Spectral Photon-Counting Computed Tomography: Technical Principles and Applications in the Assessment of Cardiovascular Diseases
Abstract
:1. Introduction
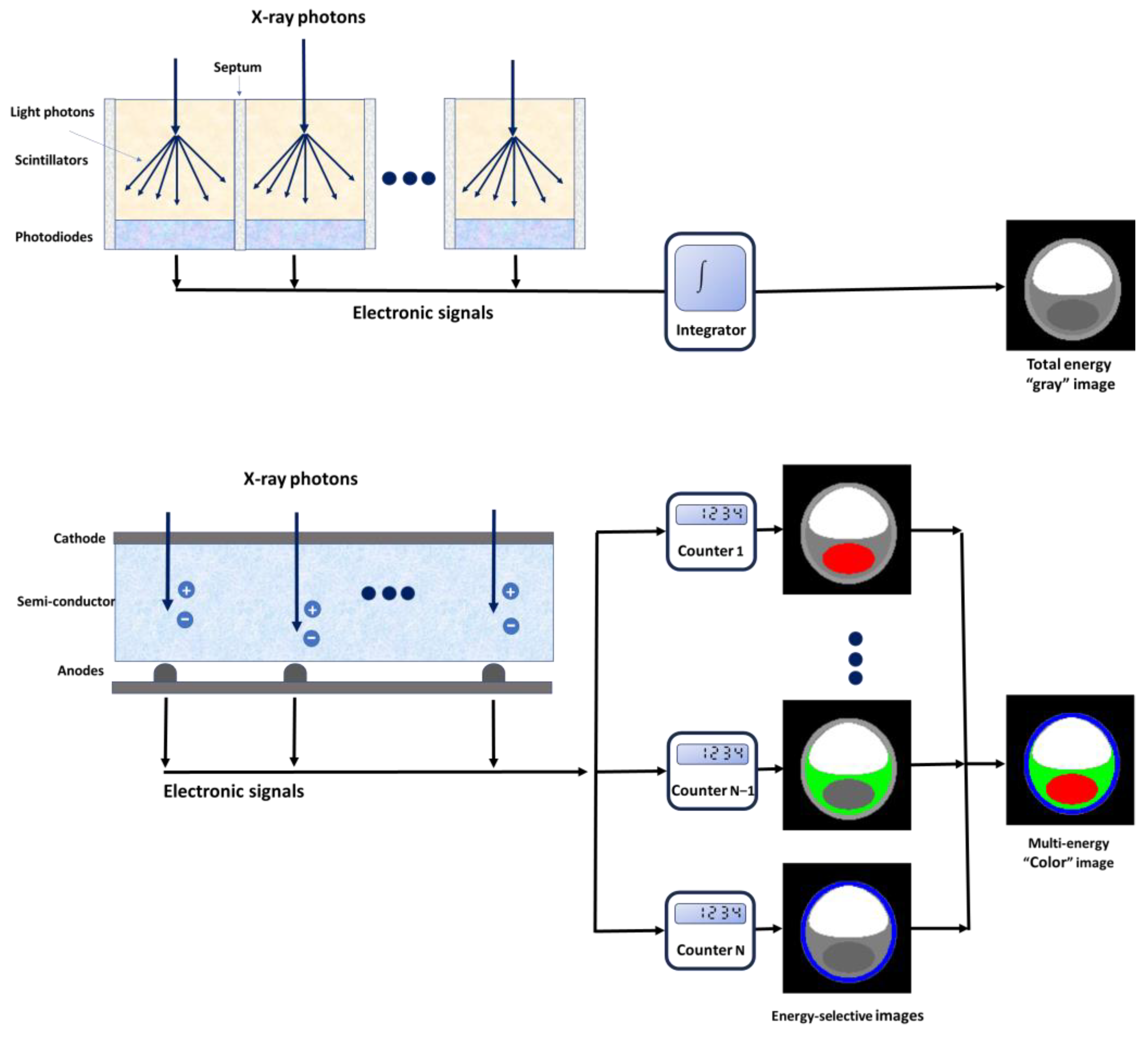
2. Spectral Photon-Counting CT Technology
2.1. Technical Aspects
2.2. Benefits Associated with PCDs
2.2.1. Improved Image Quality (Noise, Contrast, and Artifacts)
2.2.2. Multi-Energy Acquisition
2.2.3. Enhanced Spatial Resolution
2.3. Current Challenges of PCCT
2.3.1. Technical Challenges
2.3.2. Alternative to Iodine-Based Contrast Agents
3. Cardiovascular Applications
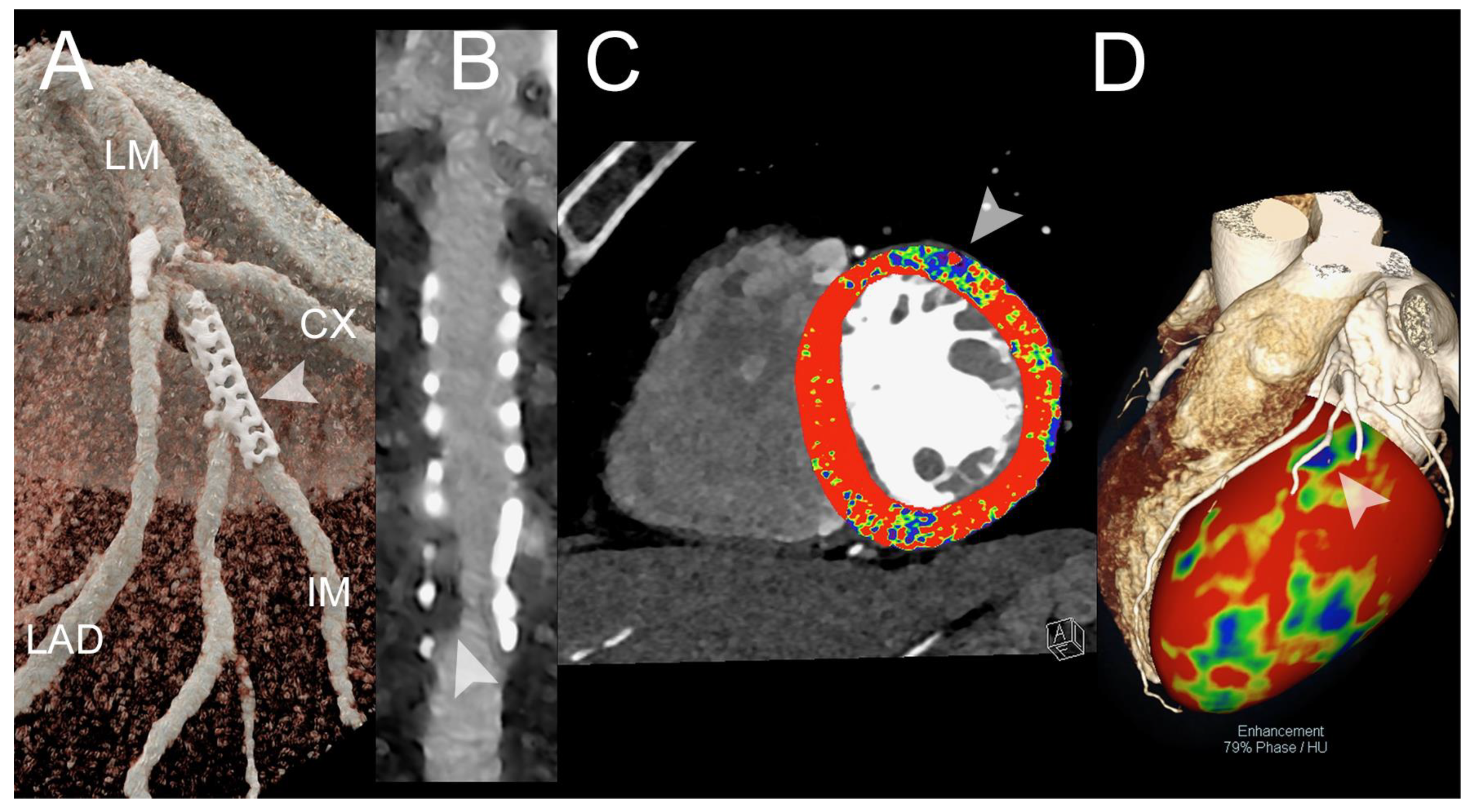
3.1. Coronary Artery Assessment
3.2. Myocardial Perfusion
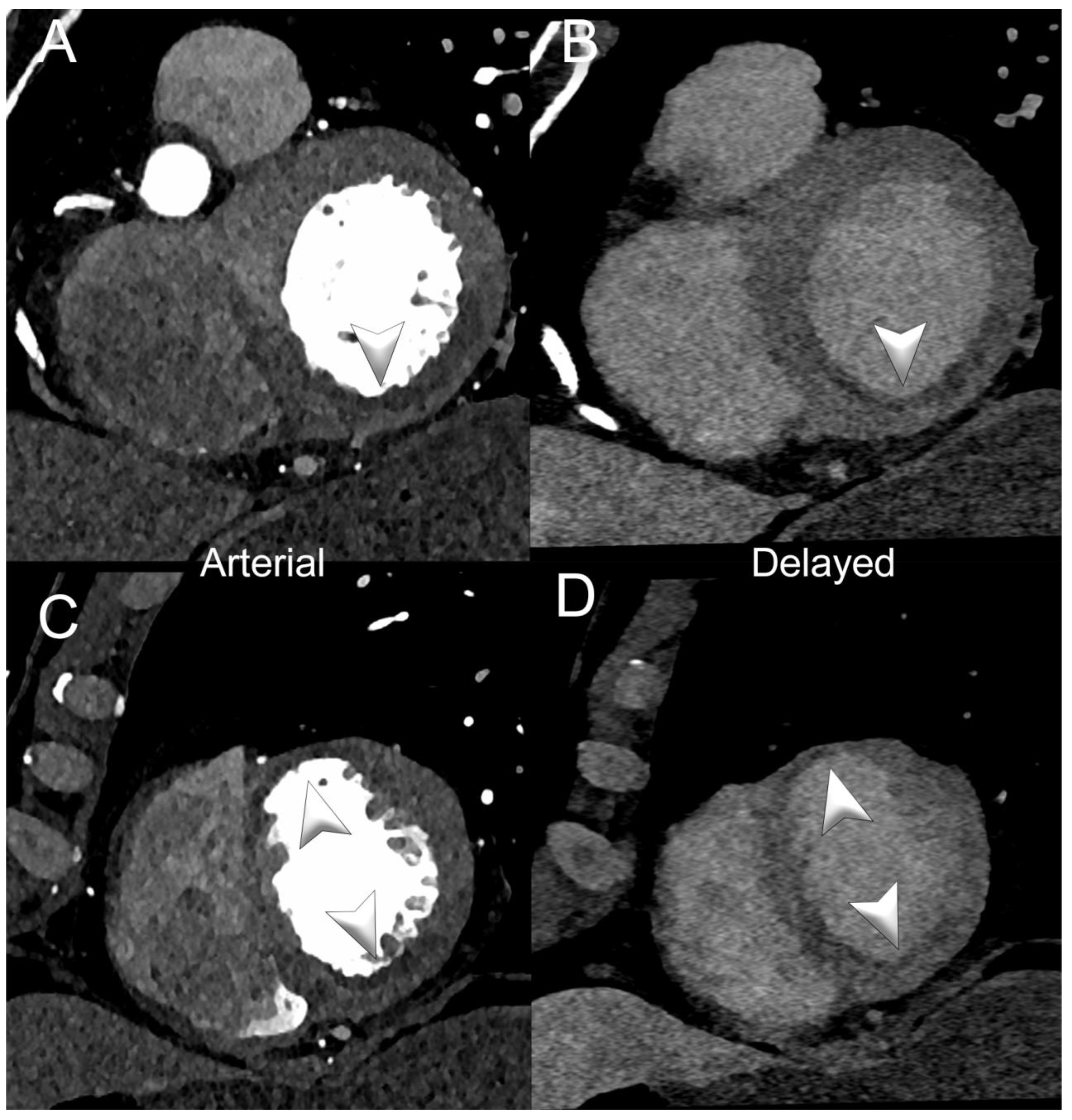
3.3. Myocardial Tissue Characterization
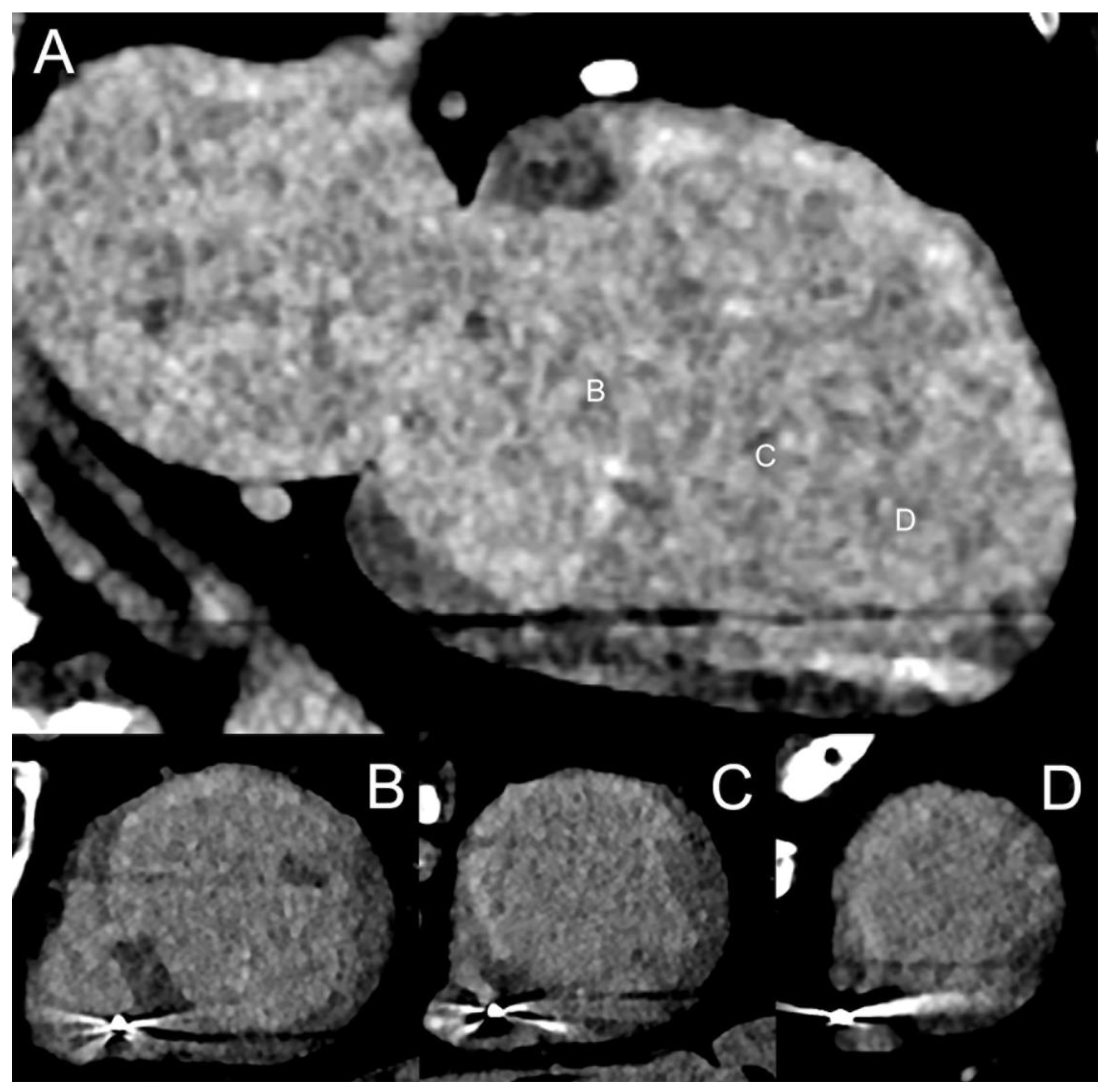
3.4. Epicardial and Pericoronary Fat
3.5. Radiomics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Biomed. Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Dell, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-Energy CT of the Heart: A Review. J. Imaging 2022, 8, 236. [Google Scholar] [CrossRef]
- Adam, S.Z.; Rabinowich, A.; Kessner, R.; Blachar, A. Spectral CT of the abdomen: Where are we now? Insights Into Imaging 2021, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Abbara, S.; Halliburton, S.S. Spectral detector CT for cardiovascular applications. Diagn. Interv. Radiol. 2017, 23, 187–193. [Google Scholar] [CrossRef]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual energy computed tomography virtual monoenergetic imaging: Technique and clinical applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: Theoretical principles and clinical applications. Radiol. Med. 2019, 124, 1281–1295. [Google Scholar] [CrossRef]
- Johnson, T.R. Dual-energy CT: General principles. AJR Am. J. Roentgenol. 2012, 199, S3–S8. [Google Scholar] [CrossRef]
- Tarkowski, P.; Czekajska-Chehab, E. Dual-Energy Heart CT: Beyond Better Angiography-Review. J. Clin. Med. 2021, 10, 5193. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Iwanczyk, J.S.; Nygård, E.; Meirav, O.; Arenson, J.; Barber, W.C.; Hartsough, N.E.; Malakhov, N.; Wessel, J.C. Photon Counting Energy Dispersive Detector Arrays for X-ray Imaging. IEEE Trans. Nucl. Sci. 2009, 56, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology. Radiographics 2019, 39, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Cademartiri, F.; Positano, V.; Celi, S.; Berti, S.; Clemente, A.; La Grutta, L.; Saba, L.; Bossone, E.; Cavaliere, C.; et al. Cardiovascular Applications of Photon-Counting CT Technology: A Revolutionary New Diagnostic Step. J. Cardiovasc. Dev. Dis. 2023, 10, 363. [Google Scholar] [CrossRef]
- Danielsson, M.; Persson, M.; Sjölin, M. Photon-counting x-ray detectors for CT. Phys. Med. Amp; Biol. 2021, 66, 03TR01. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef]
- Kreisler, D.B. Photon counting Detectors: Concept, technical Challenges, and clinical outlook. Eur. J. Radiol. 2022, 149, 110229. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, S.; Kappler, S.; Hahn, K.; Li, Z.; Halaweish, A.F.; Henning, A.; McCollough, C.H. Noise performance of low-dose CT: Comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner. J. Med. Imaging 2016, 3, 043503. [Google Scholar] [CrossRef]
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; Bluemke, D.A.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2017, 62, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, V.; Persson, M.; Pourmorteza, A.; Noël, P.B.; Fleischmann, D.; Willemink, M.J. Spectral photon-counting CT in cardiovascular imaging. J. Cardiovasc. Comput. Tomogr. 2021, 15, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Shikhaliev, P.M. Energy-resolved computed tomography: First experimental results. Phys. Med. Biol. 2008, 53, 5595–5613. [Google Scholar] [CrossRef] [PubMed]
- Shikhaliev, P.M.; Fritz, S.G. Photon counting spectral CT versus conventional CT: Comparative evaluation for breast imaging application. Phys. Med. Biol. 2011, 56, 1905–1930. [Google Scholar] [CrossRef] [PubMed]
- Silkwood, J.D.; Matthews, K.L.; Shikhaliev, P.M. Photon counting spectral breast CT: Effect of adaptive filtration on CT numbers, noise, and contrast to noise ratio. Med. Phys. 2013, 40, 051905. [Google Scholar] [CrossRef] [PubMed]
- Giersch, J.; Niederlöhner, D.; Anton, G. The influence of energy weighting on X-ray imaging quality. Nucl. Instrum. Methods Phys. Res. Sect. A: Accel. Spectrometers Detect. Assoc. Equip. [CrossRef]
- Schmidt, T.G. Optimal “image-based” weighting for energy-resolved CT. Med. Phys. 2009, 36, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Chiro, G.D. Beam hardening in X-ray reconstructive tomography. Phys. Med. Biol. 1976, 21, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Park, J.; Nam, S.; Choi, J.; Choi, Y.; Lee, S.; Lee, K.-Y.; Cho, M. Metal artifact reduction and tumor detection using photon-counting multi-energy computed tomography. PLoS ONE 2021, 16, e0247355. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Beam hardening artefacts in computed tomography with photon counting, charge integrating and energy weighting detectors: A simulation study. Phys. Med. Biol. 2005, 50, 5813–5827. [Google Scholar] [CrossRef]
- Gutjahr, R.; Halaweish, A.F.; Yu, Z.; Leng, S.; Yu, L.; Li, Z.; Jorgensen, S.M.; Ritman, E.L.; Kappler, S.; McCollough, C.H. Human Imaging with Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Investig. Radiol. 2016, 51, 421–429. [Google Scholar] [CrossRef]
- Rajagopal, J.R.; Farhadi, F.; Solomon, J.; Sahbaee, P.; Saboury, B.; Pritchard, W.F.; Jones, E.C.; Samei, E. Comparison of Low Dose Performance of Photon-Counting and Energy Integrating CT. Acad. Radiol. 2021, 28, 1754–1760. [Google Scholar] [CrossRef]
- Woeltjen, M.M.; Niehoff, J.H.; Michael, A.E.; Horstmeier, S.; Moenninghoff, C.; Borggrefe, J.; Kroeger, J.R. Low-Dose High-Resolution Photon-Counting CT of the Lung: Radiation Dose and Image Quality in the Clinical Routine. Diagnostics 2022, 12, 1441. [Google Scholar] [CrossRef]
- Niehoff, J.H.; Carmichael, A.F.; Woeltjen, M.M.; Boriesosdick, J.; Schmidt, I.L.; Michael, A.E.; Hokamp, N.G.; Piechota, H.; Borggrefe, J.; Kroeger, J.R. Clinical Low Dose Photon Counting CT for the Detection of Urolithiasis: Evaluation of Image Quality and Radiation Dose. Tomography 2022, 8, 1666–1675. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 2009, 36, 1602–1609. [Google Scholar] [CrossRef]
- Yveborg, M.; Danielsson, M.; Bornefalk, H. Theoretical comparison of a dual energy system and photon counting silicon detector used for material quantification in spectral CT. IEEE Trans. Med. Imaging 2015, 34, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Michoux, N.; Coche, E.; Dragean, C.A. Virtual unenhanced phase with spectral dual-energy CT: Is it an alternative to conventional true unenhanced phase for abdominal tissues? Diagn. Interv. Imaging 2019, 100, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Radiol. 2017, 42, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.M.; Kang, C.K.; Yoon, J.; Chae, K.J.; Goo, J.M. Effect of CT Acquisition Parameters on Iodine Density Measurement at Dual-Layer Spectral CT. AJR Am. J. Roentgenol. 2018, 211, 748–754. [Google Scholar] [CrossRef]
- Mergen, V.; Racine, D.; Jungblut, L.; Sartoretti, T.; Bickel, S.; Monnin, P.; Higashigaito, K.; Martini, K.; Alkadhi, H.; Euler, A. Virtual Noncontrast Abdominal Imaging with Photon-counting Detector CT. Radiology 2022, 305, 107–115. [Google Scholar] [CrossRef]
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First In Vivo Human Results. Investig. Radiol. 2018, 53, 135–142. [Google Scholar] [CrossRef]
- Leng, S.; Zhou, W.; Yu, Z.; Halaweish, A.; Krauss, B.; Schmidt, B.; Yu, L.; Kappler, S.; McCollough, C. Spectral performance of a whole-body research photon counting detector CT: Quantitative accuracy in derived image sets. Phys. Med. Biol. 2017, 62, 7216–7232. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Lennartz, S.; Neuhaus, V.F.; Hokamp, N.G.; Rau, R.; Le Blanc, M.; Abdullayev, N.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. CT metal artifacts in patients with total hip replacements: For artifact reduction monoenergetic reconstructions and post-processing algorithms are both efficient but not similar. Eur. Radiol. 2018, 28, 4524–4533. [Google Scholar] [CrossRef]
- Kulkarni, N.M.; Fung, A.; Kambadakone, A.R.; Yeh, B.M. Computed Tomography Techniques, Protocols, Advancements, and Future Directions in Liver Diseases. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 305–320. [Google Scholar] [CrossRef]
- Rassouli, N.; Chalian, H.; Rajiah, P.; Dhanantwari, A.; Landeras, L. Assessment of 70-keV virtual monoenergetic spectral images in abdominal CT imaging: A comparison study to conventional polychromatic 120-kVp images. Abdom. Radiol. 2017, 42, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Kappler, S.; Henning, A.; Kreisler, B.; Schoeck, F.; Stierstorfer, K.; Flohr, T. Photon counting CT at elevated X-ray tube currents: Contrast stability, image noise and multi-energy performance. In Proceedings of the Medical Imaging 2014: Physics of Medical Imaging, SPIE, San Diego, CA, USA, 15–20 February 2014; Volume 9033. [Google Scholar]
- Faby, S.; Kuchenbecker, S.; Sawall, S.; Simons, D.; Schlemmer, H.P.; Lell, M.; Kachelrieß, M. Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: A simulation study. Med. Phys. 2015, 42, 4349–4366. [Google Scholar] [CrossRef] [PubMed]
- Shikhaliev, P.M. Computed tomography with energy-resolved detection: A feasibility study. Phys. Med. Biol. 2008, 53, 1475–1495. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Kondo, S.; Kawashita, I.; Takahashi, I.; Awai, K. An introduction to photon-counting detector CT (PCD CT) for radiologists. Jpn. J. Radiol. 2022, 41, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Schirra, C.O.; Brendel, B.; Anastasio, M.A.; Roessl, E. Spectral CT: A technology primer for contrast agent development. Contrast Media Mol. Imaging 2014, 9, 62–70. [Google Scholar] [CrossRef]
- Pan, D.; Schirra, C.O.; Senpan, A.; Schmieder, A.H.; Stacy, A.J.; Roessl, E.; Thran, A.; Wickline, S.A.; Proska, R.; Lanza, G.M. An early investigation of ytterbium nanocolloids for selective and quantitative “multicolor” spectral CT imaging. ACS Nano 2012, 6, 3364–3370. [Google Scholar] [CrossRef]
- Müllner, M.; Schlattl, H.; Hoeschen, C.; Dietrich, O. Feasibility of spectral CT imaging for the detection of liver lesions with gold-based contrast agents—A simulation study. Phys. Med. 2015, 31, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bar-Ness, D.; Si-Mohamed, S.; Coulon, P.; Blevis, I.; Douek, P.; Cormode, D.P. Assessment of candidate elements for development of spectral photon-counting CT specific contrast agents. Sci. Rep. 2018, 8, 12119. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.; Cormode, D.P.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Langlois, J.-B.; Chalabreysse, L.; Coulon, P.; Blevis, I.; Roessl, E.; et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale 2017, 9, 18246–18257. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Roessl, E.; Thran, A.; Skajaa, T.; Gordon, R.E.; Schlomka, J.P.; Fuster, V.; Fisher, E.A.; Mulder, W.J.; Proksa, R.; et al. Atherosclerotic plaque composition: Analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010, 256, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Balegamire, J.; Vandamme, M.; Chereul, E.; Si-Mohamed, S.; Maache, S.A.; Almouazen, E.; Ettouati, L.; Fessi, H.; Boussel, L.; Douek, P.; et al. Iodinated polymer nanoparticles as contrast agent for spectral photon counting computed tomography. Biomater. Sci. 2020, 8, 5715–5728. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Kumar, A.; Rosario-Berríos, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noël, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274–39284. [Google Scholar] [CrossRef] [PubMed]
- Muenzel, D.; Daerr, H.; Proksa, R.; Fingerle, A.A.; Kopp, F.K.; Douek, P.; Herzen, J.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B. Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: A proof-of-concept study. Eur. Radiol. Exp. 2017, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Cork, T.E.; Lakshmanan, M.N.; Evers, R.; Davies-Venn, C.; Rice, K.A.; Thomas, M.L.; Liu, C.Y.; Kappler, S.; Ulzheimer, S.; et al. Dual-contrast agent photon-counting computed tomography of the heart: Initial experience. Int. J. Cardiovasc. Imaging 2017, 33, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Si-Mohamed, S.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Balegamire, J.; Lavenne, F.; Coulon, P.; Roessl, E.; Bartels, M.; et al. Multicolor spectral photon-counting computed tomography: In vivo dual contrast imaging with a high count rate scanner. Sci. Rep. 2017, 7, 4784. [Google Scholar] [CrossRef]
- Symons, R.; Krauss, B.; Sahbaee, P.; Cork, T.E.; Lakshmanan, M.N.; Bluemke, D.A.; Pourmorteza, A. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med. Phys. 2017, 44, 5120–5127. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Y.-X.; Lin, Y.; Zhang, J.-S.; Yang, F.; Zhou, Q.-L.; Liao, Y.-Y. Advance of Molecular Imaging Technology and Targeted Imaging Agent in Imaging and Therapy. BioMed Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Sigovan, M.; Hsu, J.C.; Tatard-Leitman, V.; Chalabreysse, L.; Naha, P.C.; Garrivier, T.; Dessouky, R.; Carnaru, M.; Boussel, L.; et al. In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT. Radiology 2021, 300, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Rajendran, K.; Gong, H.; Zhou, W.; Halaweish, A.F.; Henning, A.; Kappler, S.; Baer, M.; Fletcher, J.G.; McCollough, C.H. 150-μm Spatial Resolution Using Photon-Counting Detector Computed Tomography Technology: Technical Performance and First Patient Images. Investig. Radiol. 2018, 53, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ferda, J.; Vendiš, T.; Flohr, T.; Schmidt, B.; Henning, A.; Ulzheimer, S.; Pecen, L.; Ferdová, E.; Baxa, J.; Mírka, H. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur. J. Radiol. 2021, 137, 109614. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef] [PubMed]
- Cammin, J.; Xu, J.; Barber, W.C.; Iwanczyk, J.S.; Hartsough, N.E.; Taguchi, K. A cascaded model of spectral distortions due to spectral response effects and pulse pile-up effects in a photon-counting x-ray detector for CT. Med. Phys. 2014, 41, 041905. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zheng, B.; Liu, H. Tutorial on X-ray photon counting detector characterization. J. Xray Sci. Technol. 2018, 26, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Frey, E.C.; Wang, X.; Iwanczyk, J.S.; Barber, W.C. An analytical model of the effects of pulse pile-up on the energy spectrum recorded by energy resolved photon counting x-ray detectors. Med. Phys. 2010, 37, 3957–3969. [Google Scholar] [CrossRef]
- Wang, A.S.; Pelc, N.J. Spectral Photon Counting CT: Imaging Algorithms and Performance Assessment. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 453–464. [Google Scholar] [CrossRef]
- Flohr, T.; Schmidt, B. Technical Basics and Clinical Benefits of Photon-Counting CT. Investig. Radiol. 2023, 58, 441–450. [Google Scholar] [CrossRef]
- Amukotuwa, S.A.; Bammer, R.; Jackson, D.M.; Sutherland, T. Iodinated contrast media shortage: Insights and guidance from two major public hospitals. J. Med. Imaging Radiat. Oncol. 2022, 66, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Chicoskie, C.; Tello, R. Gadolinium-enhanced MDCT angiography of the abdomen: Feasibility and limitations. AJR Am. J. Roentgenol. 2005, 184, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Moghiseh, M.; Lowe, C.; Lewis, J.G.; Kumar, D.; Butler, A.; Anderson, N.; Raja, A. Spectral Photon-Counting Molecular Imaging for Quantification of Monoclonal Antibody-Conjugated Gold Nanoparticles Targeted to Lymphoma and Breast Cancer: An In Vitro Study. Contrast Media Mol. Imaging 2018, 2018, 2136840. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Guo, J.J.; Qin, A.P.; Yu, X.Y.; Zhang, Q.; Lei, X.P.; Huang, Y.G.; Chen, M.Y.; Li, J.X.; Zhang, Y.; et al. Bismuth chelate as a contrast agent for X-ray computed tomography. J. Nanobiotechnology 2020, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Levin, D.C.; Halpern, E.J.; Fischman, D.; Savage, M.; Walinsky, P. Accuracy of MDCT in assessing the degree of stenosis caused by calcified coronary artery plaques. AJR Am. J. Roentgenol. 2008, 191, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Koons, E.; VanMeter, P.; Rajendran, K.; Yu, L.; McCollough, C.; Leng, S. Improved quantification of coronary artery luminal stenosis in the presence of heavy calcifications using photon-counting detector CT. Proc. SPIE Int. Soc. Opt. Eng. 2022, 12031. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Douek. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Leng, S.; Halaweish, A.F.; Yu, Z.; Yu, L.; Ritman, E.L.; McCollough, C.H. Overcoming calcium blooming and improving the quantification accuracy of percent area luminal stenosis by material decomposition of multi-energy computed tomography datasets. J Med Imaging 2020, 7, 053501. [Google Scholar] [CrossRef]
- Maintz, D.; Seifarth, H.; Raupach, R.; Flohr, T.; Rink, M.; Sommer, T.; Ozgün, M.; Heindel, W.; Fischbach, R. 64-slice multidetector coronary CT angiography: In vitro evaluation of 68 different stents. Eur. Radiol. 2006, 16, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Mannil, M.; Hickethier, T.; von Spiczak, J.; Baer, M.; Henning, A.; Hertel, M.; Schmidt, B.; Flohr, T.; Maintz, D.; Alkadhi, H. Photon-Counting CT: High-Resolution Imaging of Coronary Stents. Investig. Radiol. 2018, 53, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; De Bruecker, Y.; Roosen, J.; Van Camp, L.; Cork, T.E.; Kappler, S.; Ulzheimer, S.; Sandfort, V.; Bluemke, D.A.; Pourmorteza, A. Quarter-millimeter spectral coronary stent imaging with photon-counting CT: Initial experience. J. Cardiovasc. Comput. Tomogr. 2018, 12, 509–515. [Google Scholar] [CrossRef]
- Petritsch, B.; Petri, N.; Weng, A.M.; Petersilka, M.; Allmendinger, T.; Bley, T.A.; Gassenmaier, T. Photon-Counting Computed Tomography for Coronary Stent Imaging: In Vitro Evaluation of 28 Coronary Stents. Investig. Radiol. 2021, 56, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, J.R.; Farhadi, F.; Richards, T.; Nikpanah, M.; Sahbaee, P.; Shanbhag, S.M.; Bandettini, W.P.; Saboury, B.; Malayeri, A.A.; Pritchard, W.F.; et al. Evaluation of Coronary Plaques and Stents with Conventional and Photon-counting CT: Benefits of High-Resolution Photon-counting CT. Radiol. Cardiothorac. Imaging 2021, 3, e210102. [Google Scholar] [CrossRef]
- Decker, J.A.; O’Doherty, J.; Schoepf, U.J.; Todoran, T.M.; Aquino, G.J.; Brandt, V.; Baruah, D.; Fink, N.; Zsarnoczay, E.; Flohr, T.; et al. Stent imaging on a clinical dual-source photon-counting detector CT system-impact of luminal attenuation and sharp kernels on lumen visibility. Eur. Radiol. 2023, 33, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Koons, E.K.; Thorne, J.E.; Huber, N.R.; Chang, S.; Rajendran, K.; McCollough, C.H.; Leng, S. Quantifying lumen diameter in coronary artery stents with high-resolution photon counting detector CT and convolutional neural network denoising. Med. Phys. 2023, 50, 4173–4181. [Google Scholar] [CrossRef]
- Feuerlein, S.; Roessl, E.; Proksa, R.; Martens, G.; Klass, O.; Jeltsch, M.; Rasche, V.; Brambs, H.J.; Hoffmann, M.H.; Schlomka, J.P. Multienergy photon-counting K-edge imaging: Potential for improved luminal depiction in vascular imaging. Radiology 2008, 249, 1010–1016. [Google Scholar] [CrossRef]
- Boccalini, S.; Si-Mohamed, S.A.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.A.; Villien, M.; Sigovan, M.; Dessouky, R.; Coulon, P.; et al. First In-Human Results of Computed Tomography Angiography for Coronary Stent Assessment with a Spectral Photon Counting Computed Tomography. Investig. Radiol. 2022, 57, 212–221. [Google Scholar] [CrossRef]
- Geering, L.; Sartoretti, T.; Mergen, V.; Cundari, G.; Rusek, S.; Civaia, F.; Rossi, P.; Wildberger, J.E.; Templin, C.; Manka, R.; et al. First in-vivo coronary stent imaging with clinical ultra high resolution photon-counting CT. J. Cardiovasc. Comput. Tomogr. 2023, 17, 233–235. [Google Scholar] [CrossRef]
- Bratke, G.; Hickethier, T.; Bar-Ness, D.; Bunck, A.C.; Maintz, D.; Pahn, G.; Coulon, P.; Si-Mohamed, S.; Douek, P.; Sigovan, M. Spectral Photon-Counting Computed Tomography for Coronary Stent Imaging: Evaluation of the Potential Clinical Impact for the Delineation of In-Stent Restenosis. Investig. Radiol. 2020, 55, 61–67. [Google Scholar] [CrossRef]
- Lo-Kioeng-Shioe, M.S.; Rijlaarsdam-Hermsen, D.; van Domburg, R.T.; Hadamitzky, M.; Lima, J.A.C.; Hoeks, S.E.; Deckers, J.W. Prognostic value of coronary artery calcium score in symptomatic individuals: A meta-analysis of 34, 000 subjects. Int. J. Cardiol. 2020, 299, 56–62. [Google Scholar] [CrossRef]
- Rijlaarsdam-Hermsen, D.; Lo-Kioeng-Shioe, M.S.; Kuijpers, D.; van Domburg, R.T.; Deckers, J.W.; van Dijkman, P.R.M. Prognostic value of the coronary artery calcium score in suspected coronary artery disease: A study of 644 symptomatic patients. Neth. Heart J. 2020, 28, 44–50. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R., Jr. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Ulzheimer, S.; Halliburton, S.S.; Shanneik, K.; White, R.D.; Kalender, W.A. Coronary artery calcium: A multimanufacturer international standard for quantification at cardiac CT. Radiology 2007, 243, 527–538. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, N.R.; Si-Mohamed, S.; Rodesch, P.A.; van Hamersvelt, R.W.; Greuter, M.J.W.; Boccalini, S.; Greffier, J.; Leiner, T.; Boussel, L.; Willemink, M.J.; et al. Coronary calcium scoring potential of large field-of-view spectral photon-counting CT: A phantom study. Eur. Radiol. 2022, 32, 152–162. [Google Scholar] [CrossRef]
- Chang, S.; Ren, L.; Tang, S.; Marsh, J.F., Jr.; Hsieh, S.; McCollough, C.H.; Leng, S. Technical note: Exploring the detectability of coronary calcification using ultra-high-resolution photon-counting-detector CT. Med. Phys. 2023, 50, 6836–6843. [Google Scholar] [CrossRef]
- Eberhard, M.; Mergen, V.; Higashigaito, K.; Allmendinger, T.; Manka, R.; Flohr, T.; Schmidt, B.; Euler, A.; Alkadhi, H. Coronary Calcium Scoring with First Generation Dual-Source Photon-Counting CT-First Evidence from Phantom and In-Vivo Scans. Diagnostics 2021, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Skoog, S.; Henriksson, L.; Gustafsson, H.; Sandstedt, M.; Elvelind, S.; Persson, A. Comparison of the Agatston score acquired with photon-counting detector CT and energy-integrating detector CT: Ex vivo study of cadaveric hearts. Int. J. Cardiovasc. Imaging 2022, 38, 1145–1155. [Google Scholar] [CrossRef]
- Marsh, J.F., Jr.; VanMeter, P.D.; Rajendran, K.; Leng, S.; McCollough, C.H. Ex vivo coronary calcium volume quantification using a high-spatial-resolution clinical photon-counting-detector computed tomography. J. Med. Imaging 2023, 10, 043501. [Google Scholar] [CrossRef]
- van der Werf, N.R.; van Gent, M.; Booij, R.; Bos, D.; van der Lugt, A.; Budde, R.P.J.; Greuter, M.J.W.; van Straten, M. Dose Reduction in Coronary Artery Calcium Scoring Using Mono-Energetic Images from Reduced Tube Voltage Dual-Source Photon-Counting CT Data: A Dynamic Phantom Study. Diagnostics 2021, 11, 2192. [Google Scholar] [CrossRef]
- van der Werf, N.R.; Greuter, M.J.W.; Booij, R.; van der Lugt, A.; Budde, R.P.J.; van Straten, M. Coronary calcium scores on dual-source photon-counting computed tomography: An adapted Agatston methodology aimed at radiation dose reduction. Eur. Radiol. 2022, 32, 5201–5209. [Google Scholar] [CrossRef]
- van der Werf, N.R.; Rodesch, P.A.; Si-Mohamed, S.; van Hamersvelt, R.W.; Greuter, M.J.W.; Leiner, T.; Boussel, L.; Willemink, M.J.; Douek, P. Improved coronary calcium detection and quantification with low-dose full field-of-view photon-counting CT: A phantom study. Eur. Radiol. 2022, 32, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Sandfort, V.; Mallek, M.; Ulzheimer, S.; Pourmorteza, A. Coronary artery calcium scoring with photon-counting CT: First in vivo human experience. Int. J. Cardiovasc. Imaging 2019, 35, 733–739. [Google Scholar] [CrossRef]
- Emrich, T.; Aquino, G.; Schoepf, U.J.; Braun, F.M.; Risch, F.; Bette, S.J.; Woznicki, P.; Decker, J.A.; O’Doherty, J.; Brandt, V.; et al. Coronary Computed Tomography Angiography-Based Calcium Scoring: In Vitro and In Vivo Validation of a Novel Virtual Non-iodine Reconstruction Algorithm on a Clinical, First-Generation Dual-Source Photon Counting-Detector System. Investig. Radiol. 2022, 57, 536–543. [Google Scholar] [CrossRef]
- Fink, N.; Zsarnoczay, E.; Schoepf, U.J.; O’Doherty, J.; Halfmann, M.C.; Allmendinger, T.; Hagenauer, J.; Griffith, J.P., 3rd; Vecsey-Nagy, M.; Pinos, D.; et al. Impact of Cardiac Motion on coronary artery calcium scoring using a virtual non-iodine algorithm on photon-counting detector CT: A dynamic phantom study. Int. J. Cardiovasc. Imaging 2023, 39, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Servadei, F.; Zoccai, G.B.; Giacobbi, E.; Anemona, L.; Bonanno, E.; Casella, S. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis 2013, 229, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N. Biomarkers of Atherosclerotic Plaque Instability and Rupture. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Cau, R.; Faa, G.; Nardi, V.; Balestrieri, A.; Puig, J.; Suri, J.S.; SanFilippo, R.; Saba, L. Long-COVID diagnosis: From diagnostic to advanced AI-driven models. Eur. J. Radiol. 2022, 148, 110164. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.O.; Mayrhofer, T.; Budoff, M.; Szilveszter, B.; Foldyna, B.; Hallett, T.R.; Ivanov, A.; Janjua, S.; Meyersohn, N.M.; Staziaki, P.V.; et al. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc. Imaging 2020, 13, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; Hoole, S.P.; West, N.E.J.; Goddard, M.; Rudd, J.H.F.; Bennett, M.R. Atherosclerotic Plaque Composition and Classification Identified by Coronary Computed Tomography. Circ. Cardiovasc. Imaging 2013, 6, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.M.; Davis, J.; Gopalan, D.; Rudd, J.H.F.; Clarke, S.C.; Schofield, P.M.; Bennett, M.R.; Brown, A.J.; Obaid, D.R. Characteristics of conventional high-risk coronary plaques and a novel CT defined thin-cap fibroatheroma in patients undergoing CCTA with stable chest pain. Clin. Imaging 2023, 101, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, F.; Balestrieri, A.; Cau, R.; Punzo, B.; Cavaliere, C.; Maffei, E.; Saba, L. Insight from imaging on plaque vulnerability: Similarities and differences between coronary and carotid arteries-implications for systemic therapies. Cardiovasc. Diagn. Ther. 2020, 10, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Rotzinger, D.C.; Racine, D.; Becce, F.; Lahoud, E.; Erhard, K.; Si-Mohamed, S.A.; Greffier, J.; Viry, A.; Boussel, L.; Meuli, R.A.; et al. Performance of Spectral Photon-Counting Coronary CT Angiography and Comparison with Energy-Integrating-Detector CT: Objective Assessment with Model Observer. Diagnostics 2021, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Boussel, L.; Coulon, P.; Thran, A.; Roessl, E.; Martens, G.; Sigovan, M.; Douek, P. Photon counting spectral CT component analysis of coronary artery atherosclerotic plaque samples. Br. J. Radiol. 2014, 87, 20130798. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Eberhard, M.; Manka, R.; Euler, A.; Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 2022, 9, 981012. [Google Scholar] [CrossRef]
- Vattay, B.; Szilveszter, B.; Boussoussou, M.; Vecsey-Nagy, M.; Lin, A.; Konkoly, G.; Kubovje, A.; Schwarz, F.; Merkely, B.; Maurovich-Horvat, P.; et al. Impact of virtual monoenergetic levels on coronary plaque volume components using photon-counting computed tomography. Eur. Radiol. 2023, 33, 8528–8539. [Google Scholar] [CrossRef]
- Koonce, J.D.; Vliegenthart, R.; Schoepf, U.J.; Schmidt, B.; Wahlquist, A.E.; Nietert, P.J.; Bastarrika, G.; Flohr, T.G.; Meinel, F.G. Accuracy of dual-energy computed tomography for the measurement of iodine concentration using cardiac CT protocols: Validation in a phantom model. Eur. Radiol. 2014, 24, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Ruzsics, B.; Schwarz, F.; Schoepf, U.J.; Lee, Y.S.; Bastarrika, G.; Chiaramida, S.A.; Costello, P.; Zwerner, P.L. Comparison of dual-energy computed tomography of the heart with single photon emission computed tomography for assessment of coronary artery stenosis and of the myocardial blood supply. Am. J. Cardiol. 2009, 104, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, W.; Wang, Y.; He, Y.; Yang, L.; Bi, T.; Jiao, J.; Wang, Q.; Chi, L.; Yu, Y.; et al. Incremental value of dual-energy CT to coronary CT angiography for the detection of significant coronary stenosis: Comparison with quantitative coronary angiography and single photon emission computed tomography. Int. J. Cardiovasc. Imaging 2011, 27, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Choi, J.W.; Song, M.G.; Shin, J.K.; Chee, H.K.; Chung, H.W.; Kim, D.H. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: Comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur. Radiol. 2011, 21, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Han, R.; Zhao, R.; Bai, S.; Wang, J.; Hu, J.; Lu, B. Evaluation of dual energy computed tomography iodine mapping within the myocardial blood pool for detection of acute myocardial infarction: Correlation with histopathological findings in a porcine model. Br. J. Radiol. 2018, 91, 20170569. [Google Scholar] [CrossRef] [PubMed]
- Polacin, M.; Templin, C.; Manka, R.; Alkadhi, H. Photon-counting computed tomography for the diagnosis of myocardial infarction with non-obstructive coronary artery disease. Eur. Heart J.-Case Rep. 2022, 6, ytac028. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.; Schinde, V.; Bateman, A.C.; Gallagher, P.J. Interstitial fibrosis in the dilated non-ischaemic myocardium. Heart 2003, 89, 1255–1256. [Google Scholar] [CrossRef]
- Yamada, A.; Kitagawa, K.; Nakamura, S.; Takafuji, M.; Goto, Y.; Okamoto, R.; Dohi, K.; Sakuma, H. Quantification of extracellular volume fraction by cardiac computed tomography for non-invasive assessment of myocardial fibrosis in hemodialysis patients. Sci. Rep. 2020, 10, 15367. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef]
- Abadia, A.F.; van Assen, M.; Martin, S.S.; Vingiani, V.; Griffith, L.P.; Giovagnoli, D.A.; Bauer, M.J.; Schoepf, U.J. Myocardial extracellular volume fraction to differentiate healthy from cardiomyopathic myocardium using dual-source dual-energy CT. J. Cardiovasc. Comput. Tomogr. 2020, 14, 162–167. [Google Scholar] [CrossRef]
- Kim, N.Y.; Im, D.J.; Youn, J.C.; Hong, Y.J.; Choi, B.W.; Kang, S.M.; Lee, H.J. Synthetic Extracellular Volume Fraction Derived Using Virtual Unenhanced Attenuation of Blood on Contrast-Enhanced Cardiac Dual-Energy CT in Non-ischemic Cardiomyopathy. AJR Am. J. Roentgenol. 2022, 218, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, B.; Dacher, J.N.; Durand, E.; Caudron, J.; Bauer, F.; Bubenheim, M.; Eltchaninoff, H.; Serfaty, J.M. Single-source dual energy CT to assess myocardial extracellular volume fraction in aortic stenosis before transcatheter aortic valve implantation (TAVI). Diagn. Interv. Imaging 2021, 102, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Bandula, S.; White, S.K.; Flett, A.S.; Lawrence, D.; Pugliese, F.; Ashworth, M.T.; Punwani, S.; Taylor, S.A.; Moon, J.C. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: Validation against histologic findings. Radiology 2013, 269, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; Bandula, S.; Fontana, M.; White, S.K.; Gilbertson, J.A.; Herrey, A.S.; Gillmore, J.D.; Punwani, S.; Hawkins, P.N.; Taylor, S.A.; et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J. Cardiovasc. Comput. Tomogr. 2015, 9, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Emoto, T.; Nakaura, T.; Kidoh, M.; Utsunomiya, D.; Funama, Y.; Nagayama, Y.; Takashio, S.; Ueda, M.; Yamashita, T.; et al. Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiol. Cardiothorac. Imaging 2019, 1, e180003. [Google Scholar] [CrossRef] [PubMed]
- Emoto, T.; Oda, S.; Kidoh, M.; Nakaura, T.; Nagayama, Y.; Sakabe, D.; Kakei, K.; Goto, M.; Funama, Y.; Hatemura, M.; et al. Myocardial Extracellular Volume Quantification Using Cardiac Computed Tomography: A Comparison of the Dual-energy Iodine Method and the Standard Subtraction Method. Acad. Radiol. 2021, 28, e119–e126. [Google Scholar] [CrossRef] [PubMed]
- Nacif, M.S.; Kawel, N.; Lee, J.J.; Chen, X.; Yao, J.; Zavodni, A.; Sibley, C.T.; Lima, J.A.; Liu, S.; Bluemke, D.A. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology 2012, 264, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, X.; Schoepf, U.J.; van Assen, M.; Alimohamed, I.; Griffith, L.P.; Luo, T.; Sun, Z.; Fan, Z.; Xu, L. Extracellular volume quantitation using dual-energy CT in patients with heart failure: Comparison with 3T cardiac MR. Int. J. Cardiol. 2018, 268, 236–240. [Google Scholar] [CrossRef]
- Mergen, V.; Sartoretti, T.; Klotz, E.; Schmidt, B.; Jungblut, L.; Higashigaito, K.; Manka, R.; Euler, A.; Kasel, M.; Eberhard, M.; et al. Extracellular Volume Quantification with Cardiac Late Enhancement Scanning Using Dual-Source Photon-Counting Detector CT. Investig. Radiol. 2022, 57, 406–411. [Google Scholar] [CrossRef]
- Suzuki, M.; Toba, T.; Izawa, Y.; Fujita, H.; Miwa, K.; Takahashi, Y.; Toh, H.; Kawamori, H.; Otake, H.; Tanaka, H.; et al. Prognostic Impact of Myocardial Extracellular Volume Fraction Assessment Using Dual-Energy Computed Tomography in Patients Treated with Aortic Valve Replacement for Severe Aortic Stenosis. J. Am. Heart Assoc. 2021, 10, e020655. [Google Scholar] [CrossRef]
- Aquino, G.J.; O’Doherty, J.; Schoepf, U.J.; Ellison, B.; Byrne, J.; Fink, N.; Zsarnoczay, E.; Wolf, E.V.; Allmendinger, T.; Schmidt, B.; et al. Myocardial Characterization with Extracellular Volume Mapping with a First-Generation Photon-counting Detector CT with MRI Reference. Radiology 2023, 307, e222030. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kitao, S.; Yunaga, H.; Fujii, S.; Mukai, N.; Yamamoto, K.; Ogawa, T. Myocardial Delayed Enhancement CT for the Evaluation of Heart Failure: Comparison to MRI. Radiology 2018, 288, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.W.; Kerl, J.M.; Fischer, N.; Burkhard, T.; Larson, M.C.; Ackermann, H.; Vogl, T.J. Dual-energy CT for the assessment of chronic myocardial infarction in patients with chronic coronary artery disease: Comparison with 3-T MRI. AJR Am. J. Roentgenol. 2010, 195, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006379. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 636–643. [Google Scholar] [CrossRef]
- Hoshino, M.; Yang, S.; Sugiyama, T.; Zhang, J.; Kanaji, Y.; Yamaguchi, M.; Hada, M.; Sumino, Y.; Horie, T.; Nogami, K.; et al. Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. J. Cardiovasc. Comput. Tomogr. 2020, 14, 483–489. [Google Scholar] [CrossRef]
- Yuvaraj, J.; Lin, A.; Nerlekar, N.; Munnur, R.K.; Cameron, J.D.; Dey, D.; Nicholls, S.J.; Wong, D.T.L. Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease. Cells 2021, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dang, Y.; Hu, H.; Ma, S.; Ma, Y.; Wang, K.; Liu, T.; Lu, X.; Hou, Y. Pericoronary adipose tissue attenuation assessed by dual-layer spectral detector computed tomography is a sensitive imaging marker of high-risk plaques. Quant. Imaging Med. Surg. 2021, 11, 2093–2103. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Ma, R.; Fari, R.; van der Harst, P.; De Cecco, C.N.; Stillman, A.E.; Vliegenthart, R.; van Assen, M. Evaluation of pericoronary adipose tissue attenuation on CT. Br. J. Radiol. 2023, 96, 20220885. [Google Scholar] [CrossRef]
- Risch, F.; Schwarz, F.; Braun, F.; Bette, S.; Becker, J.; Scheurig-Muenkler, C.; Kroencke, T.J.; Decker, J.A. Assessment of epicardial adipose tissue on virtual non-contrast images derived from photon-counting detector coronary CTA datasets. Eur. Radiol. 2023, 33, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Ried, E.; Allmendinger, T.; Sartoretti, T.; Higashigaito, K.; Manka, R.; Euler, A.; Alkadhi, H.; Eberhard, M. Epicardial Adipose Tissue Attenuation and Fat Attenuation Index: Phantom Study and In Vivo Measurements with Photon-Counting Detector CT. AJR Am. J. Roentgenol. 2022, 218, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Szomolanyi, P.; Jirak, D.; Materka, A.; Trattnig, S. Effects of MRI acquisition parameter variations and protocol heterogeneity on the results of texture analysis and pattern discrimination: An application-oriented study. Med. Phys. 2009, 36, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Ayx, I.; Tharmaseelan, H.; Hertel, A.; Nörenberg, D.; Overhoff, D.; Rotkopf, L.T.; Riffel, P.; Schoenberg, S.O.; Froelich, M.F. Comparison Study of Myocardial Radiomics Feature Properties on Energy-Integrating and Photon-Counting Detector CT. Diagnostics 2022, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Ayx, I.; Tharmaseelan, H.; Hertel, A.; Norenberg, D.; Overhoff, D.; Rotkopf, L.T.; Riffel, P.; Schoenberg, S.O.; Froelich, M.F. Myocardial Radiomics Texture Features Associated with Increased Coronary Calcium Score-First Results of a Photon-Counting CT. Diagnostics 2022, 12, 1663. [Google Scholar] [CrossRef]
- Dunning, C.A.S.; Rajiah, P.S.; Hsieh, S.S.; Esquivel, A.; Yalon, M.; Weber, N.M.; Gong, H.; Fletcher, J.G.; McCollough, C.H.; Leng, S. Classification of high-risk coronary plaques using radiomic analysis of multi-energy photon-counting-detector computed tomography (PCD-CT) images. Proc. SPIE Int. Soc. Opt. Eng. 2023, 12465, 654–661. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Maffei, E.; Clemente, A.; De Gori, C.; Occhipinti, M.; Positano, V.; Berti, S.; La Grutta, L.; Saba, L.; Cau, R.; et al. Spectral Photon-Counting Computed Tomography: Technical Principles and Applications in the Assessment of Cardiovascular Diseases. J. Clin. Med. 2024, 13, 2359. https://doi.org/10.3390/jcm13082359
Meloni A, Maffei E, Clemente A, De Gori C, Occhipinti M, Positano V, Berti S, La Grutta L, Saba L, Cau R, et al. Spectral Photon-Counting Computed Tomography: Technical Principles and Applications in the Assessment of Cardiovascular Diseases. Journal of Clinical Medicine. 2024; 13(8):2359. https://doi.org/10.3390/jcm13082359
Chicago/Turabian StyleMeloni, Antonella, Erica Maffei, Alberto Clemente, Carmelo De Gori, Mariaelena Occhipinti, Vicenzo Positano, Sergio Berti, Ludovico La Grutta, Luca Saba, Riccardo Cau, and et al. 2024. "Spectral Photon-Counting Computed Tomography: Technical Principles and Applications in the Assessment of Cardiovascular Diseases" Journal of Clinical Medicine 13, no. 8: 2359. https://doi.org/10.3390/jcm13082359












