Risk Factors and Modifiers for Cardiovascular Disease Assessment of Patients with Heterozygous Familial Hypercholesterolaemia
Abstract
1. Introduction
- Determine the clinical characteristics of a cohort of patients attending a Lipid Clinic over a six-month period.
- Identify HeFH patients with either a clinical diagnosis or genetically confirmed diagnosis.
- Profile the prevalence and both conventional CVD risk factors and risk modifiers as per current ESC guidance in the cohort with comparative HeFH subgroup analysis.
2. Patients and Methods
2.1. Setting and Study Design
2.2. Consent Process and Anonymisation
2.3. Data Collection and Extraction
- Patient’s height, weight and body mass index (BMI) as assessed by nursing staff at each clinic visit and recorded into patient charts. Exceptions included, but were not limited to, telephone consultations and/or the patient’s refusal or inability to ambulate (wheelchair users).
- Family history of CVD and age of CVD to ascertain whether premature CVD was present.
- Physical examination findings including weight, height, BMI, and lipid deposition (xanthoma, xanthelasma and corneal arcus) which was assessed by senior clinicians.
- Patient physical activity was noted as part of standard history taking in the clinic. Categories included the following: walking, running, cycling, gym, cardio, resistance training, mixed, team-based sports, none and other.
2.4. Definitions
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Cohort Characteristics
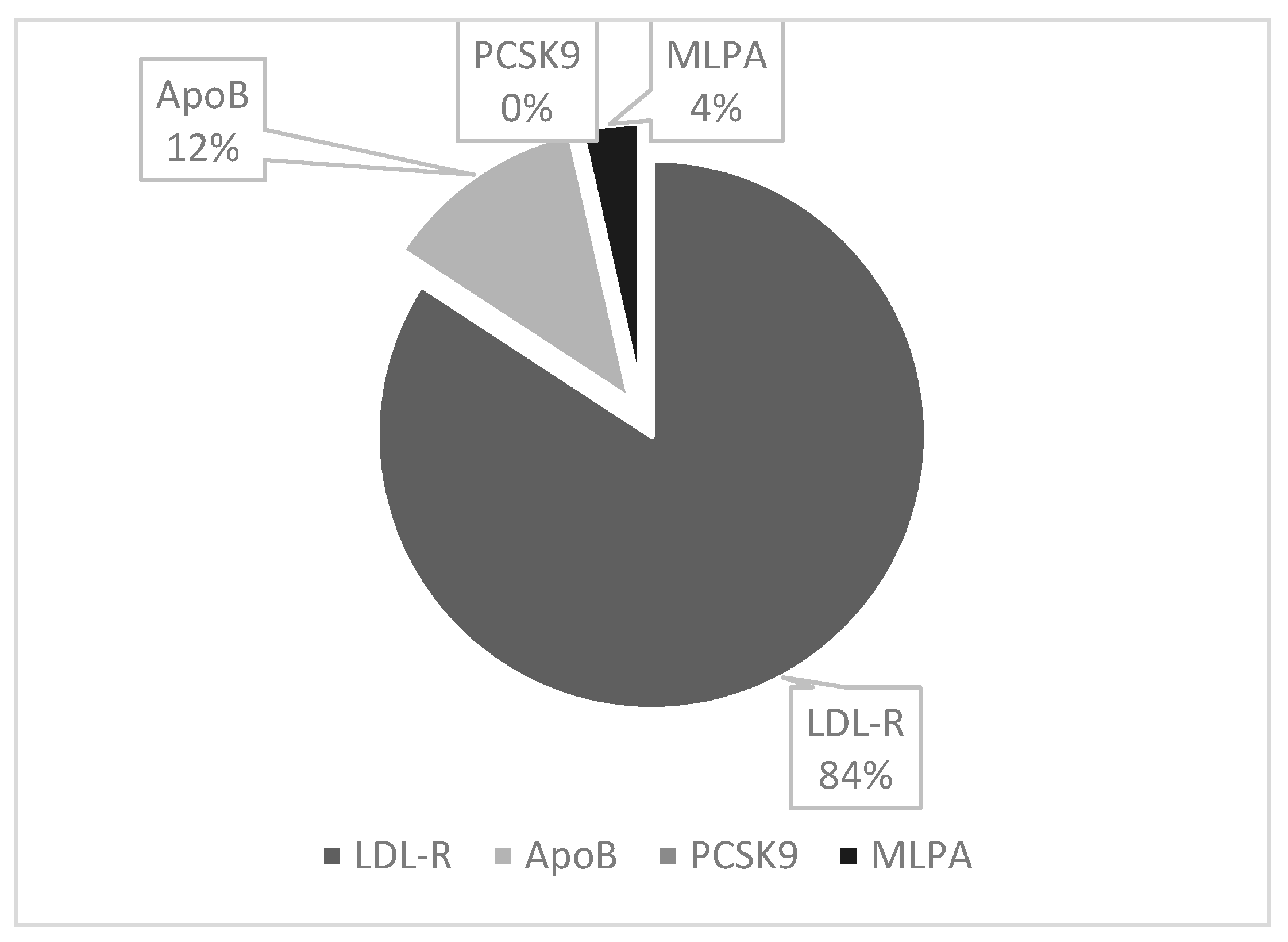
3.2. Heterozygous Familial Hypercholesterolemia Phenotype (FH-P) and Phenotype and Genotype (FH-PG)
3.3. HeFH Subgroup Risk Assessment
3.4. Physical Examination Findings
4. Discussion
HeFH CV Risk Assessment: Current Limitations and Future Opportunities
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Talmud, P.J.; Shah, S.; Whittall, R.; Futema, M.; Howard, P.; A Cooper, J.; Harrison, S.C.; Li, K.; Drenos, F.; Karpe, F.; et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: A case-control study. Lancet 2013, 381, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; O’kane, M.; McGilligan, V.; Watterson, S. The genetics and screening of familial hypercholesterolaemia. J. Biomed. Sci. 2016, 23, 39. [Google Scholar] [CrossRef]
- SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [CrossRef] [PubMed]
- Brunham, L.R.; Ruel, I.; Aljenedil, S.; Rivière, J.-B.; Baass, A.; Tu, J.V.; Mancini, G.J.; Raggi, P.; Gupta, M.; Couture, P.; et al. Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: Update 2018. Can. J. Cardiol. 2018, 34, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Yoon, M.; Kang, H.-J.; Kim, B.J.; Choi, S.H.; Jeong, I.-K.; Lee, S.-H. 2022 Consensus statement on the management of familial hypercholesterolemia in Korea. Korean J. Intern. Med. Korean Assoc. Intern. Med. 2022, 37, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Akioyamen, L.E.; Genest, J.; Chu, A.; Inibhunu, H.; Ko, D.T.; Tu, J.V. Risk factors for cardiovascular disease in heteterolemia: A systematic review and meta-analysis. J. Clin. Lipidol. 2019, 13, 15–30. Available online: https://www.sciencedirect.com/science/article/pii/S193328741830432X (accessed on 10 October 2023). [CrossRef]
- Sijbrands, E.J.G.; Kaprio, J.; Westendorp, R.G.J.; Defesche, J.C.; de Meier, P.H.E.M.; Smelt, A.H.M.; Kastelein, J.J. Mortality over two centuries in large pedigree with familial hypercholesterolaemia: Family tree mortality studyCommentary: Role of other genes and environment should not be overlooked in monogenic disease. BMJ 2001, 322, 1019–1023. Available online: https://www.bmj.com/content/322/7293/1019 (accessed on 17 October 2023). [CrossRef]
- Santos, R.D. Phenotype vs. genotype in severe familial hypercholesterolemia: What matters most for the clinician? Curr. Opin. Lipidol. 2017, 28, 130–135. [Google Scholar] [CrossRef]
- Vuorio, A.; Watts, G.F.; Schneider, W.J.; Tsimikas, S.; Kovanen, P.T. Familial hypercholesterolemia and elevated lipoprotein(a): Double heritable risk and new therapeutic opportunities. J. Intern. Med. 2020, 287, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Banach, M.; Pirro, M. Why patients with familial hypercholesterolemia are at high cardiovascular risk? Beyond LDL-C levels. Trends Cardiovasc. Med. 2021, 31, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cardiff University. LDL-C Estimator. Available online: https://fhwalescriteria.co.uk/ldl_estimator (accessed on 21 August 2023).
- MDCalc. Dutch Criteria for Familial Hypercholesterolemia (FH). Available online: https://www.mdcalc.com/calc/3818/dutch-criteria-familial-hypercholesterolemia-fh (accessed on 21 August 2023).
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Galema-Boers, A.M.; Lenzen, M.J.; Engelkes, S.R.; Sijbrands, E.J.; van Lennep, J.E.R. Cardiovascular risk in patients with familial hypercholesterolemia using optimal lipid-lowering therapy. J. Clin. Lipidol. 2018, 12, 409–416. Available online: https://www.sciencedirect.com/science/article/pii/S1933287417305494 (accessed on 1 October 2023). [CrossRef] [PubMed]
- Sijbrands, E.J.; Westendorp, R.G.; Lombardi, M.P.; Havekes, L.M.; Frants, R.R.; Kastelein, J.J.; Smelt, A.H. Additional risk factors influence excess mortality in heterozygous familial hypercholesterolaemia. Atherosclerosis 2000, 149, 421–425. Available online: https://www.sciencedirect.com/science/article/pii/S0021915099003366 (accessed on 17 October 2023). [CrossRef] [PubMed]
- A Healthy Weight for Ireland 2016–2025: Obesity Policy and Action Plan. Available online: https://www.hse.ie/eng/about/who/cspd/ncps/obesity/a-healthy-weight-for-ireland-obesity-policy-and-action-plan.pdf (accessed on 9 October 2023).
- Chang, C.-K.; Hayes, R.D.; Perera, G.; Broadbent, M.T.M.; Fernandes, A.C.; Lee, W.E.; Hotopf, M.; Stewart, R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE 2011, 6, e19590. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Hancock, K.J.; Kisely, S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: Retrospective analysis of population based registers. BMJ Br. Med. J. 2013, 346, f2539. Available online: http://www.bmj.com/content/346/bmj.f2539.abstract (accessed on 5 October 2023). [CrossRef] [PubMed]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, M.S. Psychological issues and cognitive impairment in adults with familial hypercholesterolemia. Fam. Pract. 2017, 34, 520–524. [Google Scholar] [CrossRef]
- Arslan, A.; Şimşek, Ö.; Turhan, A.; Çarlioğlu, A.; Arıkan, Ş.; Utlu, M.; Kartal, E. Non-alcoholic fatty liver disease in patients with familial hypercholesterolemia. Ortadoğu Tıp Derg. 2020, 12, 219–224. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Urowitz, M.B.; Ibañez, D.; Nikpour, M.; Gladman, D.D. Risk factors for development of coronary artery disease in women with systemic lupus erythematosus. J. Rheumatol. 2009, 36, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Yu, Y.; Haynes, K.; Love, T.J.; Maliha, S.; Jiang, Y.; Troxel, A.B.; Hennessy, S.; E Kimmel, S.; Margolis, D.J.; et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A population-based cohort study. Ann. Rheum. Dis. 2015, 74, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Deshpande, A.R.; Pechman, D.M.; Tamariz, L.; Abreu, M.T.; A Sussman, D. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am. J. Gastroenterol. 2011, 106, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Escate, R.; Mata, P.; Cepeda, J.M.; Padró, T.; Badimon, L. miR-505-3p controls chemokine receptor up-regulation in macrophages: Role in familial hypercholesterolemia. FASEB J. 2018, 32, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Kruse, G.; Kutikova, L.; Wong, B.; Villa, G.; Ray, K.; Mata, P.; Bruckert, E. Cardiovascular Disease Risk And Risk Factors Associated with Familial Hypercholesterolemia: A Systematic Review. Value Health 2017, 20, A606. [Google Scholar] [CrossRef]
- Wong, B.; Kruse, G.; Kutikova, L.; Ray, K.K.; Mata, P.; Bruckert, E. Cardiovascular Disease Risk Associated with Familial Hypercholesterolemia: A Systematic Review of the Literature. Clin. Ther. 2016, 38, 1696–1709. Available online: https://www.sciencedirect.com/science/article/pii/S014929181630340X (accessed on 17 October 2023). [CrossRef]
- Wang, L.; Guo, J.; Tian, Z.; Seery, S.; Jin, Y.; Zhang, S. Developing a Hybrid Risk Assessment Tool for Familial Hypercholesterolemia: A Machine Learning Study of Chinese Arteriosclerotic Cardiovascular Disease Patients. Front. Cardiovasc. Med. 2022, 9, 893986. [Google Scholar] [CrossRef]



| Demographics | Gender | Age | Height | Weight |
|---|---|---|---|---|
| BMI | Referral Details | |||
| Comorbidities | Hypertension | Established CVD | T2DM | Obesity |
| NAFLD | Inflammatory Disease | HIV | Post HSCT | |
| CKD 3–5 | OSA | Major Psychiatric Disorder | ||
| Lifestyle Factors | Smoking Status | Psychosocial Stress | Physical Activity | |
| Lipid Lowering Therapy | Generic Name | Dose | Frequency | |
| Laboratory Investigations | Lipid Profiles | LFTs | TSH | FH Genetic Testing |
| HbA1c | CK | Renal Function | ||
| Imaging | Coronary Artery Calcium Score | Carotid Plaque on Ultrasound | ||
| Physical Examination Findings | Xanthoma | Xanthelasma | Corneal Arcus |
| Study Cohort | FH-P | p | FH-P/G | p | |
|---|---|---|---|---|---|
| Number of patients | 370 | 98 (26%) | - | 57 (15%) | - |
| Age | 49.43 ± 13.59 | 45.70 ± 13.13 | 0.001 | 40.93 ± 13.15 | 0.000 |
| Male | 168 (45%) | 38 (39%) | 0.124 | 22 (39%) | 0.262 |
| Female | 202 (55%) | 60 (61%) | 35 (61%) |
| Whole Cohort n = 370 | FH-P n = 98 | p | FH-PG n = 57 | p | |
|---|---|---|---|---|---|
| Smoking Status | |||||
| Never Smoker | 208 (56%) | 53 (54%) | 0.619 | 36 (63%) | 0.251 |
| Current Smoker | 57 (15%) | 17 (17%) | 0.535 | 8 (14%) | 0.755 |
| Ex-smoker | 100 (27%) | 27 (28%) | 0.892 | 12 (21%) | 0.269 |
| Lipid Profile | |||||
| Baseline TC | 7.95 ± 1.92 | 8.99 ± 2.02 | 0.000 | 9.49 ± 2.39 | 0.000 |
| Baseline LDL-C | 5.57 ± 1.83 | 6.69 ± 1.88 | 0.000 | 7.22 ± 2.15 | 0.000 |
| Baseline TG | 3.68 ± 4.96 | 2.02 ± 2.33 | 0.000 | 2.02 ± 3.02 | 0.001 |
| Lp (a) > 70 nmol/L | 127 (34%) | 33 (34%) | 0.874 | 16 (28%) | 0.280 |
| Lp (a) > 430 nmol/L | 18 (5%) | 3 (3%) | 0.333 | 2 (4%) | 0.605 |
| Co-morbidities | |||||
| Established CVD | 85 (23%) | 27 (28%) | 0.209 | 13 (23%) | 0.974 |
| Hypertension | 125 (34%) | 25 (26%) | 0.043 | 11 (19%) | 0.012 |
| Type 2 Diabetes Mellitus | 42 (11%) | 7 (7%) | 0.126 | 4 (7%) | 0.262 |
| Family History of Premature CVD | 145 (39%) | 55 (56%) | 0.000 | 35 (61%) | 0.000 |
| Co-morbidities | |||||
| Obesity | 121 (33%) | 27 (28%) | 0.205 | 15 (26%) | 0.264 |
| Non-Alcoholic Fatty Liver Disease | 43 (12%) | 9 (9%) | 0.380 | 6 (11%) | 0.779 |
| Atrial Fibrillation | 4 (1%) | 1 (1%) | 0.946 | - | 0.391 |
| Left Ventricular Hypertrophy | 3 (1%) | - | 0.297 | - | 0.458 |
| Inflammatory Disease | 35 (9%) | 7 (7%) | 0.361 | 4 (7%) | 0.493 |
| HIV | 3 (1%) | - | 0.297 | - | 0.458 |
| Post Haematopoietic Stem Cell Transplant | 8 (2%) | - | 0.086 | - | 0.222 |
| Chronic Kidney Disease Stage 3–5 | 9 (2%) | - | 0.068 | - | 0.195 |
| Obstructive Sleep Apnoea | 7 (2%) | 2 (2%) | 0.900 | 2 (4%) | 0.330 |
| Major Psychiatric Disorder | 55 (15%) | 12 (12%) | 0.395 | 7 (12%) | 0.551 |
| Lifestyle Factors | |||||
| Psychosocial Stress | 7 (2%) | 2 (2%) | 0.900 | - | 0.254 |
| Sedentary Lifestyle | 62 (17%) | 13 (13%) | 0.280 | 3 (5%) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malone, R.; Savage, S.; Crowley, V.; Hennessy, M.; O’Connor, P.; Kennedy, C. Risk Factors and Modifiers for Cardiovascular Disease Assessment of Patients with Heterozygous Familial Hypercholesterolaemia. J. Clin. Med. 2024, 13, 2270. https://doi.org/10.3390/jcm13082270
Malone R, Savage S, Crowley V, Hennessy M, O’Connor P, Kennedy C. Risk Factors and Modifiers for Cardiovascular Disease Assessment of Patients with Heterozygous Familial Hypercholesterolaemia. Journal of Clinical Medicine. 2024; 13(8):2270. https://doi.org/10.3390/jcm13082270
Chicago/Turabian StyleMalone, Richard, Sarah Savage, Vivion Crowley, Martina Hennessy, Patricia O’Connor, and Cormac Kennedy. 2024. "Risk Factors and Modifiers for Cardiovascular Disease Assessment of Patients with Heterozygous Familial Hypercholesterolaemia" Journal of Clinical Medicine 13, no. 8: 2270. https://doi.org/10.3390/jcm13082270
APA StyleMalone, R., Savage, S., Crowley, V., Hennessy, M., O’Connor, P., & Kennedy, C. (2024). Risk Factors and Modifiers for Cardiovascular Disease Assessment of Patients with Heterozygous Familial Hypercholesterolaemia. Journal of Clinical Medicine, 13(8), 2270. https://doi.org/10.3390/jcm13082270







