (0.1) | |
| >5 | 0 (0) | 1 (0.3) | 1 (0.1) | |
| Myocardial infarction | 2 (0.4) | 1 (0.3) | 3 (0.4) | 0.999 |
| Acute mesenteric ischemia | 3 (0.6) | 8 (2.7) | 11 (1.4) | 0.024 |
| Large intestine resection | 1 (0.2) | 7 (2.4) | 8 (1.0) | 0.005 |
| Small intestine resection | 2 (0.4) | 3 (1.0) | 5 (0.6) | 0.369 |
| Acute lower limb ischemia | 32 (6.5) | 42 (14.2) | 74 (9.4) | <0.001 |
| Crural fasciotomy | 1 (0.2) | 4 (1.4) | 5 (0.6) | 0.068 |
| Major amputation | 0 (0) | 0 (0.0) | 0 (0) | NA |
| Destination after discharge | <0.001 | |||
| Home | 443 (90) | 240 (81.4) | 683 (87) | |
| Rehabilitation | 21 (4.3) | 37 (12.5) | 58 (7.4) | |
| Acute care hospital | 14 (2.8) | 8 (2.7) | 22 (2.8) | |
| Nursing home | 8 (1.6) | 0 (0.0) | 8 (1.0) | |
| Other | 2 (0.4) | 1 (0.3) | 3 (0.4) | |
| Hospital mortality | 4 (0.8) | 9 (3.1) | 13 (1.7) | 0.022 |
| Variable | Endovascular Repair (n = 91) | Open Repair (n = 73) | Total (n = 164) | p Value |
|---|---|---|---|---|
| Length of stay ICU, hours | 0 (0, 43) | 46 (15, 109) | 23 (0, 83) | <0.001 |
| Length of hospital stay, days | 11 (6, 19) | 16 (10, 28) | 13 (7, 21) | 0.003 |
| Packed red blood cells | 0.171 | |||
| 0 | 43 (47) | 26 (36) | 69 (42) | |
| 1–5 | 30 (33) | 24 (33) | 54 (33) | |
| >5 | 18 (20) | 23 (32) | 41 (25) | |
| Fresh frozen plasma | 0.799 | |||
| 0 | 85 (93) | 67 (92) | 152 (93) | |
| 1–5 | 4 (4.4) | 5 (6.8) | 9 (5.5) | |
| >5 | 2 (2.2) | 1 (1.4) | 3 (1.8) | |
| Platelet transfusion | 0.254 | |||
| 0 | 88 (97) | 73 (100) | 161 (98) | |
| 1–5 | 3 (3.3) | 0 (0) | 3 (1.8) | |
| >5 | 0 (0) | 0 (0) | 0 (0) | |
| Myocardial infarction | 2 (2.2) | 6 (8.2) | 8 (4.9) | 0.141 |
| Acute mesenteric ischemia | 2 (2.2) | 9 (12) | 11 (6.7) | 0.012 |
| Large intestine resection | 2 (2.2) | 6 (8.2) | 8 (4.9) | 0.141 |
| Small intestine resection | 1 (1.1) | 4 (5.5) | 5 (3.0) | 0.173 |
| Acute lower limb ischemia | 9 (9.9) | 14 (19) | 23 (14) | 0.089 |
| Crural fasciotomy | 2 (2.2) | 4 (5.5) | 6 (3.7) | 0.408 |
| Major amputation | 2 (2.2) | 2 (2.7) | 4 (2.4) | >0.999 |
| Destination after discharge | 0.201 | |||
| Home | 54 (59) | 37 (51) | 91 (55) | |
| Rehabilitation | 13 (14) | 15 (21) | 28 (17) | |
| Acute care hospital | 11 (12) | 4 (5.5) | 15 (9.1) | |
| Nursing home | 3 (3.3) | 1 (1.4) | 4 (2.4) | |
| Other | 1 (1.1) | 2 (2.7) | 3 (1.8) | |
| Hospital mortality | 9 (9.9) | 14 (19) | 23 (14) | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozalka, R.; Menges, A.-L.; Zimmermann, A.; Meuli, L. Hospital Incidence and Treatment Outcomes of Patients with Aneurysms and Dissections of the Iliac Artery in Switzerland—A Secondary Analysis of Swiss DRG Statistics Data. J. Clin. Med. 2024, 13, 2267. https://doi.org/10.3390/jcm13082267
Bozalka R, Menges A-L, Zimmermann A, Meuli L. Hospital Incidence and Treatment Outcomes of Patients with Aneurysms and Dissections of the Iliac Artery in Switzerland—A Secondary Analysis of Swiss DRG Statistics Data. Journal of Clinical Medicine. 2024; 13(8):2267. https://doi.org/10.3390/jcm13082267
Chicago/Turabian StyleBozalka, Roland, Anna-Leonie Menges, Alexander Zimmermann, and Lorenz Meuli. 2024. "Hospital Incidence and Treatment Outcomes of Patients with Aneurysms and Dissections of the Iliac Artery in Switzerland—A Secondary Analysis of Swiss DRG Statistics Data" Journal of Clinical Medicine 13, no. 8: 2267. https://doi.org/10.3390/jcm13082267





