Detraining among Athletes—Is Withdrawal of Adaptive Cardiovascular Changes a Hint for the Differential Diagnosis of Physically Active People?
Abstract
:1. Introduction
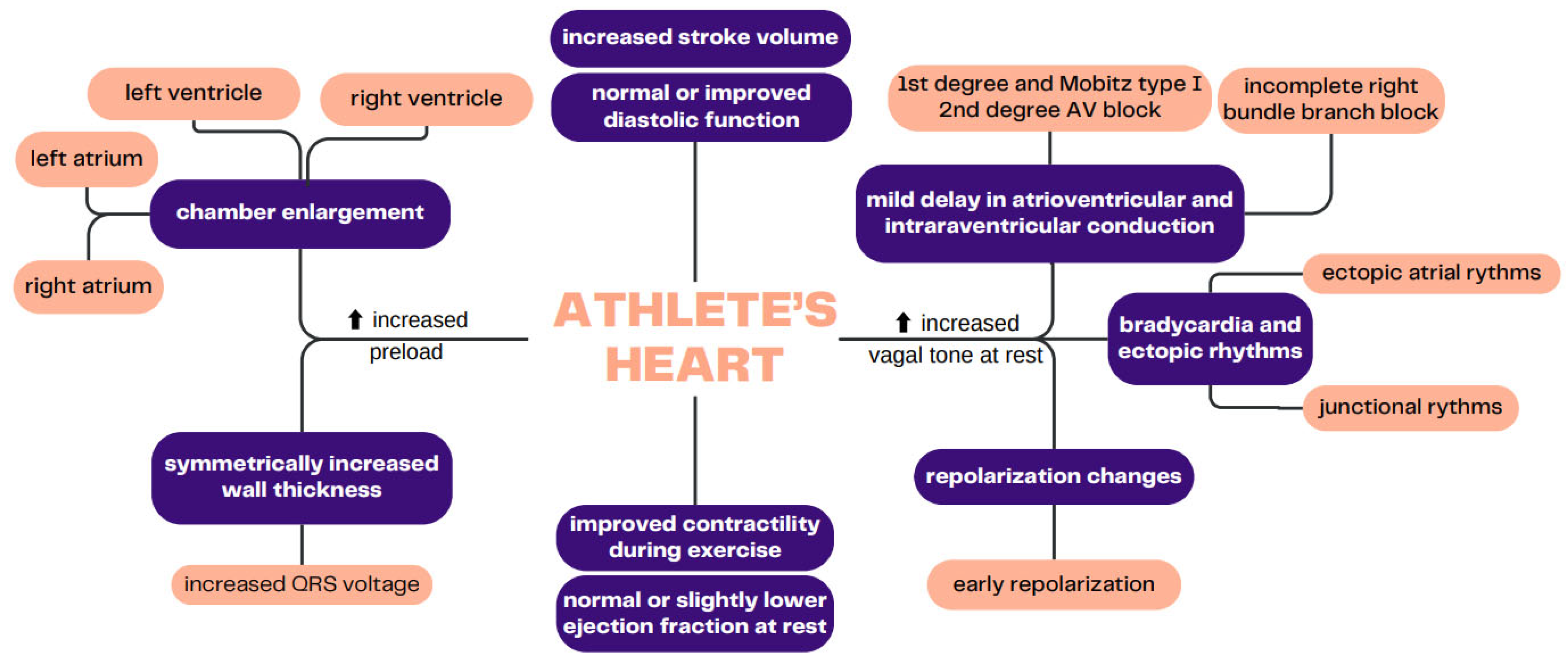
2. The Prolonged Impact of Endurance Training on the CV System
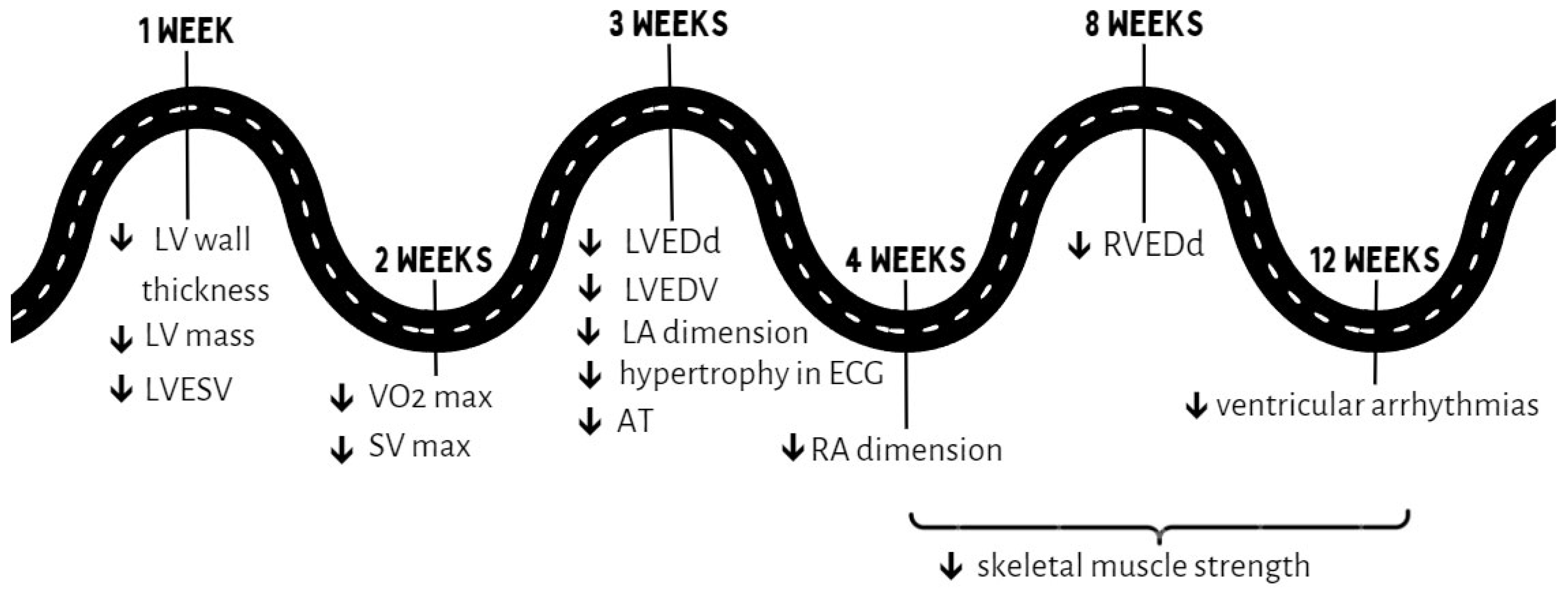
3. Effects of Detraining in Endurance Sports
3.1. Cardiac Morphology
3.2. Electrocardiographic Parameters
3.3. Functional Parameters
4. Effects of Detraining in Resistance Sports
5. Cardiovascular Diseases
6. Summary
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; ESC Scientific Document Group; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Boraita, A.; Sánchez-Testal, M.V.; Diaz-Gonzalez, L.; Heras, M.E.; Alcocer-Ayuga, M.; de la Rosa, A.; Rabadán, M.; Abdul-Jalbar, B.; Pérez de Isla, L.; Santos-Lozano, A.; et al. Apparent ventricular dysfunction in elite young athletes: Another form of cardiac adaptation of the athlete’s heart. J. Am. Soc. Echocardiogr. 2019, 32, 987–996. [Google Scholar] [CrossRef]
- Encarnação, I.G.A.; Viana, R.B.; Soares, S.R.S.; Freitas, E.D.S.; de Lira, C.A.B.; Ferreira-Junior, J.B. Effects of detraining on muscle strength and hypertrophy induced by resistance training: A systematic review. Muscles 2022, 1, 1–15. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; De Luca, R.; Di Paolo, F.M.; Spataro, A.; Culasso, F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.H., 3rd; Coyle, E.F.; Bloomfield, S.A.; Ehsani, A.A. Effects of physical deconditioning after intense endurance training on left ventricular dimensions and stroke volume. J. Am. Coll. Cardiol. 1986, 7, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.A.; Hagberg, J.M.; Hickson, R.C. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am. J. Cardiol. 1978, 42, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Petretta, M.; Cavallaro, V.; Bianchi, V.; La Mura, G.; Conforti, G.; Breglio, R.; Valva, G.; Morgano, G.; Bonaduce, D. Modificazionicardiacheindotte dal decondizionamentonell’atleta: Studio ecocardiografico ed elettrocardiografico [Cardiac changes induced by deconditioning in athletes: An echocardiographic and electrocardiographic study]. G. Ital. Cardiol. 1991, 21, 1167–1177. (In Italian) [Google Scholar]
- Maron, B.J.; Pelliccia, A.; Spataro, A.; Granata, M. Reduction in left ventricular wall thickness after deconditioning in highly trained Olympic athletes. Br. Heart J. 1993, 69, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Pedlar, C.R.; Brown, M.G.; Shave, R.E.; Otto, J.M.; Drane, A.; Michaud-Finch, J.; Contursi, M.; Wasfy, M.M.; Hutter, A.; Picard, M.H.; et al. Cardiovascular response to prescribed detraining among recreational athletes. J. Appl. Physiol. 2018, 124, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Swoboda, P.P.; Garg, P.; Levelt, E.; Broadbent, D.A.; Zolfaghari-Nia, A.; Foley, A.J.R.; Fent, G.J.; Chew, P.G.; Brown, L.A.; Saunderson, C.E.; et al. Regression of left ventricular mass in athletes undergoing complete detraining is mediated by decrease in intracellular but not extracellular compartments. Circ. Cardiovasc. Imaging 2019, 12, e009417. [Google Scholar] [CrossRef] [PubMed]
- Giada, F.; Bertaglia, E.; De Piccoli, B.; Franceschi, M.; Sartori, F.; Raviele, A.; Pascotto, P. Cardiovascular adaptations to endurance training and detraining in young and older athletes. Int. J. Cardiol. 1998, 65, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Petek, B.J.; Groezinger, E.Y.; Pedlar, C.R.; Baggish, A.L. Cardiac effects of detraining in athletes: A narrative review. Ann. Phys. Rehabil. Med. 2022, 65, 101581. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.B.; Wang, F.; Berkstresser, B.; Kim, J.; Wang, T.J.; Lewis, G.D.; Hutter, A.M., Jr.; Picard, M.H.; Baggish, A.L. Regression of “gray zone” exercise-induced concentric left ventricular hypertrophy during prescribed detraining. J. Am. Coll. Cardiol. 2012, 59, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, F.S.; Martuchi, S.E.; Negrão, C.E.; Brum, P.C. Loss of resting bradycardia with detraining is associated with intrinsic heart rate changes. Braz. J. Med. Biol. Res. 2005, 38, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, A.; Pavlik, G.; Olexó, Z. The effect of detraining on echocardiographic parameters due to injury. Acta Physiol. Hung. 1999, 86, 223–227. [Google Scholar] [PubMed]
- Chen, Y.T.; Hsieh, Y.Y.; Ho, J.Y.; Lin, T.Y.; Lin, J.C. Two weeks of detraining reduces cardiopulmonary function and muscular fitness in endurance athletes. Eur. J. Sport Sci. 2022, 22, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Hemmert, M.K.; Coggan, A.R. Effects of detraining on cardiovascular responses to exercise: Role of blood volume. J. Appl. Physiol 1986, 60, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ready, A.E.; Quinney, H.A. Alterations in anaerobic threshold as the result of endurance training and detraining. Med. Sci. Sports Exerc. 1982, 14, 292–296. [Google Scholar] [CrossRef]
- Coyle, E.F.; Martin, W.H., 3rd; Sinacore, D.R.; Joyner, M.J.; Hagberg, J.M.; Holloszy, J.O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Pan, T.; Jiang, Y.; Shen, Y. Effects of short- and long-term detraining on maximal oxygen uptake in athletes: A systematic review and meta-analysis. Biomed. Res. Int. 2022, 2022, 2130993. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R.C.; Rosenkoetter, M.A. Reduced training frequencies and maintenance of increased aerobic power. Med. Sci. Sports Exerc. 1981, 13, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Mujika, I.; Sharp, M.A.; Foulis, S.A. Maintaining physical performance: The minimal dose of exercise needed to preserve endurance and strength over time. J. Strength Cond. Res. 2021, 35, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, J.T.; Hurlbut, D.E.; Martel, G.F.; Tracy, B.L.; Ivey, F.M.; Metter, E.J.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Age and gender responses to strength training and detraining. Med. Sci. Sports Exerc. 2000, 32, 1505–1512. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Marcus, R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin. Physiol. 1997, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.S.; Baroni, B.M.; Radaelli, R.; Lanferdini, F.J.; Cunha Gdos, S.; Reischak-Oliveira, Á.; Vaz, M.A.; Pinto, R.S. Effects of strength training and detraining on knee extensor strength, muscle volume and muscle quality in elderly women. Age 2013, 35, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Staron, R.S.; Leonardi, M.J.; Karapondo, D.L.; Malicky, E.S.; Falkel, J.E.; Hagerman, F.C.; Hikida, R.S. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J. Appl. Physiol. 1991, 70, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Houmard, J.A.; Stevenson, J.R.; Fraser, D.D.; Johns, R.A.; Israel, R.G. The effects of detraining on power athletes. Med. Sci. Sports Exerc. 1993, 25, 929–935. [Google Scholar] [PubMed]
- García-Pallarés, J.; Carrasco, L.; Díaz, A.; Sánchez-Medina, L. Post-season detraining effects on physiological and performance parameters in top-level kayakers: Comparison of two recovery strategies. J. Sports Sci. Med. 2009, 8, 622–628. [Google Scholar]
- Vuori, I.; Heinonen, A.; Sievänen, H.; Kannus, P.; Pasanen, M.; Oja, P. Effects of unilateral strength training and detraining on bone mineral density and content in young women: A study of mechanical loading and deloading on human bones. Calcif. Tissue Int. 1994, 55, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Delshad, M.; Ghanbarian, A.; Mehrabi, Y.; Sarvghadi, F.; Ebrahim, K. Effect of strength training and short-term detraining on muscle mass in women aged over 50 years old. Int. J. Prev. Med. 2013, 4, 1386–1394. [Google Scholar] [PubMed]
- Padilha, C.S.; Ribeiro, A.S.; Fleck, S.J.; Nascimento, M.A.; Pina, F.L.; Okino, A.M.; Venturini, D.; Barbosa, D.S.; Mayhew, J.L.; Cyrino, E.S. Effect of resistance training with different frequencies and detraining on muscular strength and oxidative stress biomarkers in older women. Age 2015, 37, 104. [Google Scholar] [CrossRef] [PubMed]
- Blocquiaux, S.; Gorski, T.; Van Roie, E.; Ramaekers, M.; Van Thienen, R.; Nielens, H.; Delecluse, C.; De Bock, K.; Thomis, M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp. Gerontol. 2020, 133, 110860. [Google Scholar] [CrossRef] [PubMed]
- Borland, M.; Bergfeldt, L.; Cider, Å.; Rosenkvist, A.; Jakobsson, M.; Olsson, K.; Lundwall, A.; Andersson, L.; Nordeman, L. Effects of 3 months of detraining following cardiac rehabilitation in patients with atrial fibrillation. Eur. Rev. Aging Phys. Act. 2022, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Tofas, T.; Fatouros, I.G.; Draganidis, D.; Deli, C.K.; Chatzinikolaou, A.; Tziortzis, C.; Panayiotou, G.; Koutedakis, Y.; Jamurtas, A.Z. Effects of cardiovascular, resistance and combined exercise training on cardiovascular, performance and blood redox parameters in coronary artery disease patients: An 8-Month training-detraining randomized intervention. Antioxidants 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Schlesinger, S.; Leitzmann, M.F.; Tonstad, S.; Norat, T.; Riboli, E.; Vatten, L.J. Physical activity and the risk of heart failure: A systematic review and dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Hadžović-Džuvo, A.; Valjevac, A.; Lepara, O.; Pjanić, S.; Hadžimuratović, A.; Mekić, A. Oxidative stress status in elite athletes engaged in different sport disciplines. Bosn. J. Basic Med. Sci. 2014, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gandía, V.; Ramos-Campo, D.J.; García-Sánchez, E.; Luque-Rubia, A.J.; López, A.; López-Román, F.J. Training, detraining and retraining effects of moderate vs. high intensity exercise training programme on cardiovascular risk factors. J. Hypertens. 2023, 41, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Merghani, A.; Malhotra, A.; Sharma, S. The U-shaped relationship between exercise and cardiac morbidity. Trends Cardiovasc. Med. 2016, 26, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Lemme, E.; Maestrini, V.; Di Paolo, F.M.; Pisicchio, C.; Di Gioia, G.; Caselli, S. Does sport participation worsen the clinical course of hypertrophic cardiomyopathy? Clinicaloutcome of hypertrophiccardiomyopathy in athletes. Circulation 2018, 137, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Borrazzo, C.; Caselli, S.; Lemme, E.; Musumeci, M.B.; Maestrini, V.; Francia, P.; Russo, D.; Pelliccia, M.; Olivotto, I.; et al. Neither athletic training nor detraining affects LV hypertrophy in adult, low-risk patients with HCM. JACC Cardiovasc. Imaging 2022, 15, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden death in sports: Insights from a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Bhonsale, A.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J. Am. Coll. Cardiol. 2013, 62, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Palermi, S.; D’Ascenzi, F.; Bonifazi, M.; Zorzi, A.; Corrado, D. Premature ventricular beats in athletes: To detrain or not to detrain? Br. J. Sports Med. 2024, 58, 407–408. [Google Scholar] [CrossRef]
- Biffi, A.; Maron, B.J.; Verdile, L.; Fernando, F.; Spataro, A.; Marcello, G.; Ciardo, R.; Ammirati, F.; Colivicchi, F.; Pelliccia, A. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J. Am. Coll. Cardiol. 2004, 44, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Gasperetti, A.; Dello Russo, A.; Busana, M.; Dessanai, M.; Pizzamiglio, F.; Saguner, A.M.; TeRiele, A.S.J.M.; Sommariva, E.; Vettor, G.; Bosman, L.; et al. Novel risk calculator performance in athletes with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2020, 17, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Orgeron, G.; Tichnell, C.; Murray, B.; Crosson, J.; Monfredi, O.; Cadrin-Tourigny, J.; Tandri, H.; Calkins, H.; James, C.A. Impact of exercise restriction on arrhythmic risk among patients with arrhythmogenic right ventricular cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e008843. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, C.; Speranza, G.; Magliarditi, A.; Pugliatti, P.; Andò, G.; Coglitore, S. Detraining-related changes in left ventricular wall thickness and longitudinal strain in a young athlete likely to have hypertrophic cardiomyopathy. J. Sports Sci. Med. 2012, 11, 557–561. [Google Scholar] [PubMed]
- Di Gioia, G.; Maestrini, V.; Colella, A.; Mango, R.; Segreti, A.; Squeo, M.R.; Lemme, E.; Pelliccia, A. Reversible apical hypertrophy in a young competitive athlete with familiar hypertrophic cardiomyopathy. Am. J. Case Rep. 2023, 24, e939058. [Google Scholar] [CrossRef] [PubMed]
- Vessella, T.; Cardillo, R.; Bianco, M.; Palmieri, V.; Zeppilli, P. Marked negative T waves in athletes: ECG normalization after detraining. J. Sports Med. Phys. Fit. 2013, 53, 520–523. [Google Scholar]
- Ghani, S.; Sharma, S. Electrocardiographic changes in an athlete before and after detraining. BMJ Case Rep. 2012, 2012, bcr0120125520. [Google Scholar] [CrossRef] [PubMed]
- Klempfner, R.; Kamerman, T.; Schwammenthal, E.; Nahshon, A.; Hay, I.; Goldenberg, I.; Dov, F.; Arad, M. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: Results of a structured exercise training program in a cardiac rehabilitation center. Eur. J. Prev. Cardiol. 2015, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Chen, W.C.; Wu, C.H.; Chen, S.Y.; Lee, C.M. The effectiveness of cardiac rehabilitation in non-ischemic dilated cardiomyopathy patients: A pilot study. J. Formos. Med. Assoc. 2020, 119, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Joo, C.H. The effects of short term detraining and retraining on physical fitness in elite soccer players. PLoS ONE 2018, 13, e0196212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zujko-Kowalska, K.; Kamiński, K.A.; Małek, Ł. Detraining among Athletes—Is Withdrawal of Adaptive Cardiovascular Changes a Hint for the Differential Diagnosis of Physically Active People? J. Clin. Med. 2024, 13, 2343. https://doi.org/10.3390/jcm13082343
Zujko-Kowalska K, Kamiński KA, Małek Ł. Detraining among Athletes—Is Withdrawal of Adaptive Cardiovascular Changes a Hint for the Differential Diagnosis of Physically Active People? Journal of Clinical Medicine. 2024; 13(8):2343. https://doi.org/10.3390/jcm13082343
Chicago/Turabian StyleZujko-Kowalska, Kinga, Karol Adam Kamiński, and Łukasz Małek. 2024. "Detraining among Athletes—Is Withdrawal of Adaptive Cardiovascular Changes a Hint for the Differential Diagnosis of Physically Active People?" Journal of Clinical Medicine 13, no. 8: 2343. https://doi.org/10.3390/jcm13082343
APA StyleZujko-Kowalska, K., Kamiński, K. A., & Małek, Ł. (2024). Detraining among Athletes—Is Withdrawal of Adaptive Cardiovascular Changes a Hint for the Differential Diagnosis of Physically Active People? Journal of Clinical Medicine, 13(8), 2343. https://doi.org/10.3390/jcm13082343







