Neutrophil Extracellular Traps and Respiratory Disease
Abstract
:1. Introduction
2. Lung Immunology
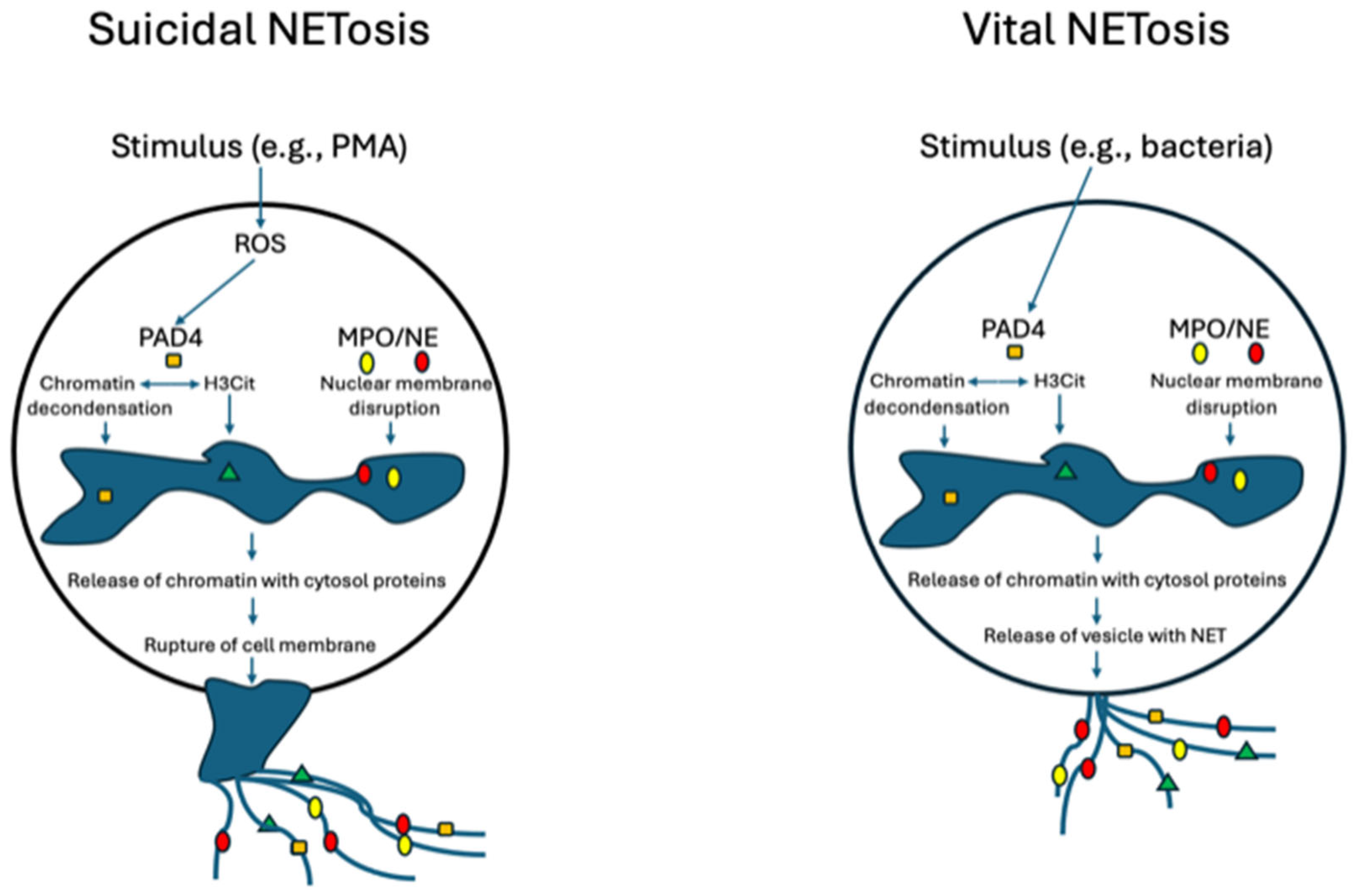
3. Mechanisms of the Formation of Neutrophil Extracellular Traps
4. Triggers of Extracellular Trap Formation
4.1. Bacteria
4.2. Viruses
4.3. Fungi
4.4. Non-Infectious Triggers
5. Detection of Neutrophil Extracellular Traps
6. Specific Respiratory Conditions
6.1. Cystic Fibrosis
6.2. Chronic Obstructive Pulmonary Disease
6.3. Bronchiectasis
6.4. Asthma
6.5. Pneumonia and Acute Respiratory Distress Syndrome (ARDS)
6.6. COVID-19
6.7. Chronic Rhinosinusitis (CRS)
6.8. Lung Cancer
6.9. Interstitial Lung Disease/Pulmonary Fibrosis
7. Treatment
7.1. DNase
7.2. Protease Inhibitors
7.3. Inhibitors of Leukotriene B4 and CXCR2
7.4. Macrolides
7.5. Other Therapies
7.6. Combined and Individually Tailored Therapeutics
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Singh, S.; Segal, L.N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The lung microbiome: Progress and promise. J. Clin. Investig. 2021, 131, e151725. [Google Scholar] [CrossRef]
- Heung, L.J.; Wiesner, D.L.; Wang, K.; Rivera, A.; Hohl, T.M. Immunity to fungi in the lung. Semin. Immunol. 2023, 66, 101728. [Google Scholar] [CrossRef]
- Mettelman, R.C.; Allen, E.K.; Thomas, P.G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 2022, 55, 749–780. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.M.; Caramori, G.; Stefano, A.D. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.E.; Grinstein, S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci. STKE 2007, 2007, pe11. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar66890. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef] [PubMed]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar] [CrossRef] [PubMed]
- Radermecker, C.; Sabatel, C.; Vanwinge, C.; Ruscitti, C.; Marechal, P.; Perin, F.; Schyns, J.; Rocks, N.; Toussaint, M.; Cataldo, D.; et al. Locally instructed CXCR4(hi) neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat. Immunol. 2019, 20, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Kaczmarek, E.; Lee, Y.T.; Tang, I.T.; Isal, B.; Adibnia, Y.; Sandler, N.; Grimm, M.J.; Segal, B.H.; Otterbein, L.E.; et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS ONE 2015, 10, e0120549. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Wang, Y.; Guo, H.; Fan, H.; Zhang, C.; Hou, L.; Liu, Z. Autophagy-driven NETosis is a double-edged sword—Review. Biomed. Pharmacother. 2020, 126, 110065. [Google Scholar] [CrossRef]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm (2020) 2022, 3, e162. [Google Scholar] [CrossRef]
- Denning, N.L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.W.; Jacobs, W.R., Jr. Mycobacterium tuberculosis exploits human interferon gamma to stimulate macrophage extracellular trap formation and necrosis. J. Infect. Dis. 2013, 208, 109–119. [Google Scholar] [CrossRef]
- King, P.T.; Sharma, R.; O’Sullivan, K.; Selemidis, S.; Lim, S.; Radhakrishna, N.; Lo, C.; Prasad, J.; Callaghan, J.; McLaughlin, P.; et al. Nontypeable Haemophilus influenzae induces sustained lung oxidative stress and protease expression. PLoS ONE 2015, 10, e0120371. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.H.; Hawkins, C.L. Role of macrophage extracellular traps in innate immunity and inflammatory disease. Biochem. Soc. Trans. 2022, 50, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Simon, D.; Simon, H.U. Eosinophil extracellular DNA traps: Molecular mechanisms and potential roles in disease. Curr. Opin. Immunol. 2012, 24, 736–739. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, M.; Zhao, R.; Li, Y.; Li, C.; Hou, X.; Sun, B.; Liu, B.; Xiang, M.; Lin, J. Eosinophil extracellular traps in asthma: Implications for pathogenesis and therapy. Respir. Res. 2023, 24, 231. [Google Scholar] [CrossRef]
- Mollerherm, H.; von Kockritz-Blickwede, M.; Branitzki-Heinemann, K. Antimicrobial Activity of Mast Cells: Role and Relevance of Extracellular DNA Traps. Front. Immunol. 2016, 7, 265. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Kuebler, W.M. Significance of Mast Cell Formed Extracellular Traps in Microbial Defense. Clin. Rev. Allergy Immunol. 2022, 62, 160–179. [Google Scholar] [CrossRef]
- Hong, W.; Juneau, R.A.; Pang, B.; Swords, W.E. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J. Innate Immun. 2009, 1, 215–224. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Simpson, A.J.; Aziz, R.K.; Liu, G.Y.; Kristian, S.A.; Kotb, M.; Feramisco, J.; Nizet, V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 2006, 16, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Young, R.L.; Malcolm, K.C.; Kret, J.E.; Caceres, S.M.; Poch, K.R.; Nichols, D.P.; Taylor-Cousar, J.L.; Saavedra, M.T.; Randell, S.H.; Vasil, M.L.; et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: Evidence of acquired resistance within the CF airway, independent of CFTR. PLoS ONE 2011, 6, e23637. [Google Scholar] [CrossRef]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef]
- Keir, H.R.; Shoemark, A.; Dicker, A.J.; Perea, L.; Pollock, J.; Giam, Y.H.; Suarez-Cuartin, G.; Crichton, M.L.; Lonergan, M.; Oriano, M.; et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: An international, observational, multicohort study. Lancet Respir. Med. 2021, 9, 873–884. [Google Scholar] [CrossRef]
- Pattison, S.H.; Gibson, D.S.; Johnston, E.; Peacock, S.; Rivera, K.; Tunney, M.M.; Pappin, D.J.; Elborn, J.S. Proteomic profile of cystic fibrosis sputum cells in adults chronically infected with Pseudomonas aeruginosa. Eur. Respir. J. 2017, 50, 1601569. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aleman, S.; Bustamante, A.E.; Jimenez-Valdes, R.J.; Gonzalez, G.M.; Sanchez-Gonzalez, A. Pseudomonas aeruginosa isolates from cystic fibrosis patients induce neutrophil extracellular traps with different morphologies that could correlate with their disease severity. Int. J. Med. Microbiol. 2020, 310, 151451. [Google Scholar] [CrossRef]
- Grinberg, N.; Elazar, S.; Rosenshine, I.; Shpigel, N.Y. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 2008, 76, 2802–2807. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Almeida, F.S.; Andrade, A.G.; Comberlang, F.C.; Cardoso, L.L.; Vanderley, S.E.R.; Keesen, T.S.L. Mycobacterium tuberculosis in a Trap: The Role of Neutrophil Extracellular Traps in Tuberculosis. Int. J. Mol. Sci. 2023, 24, 1385. [Google Scholar] [CrossRef] [PubMed]
- Beiter, K.; Wartha, F.; Albiger, B.; Normark, S.; Zychlinsky, A.; Henriques-Normark, B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 2006, 16, 401–407. [Google Scholar] [CrossRef]
- Storisteanu, D.M.; Pocock, J.M.; Cowburn, A.S.; Juss, J.K.; Nadesalingam, A.; Nizet, V.; Chilvers, E.R. Evasion of Neutrophil Extracellular Traps by Respiratory Pathogens. Am. J. Respir. Cell Mol. Biol. 2017, 56, 423–431. [Google Scholar] [CrossRef]
- Zafarani, A.; Razizadeh, M.H.; Haghi, A. Neutrophil extracellular traps in influenza infection. Heliyon 2023, 9, e23306. [Google Scholar] [CrossRef]
- Toussaint, M.; Jackson, D.J.; Swieboda, D.; Guedan, A.; Tsourouktsoglou, T.D.; Ching, Y.M.; Radermecker, C.; Makrinioti, H.; Aniscenko, J.; Bartlett, N.W.; et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat. Med. 2017, 23, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; de Jong, R.; Bonsing, J.G.; van Woensel, J.B.M.; Antonis, A.F.G.; Bem, R.A. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax 2018, 73, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetite, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef] [PubMed]
- Narayana Moorthy, A.; Narasaraju, T.; Rai, P.; Perumalsamy, R.; Tan, K.B.; Wang, S.; Engelward, B.; Chow, V.T. In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front. Immunol. 2013, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, L.; Zhang, Y.; Pu, L.; Liu, J.; Li, X.; Chen, Z.; Hao, Y.; Wang, B.; Han, J.; et al. High Level of Neutrophil Extracellular Traps Correlates With Poor Prognosis of Severe Influenza A Infection. J. Infect. Dis. 2018, 217, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Bruns, S.; Kniemeyer, O.; Hasenberg, M.; Aimanianda, V.; Nietzsche, S.; Thywissen, A.; Jeron, A.; Latge, J.P.; Brakhage, A.A.; Gunzer, M. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010, 6, e1000873. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Alflen, A.; Aranda Lopez, P.; Hartmann, A.K.; Maxeiner, J.; Bosmann, M.; Sharma, A.; Platten, J.; Ries, F.; Beckert, H.; Ruf, W.; et al. Neutrophil extracellular traps impair fungal clearance in a mouse model of invasive pulmonary aspergillosis. Immunobiology 2020, 225, 151867. [Google Scholar] [CrossRef]
- Shin, S.H.; Ye, M.K.; Lee, D.W.; Choi, M.H.; Geum, S.Y. Aspergillus Enhances Eosinophil and Neutrophil Extracellular DNA Trap Formation in Chronic Rhinosinusitis. Int. J. Mol. Sci. 2023, 24, 7264. [Google Scholar] [CrossRef]
- Gupta, S.; Kaplan, M.J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 2016, 12, 402–413. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Allam, R.; Kumar, S.V.; Darisipudi, M.N.; Anders, H.J. Extracellular histones in tissue injury and inflammation. J. Mol. Med. 2014, 92, 465–472. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Gamberucci, A.; Lucherini, O.M.; Ali, A.; Simpatico, A.; Lorenzini, S.; Lazzerini, P.E.; Tripodi, S.; Frediani, B.; Selvi, E. Neutrophil extracellular traps release in gout and pseudogout depends on the number of crystals regardless of leukocyte count. Rheumatology 2021, 60, 4920–4928. [Google Scholar] [CrossRef]
- King, P.T.; Sharma, R.; O’Sullivan, K.M.; Callaghan, J.; Dousha, L.; Thomas, B.; Ruwanpura, S.; Lim, S.; Farmer, M.W.; Jennings, B.R.; et al. Deoxyribonuclease 1 reduces pathogenic effects of cigarette smoke exposure in the lung. Sci. Rep. 2017, 7, 12128. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Krautgartner, W.D.; Vitkov, L. Visualization of neutrophil extracellular traps in TEM. Micron 2008, 39, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Manzenreiter, R.; Kienberger, F.; Marcos, V.; Schilcher, K.; Krautgartner, W.D.; Obermayer, A.; Huml, M.; Stoiber, W.; Hector, A.; Griese, M.; et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cyst. Fibros. 2012, 11, 84–92. [Google Scholar] [CrossRef]
- Zharkova, O.; Tay, S.H.; Lee, H.Y.; Shubhita, T.; Ong, W.Y.; Lateef, A.; MacAry, P.A.; Lim, L.H.K.; Connolly, J.E.; Fairhurst, A.M. A Flow Cytometry-Based Assay for High-Throughput Detection and Quantification of Neutrophil Extracellular Traps in Mixed Cell Populations. Cytom. A 2019, 95, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Shimizu, S.; Matsuo, J.; Nishibata, Y.; Kusunoki, Y.; Hattanda, F.; Shida, H.; Nakazawa, D.; Tomaru, U.; Atsumi, T.; et al. Measurement of NET formation in vitro and in vivo by flow cytometry. Cytom. A 2017, 91, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhao, X.; Li, W.; Wang, F.; Tian, J.; Wang, N.; Gao, X.; Zhang, J.; Wu, J.; Mang, G.; et al. Neutrophil extracellular traps: A novel therapeutic target for intracranial hemorrhage. Thromb. Res. 2022, 219, 1–13. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Staab, D.; Zychlinsky, A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS ONE 2011, 6, e28526. [Google Scholar] [CrossRef]
- de Buhr, N.; von Kockritz-Blickwede, M. Detection, Visualization, and Quantification of Neutrophil Extracellular Traps (NETs) and NET Markers. Methods Mol. Biol. 2020, 2087, 425–442. [Google Scholar] [CrossRef]
- Sharma, R.; O’Sullivan, K.M.; Holdsworth, S.R.; Bardin, P.G.; King, P.T. Visualizing Macrophage Extracellular Traps Using Confocal Microscopy. J. Vis. Exp. 2017, 128, e56459. [Google Scholar] [CrossRef]
- Ong, T.; Ramsey, B.W. Cystic Fibrosis: A Review. JAMA 2023, 329, 1859–1871. [Google Scholar] [CrossRef]
- Gray, J.C.; Treloar Padovano, H.; Wemm, S.E.; Miranda, R., Jr. Predictors of Topiramate Tolerability in Heavy Cannabis-Using Adolescents and Young Adults: A Secondary Analysis of a Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Psychopharmacol. 2018, 38, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Reid, P.A.; McAllister, D.A.; Boyd, A.C.; Innes, J.A.; Porteous, D.; Greening, A.P.; Gray, R.D. Measurement of serum calprotectin in stable patients predicts exacerbation and lung function decline in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2015, 191, 233–236. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Dousha, L.; Clarke, N.; Schaefer, J.; Carzino, R.; Sharma, R.; Wan, K.L.; Anantharajah, A.; O’Sullivan, K.; Lu, Z.X.; et al. Phagocyte extracellular traps in children with neutrophilic airway inflammation. ERJ Open Res. 2021, 7, 00883-2020. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, A.S.; Kho, S.; Binks, M.J.; Pizzutto, S.; Chang, A.B.; Beissbarth, J.; Minigo, G.; Marsh, R.L. Extracellular traps are evident in Romanowsky-stained smears of bronchoalveolar lavage from children with non-cystic fibrosis bronchiectasis. Respirology 2023, 28, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Moriceau, S.; Lenoir, G.; Witko-Sarsat, V. In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: Evidence for an innate neutrophil disturbance. J. Innate Immun. 2010, 2, 260–266. [Google Scholar] [CrossRef]
- Gehrig, S.; Duerr, J.; Weitnauer, M.; Wagner, C.J.; Graeber, S.Y.; Schatterny, J.; Hirtz, S.; Belaaouaj, A.; Dalpke, A.H.; Schultz, C.; et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am. J. Respir. Crit. Care Med. 2014, 189, 1082–1092. [Google Scholar] [CrossRef]
- Hogg, J.C.; Senior, R.M. Chronic obstructive pulmonary disease—Part 2: Pathology and biochemistry of emphysema. Thorax 2002, 57, 830–834. [Google Scholar] [CrossRef]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha(1)-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Baltazar-Garcia, E.A.; Vargas-Guerrero, B.; Gasca-Lozano, L.E.; Gurrola-Diaz, C.M. Molecular changes underlying pulmonary emphysema and chronic bronchitis in Chronic Obstructive Pulmonary Disease: An updated review. Histol. Histopathol. 2023, 18699. [Google Scholar] [CrossRef]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.D.; Steinbacher, P.; Winterberg, N.; Bathke, A.C.; Klappacher, M.; Studnicka, M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015, 16, 59. [Google Scholar] [CrossRef]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- King, P.T. The Role of the Immune Response in the Pathogenesis of Bronchiectasis. Biomed. Res. Int. 2018, 2018, 6802637. [Google Scholar] [CrossRef] [PubMed]
- Angrill, J.; Agusti, C.; De Celis, R.; Filella, X.; Rano, A.; Elena, M.; De La Bellacasa, J.P.; Xaubet, A.; Torres, A. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am. J. Respir. Crit. Care Med. 2001, 164, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Smith, M.P.; McHugh, B.J.; Doherty, C.; Govan, J.R.; Hill, A.T. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2012, 186, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Keir, H.R.; Chalmers, J.D. Neutrophil extracellular traps in chronic lung disease: Implications for pathogenesis and therapy. Eur. Respir. Rev. 2022, 31, 210241. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Moffitt, K.L.; Suarez-Cuartin, G.; Sibila, O.; Finch, S.; Furrie, E.; Dicker, A.; Wrobel, K.; Elborn, J.S.; Walker, B.; et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2017, 195, 1384–1393. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2022, 59, 2102730. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Dunican, E.M.; Charbit, A.R.; Raymond, W.; Looney, M.R.; Peters, M.C.; Gordon, E.D.; Woodruff, P.G.; Lefrancais, E.; Phillips, B.R.; et al. Extracellular DNA, Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 1076–1085. [Google Scholar] [CrossRef]
- Wan, R.; Jiang, J.; Hu, C.; Chen, X.; Chen, C.; Zhao, B.; Hu, X.; Zheng, Z.; Li, Y. Neutrophil extracellular traps amplify neutrophil recruitment and inflammation in neutrophilic asthma by stimulating the airway epithelial cells to activate the TLR4/NF-kappaB pathway and secrete chemokines. Aging 2020, 12, 16820–16836. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, J.; Lv, T.; Song, Y. A Narrative Review: The Role of NETs in Acute Respiratory Distress Syndrome/Acute Lung Injury. Int. J. Mol. Sci. 2024, 25, 1464. [Google Scholar] [CrossRef]
- Bianchi, M.; Hakkim, A.; Brinkmann, V.; Siler, U.; Seger, R.A.; Zychlinsky, A.; Reichenbach, J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 2009, 114, 2619–2622. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, S.D.; Kook, M.; Lee, H.Y.; Ghim, J.; Choi, Y.; Zabel, B.A.; Ryu, S.H.; Bae, Y.S. Phospholipase D2 drives mortality in sepsis by inhibiting neutrophil extracellular trap formation and down-regulating CXCR2. J. Exp. Med. 2015, 212, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Paunel-Gorgulu, A.; Flohe, S.; Hoffmann, A.; Witte, I.; MacKenzie, C.; Baldus, S.E.; Windolf, J.; Logters, T.T. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care 2012, 16, R137. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.; Nascimento, D.C.; Sonego, F.; Castanheira, F.V.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, H.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016, 6, 37252. [Google Scholar] [CrossRef]
- McDonald, B.; Davis, R.P.; Kim, S.J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Qu, M.; Yu, Y.; Chen, Z.; Zhu, S.; Guo, K.; Chen, W.; Miao, C. Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury. Front. Cell Infect. Microbiol. 2021, 11, 677902. [Google Scholar] [CrossRef]
- Lefrancais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 2018, 3, e98178. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Giaglis, S.; Hahn, S.; Blum, C.A.; Baumgartner, C.; Kutz, A.; van Breda, S.V.; Mueller, B.; Schuetz, P.; Christ-Crain, M.; et al. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: Secondary analysis of a randomised controlled trial. Eur. Respir. J. 2018, 51, 1701389. [Google Scholar] [CrossRef]
- Pu, S.; Wang, D.; Liu, D.; Zhao, Y.; Qi, D.; He, J.; Zhou, G. Effect of sivelestat sodium in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. BMC Pulm. Med. 2017, 17, 148. [Google Scholar] [CrossRef]
- Reusch, N.; De Domenico, E.; Bonaguro, L.; Schulte-Schrepping, J.; Bassler, K.; Schultze, J.L.; Aschenbrenner, A.C. Neutrophils in COVID-19. Front. Immunol. 2021, 12, 652470. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, A.; Logullo, J.; Menezes, C.C.B.; de Souza Carvalho Giangiarulo, T.C.; Dos Reis, M.C.; de Castro, G.M.M.; da Silva Fontes, Y.; Todeschini, A.R.; Freire-de-Lima, L.; Decote-Ricardo, D.; et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci. Rep. 2020, 10, 19630. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Dassler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol. Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Hussaniy, H.A.; Al-Harcan, N.A.H.; Alexiou, A.; Batiha, G.E. Neutrophil Extracellular Traps (NETs) and COVID-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022, 104, 108516. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yang, J.; Zou, J.; Bi, Z.; He, C.; Lei, H.; He, X.; Li, X.; Alu, A.; Ren, W.; et al. Histones released by NETosis enhance the infectivity of SARS-CoV-2 by bridging the spike protein subunit 2 and sialic acid on host cells. Cell Mol. Immunol. 2022, 19, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.G.; Chau, A.S.; Egeblad, M.; Barnes, B.J.; Janowitz, T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: A case series. Mol. Med. 2020, 26, 91. [Google Scholar] [CrossRef]
- Toma, A.; Darwish, C.; Taylor, M.; Harlacher, J.; Darwish, R. The Use of Dornase Alfa in the Management of COVID-19-Associated Adult Respiratory Distress Syndrome. Crit. Care Res. Pract. 2021, 2021, 8881115. [Google Scholar] [CrossRef]
- Sedaghat, A.R.; Phillips, K.M. Chronic rhinosinusitis disease control: A review of the history and the evidence. Expert Rev. Clin. Immunol. 2023, 19, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Kim, D.W. Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 5034. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Lim, H.S.; Park, J.A.; Jo, A.; Ryu, H.T.; Kim, D.W.; Kim, J.K.; Hong, S.N.; Shin, H.W.; Kim, D.W. Effects of Neutrophil and Eosinophil Extracellular Trap Formation on Refractoriness in Chronic Rhinosinusitis With Nasal Polyps. Allergy Asthma Immunol. Res. 2023, 15, 94–108. [Google Scholar] [CrossRef]
- Hwang, J.W.; Kim, J.H.; Kim, H.J.; Choi, I.H.; Han, H.M.; Lee, K.J.; Kim, T.H.; Lee, S.H. Neutrophil extracellular traps in nasal secretions of patients with stable and exacerbated chronic rhinosinusitis and their contribution to induce chemokine secretion and strengthen the epithelial barrier. Clin. Exp. Allergy 2019, 49, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sima, Y.; Zhao, Y.; Zhang, N.; Zheng, M.; Du, K.; Wang, M.; Wang, Y.; Hao, Y.; Li, Y.; et al. Endotypes of chronic rhinosinusitis based on inflammatory and remodeling factors. J. Allergy Clin. Immunol. 2023, 151, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Delemarre, T.; Bochner, B.S.; Simon, H.U.; Bachert, C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2021, 148, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.Z.; Yao, Y.; Li, J.P.; Chai, N.L.; Linghu, E.Q. The Role of Neutrophil Extracellular Traps in Cancer. Front. Oncol. 2021, 11, 714357. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Kuttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e858. [Google Scholar] [CrossRef]
- Yazdani, H.O.; Roy, E.; Comerci, A.J.; van der Windt, D.J.; Zhang, H.; Huang, H.; Loughran, P.; Shiva, S.; Geller, D.A.; Bartlett, D.L.; et al. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019, 79, 5626–5639. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, D.; Assinger, A. Platelet-Leukocyte Interplay in Cancer Development and Progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Orlichenko, L.; Schapiro, N.E.; Loughran, P.; Gianfrate, G.C.; Ellis, J.T.; Singhi, A.D.; Kang, R.; Tang, D.; Lotze, M.T.; et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015, 22, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L. The pro-tumor effect and the anti-tumor effect of neutrophils extracellular traps. Biosci. Trends 2020, 13, 469–475. [Google Scholar] [CrossRef]
- Triner, D.; Devenport, S.N.; Ramakrishnan, S.K.; Ma, X.; Frieler, R.A.; Greenson, J.K.; Inohara, N.; Nunez, G.; Colacino, J.A.; Mortensen, R.M.; et al. Neutrophils Restrict Tumor-Associated Microbiota to Reduce Growth and Invasion of Colon Tumors in Mice. Gastroenterology 2019, 156, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Y.; Zhang, S.; Zhou, L.; Yan, G.; Wang, Y.; Zhang, M.; Wang, M.; Lin, H.; Tong, Q.; et al. Emodin regulates neutrophil phenotypes to prevent hypercoagulation and lung carcinogenesis. J. Transl. Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial lung diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, M.; Liu, B.; Ma, Z.; Yang, Q. Neutrophil extracellular traps and pulmonary fibrosis: An update. J. Inflamm. 2023, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jia, X.; Zhang, Q.; Zhang, L.; Yang, J.; Hu, C.; Shi, J.; Jiang, X.; Lu, J.; Shen, H. Neutrophil extracellular traps activate lung fibroblast to induce polymyositis-related interstitial lung diseases via TLR9-miR-7-Smad2 pathway. J. Cell Mol. Med. 2020, 24, 1658–1669. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Frangou, E.; Chrysanthopoulou, A.; Mitsios, A.; Kambas, K.; Arelaki, S.; Angelidou, I.; Arampatzioglou, A.; Gakiopoulou, H.; Bertsias, G.K.; Verginis, P.; et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann. Rheum. Dis. 2019, 78, 238–248. [Google Scholar] [CrossRef]
- Suzuki, M.; Ikari, J.; Anazawa, R.; Tanaka, N.; Katsumata, Y.; Shimada, A.; Suzuki, E.; Tatsumi, K. PAD4 Deficiency Improves Bleomycin-induced Neutrophil Extracellular Traps and Fibrosis in Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2020, 63, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Chirivi, R.G.S.; van Rosmalen, J.W.G.; van der Linden, M.; Euler, M.; Schmets, G.; Bogatkevich, G.; Kambas, K.; Hahn, J.; Braster, Q.; Soehnlein, O.; et al. Therapeutic ACPA inhibits NET formation: A potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol. Immunol. 2021, 18, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Latz, E. Intracellular DNA recognition. Nat. Rev. Immunol. 2010, 10, 123–130. [Google Scholar] [CrossRef]
- Konstan, M.W.; Ratjen, F. Effect of dornase alfa on inflammation and lung function: Potential role in the early treatment of cystic fibrosis. J. Cyst. Fibros. 2012, 11, 78–83. [Google Scholar] [CrossRef]
- Thomas, G.M.; Carbo, C.; Curtis, B.R.; Martinod, K.; Mazo, I.B.; Schatzberg, D.; Cifuni, S.M.; Fuchs, T.A.; von Andrian, U.H.; Hartwig, J.H.; et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 2012, 119, 6335–6343. [Google Scholar] [CrossRef]
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 Regulated Poly I:C-Induced Neutrophil Extracellular Traps and Acute Lung Injury Partly Through p38 MAP Kinase. Front. Microbiol. 2018, 9, 3174. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.; Thanabalasuriar, A.; Lee, W.Y.; Sanz, M.J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015, 6, 6673. [Google Scholar] [CrossRef]
- Park, H.H.; Park, W.; Lee, Y.Y.; Kim, H.; Seo, H.S.; Choi, D.W.; Kwon, H.K.; Na, D.H.; Kim, T.H.; Choy, Y.B.; et al. Bioinspired DNase-I-Coated Melanin-Like Nanospheres for Modulation of Infection-Associated NETosis Dysregulation. Adv. Sci. 2021, 8, e2103748. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.E.; Barker, A.F.; Ilowite, J.S.; Fick, R.B. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998, 113, 1329–1334. [Google Scholar] [CrossRef]
- Dubois, A.V.; Gauthier, A.; Brea, D.; Varaigne, F.; Diot, P.; Gauthier, F.; Attucci, S. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am. J. Respir. Cell Mol. Biol. 2012, 47, 80–86. [Google Scholar] [CrossRef]
- The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am. J. Respir. Crit. Care Med. 1998, 158, 49–59. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Carroll, T.P.; Franciosi, A.N.; Sweeney, J.; Hobbs, B.D.; Kowlessar, V.; Gunaratnam, C.; Reeves, E.P.; McElvaney, N.G. Consequences of Abrupt Cessation of Alpha(1)-Antitrypsin Replacement Therapy. N. Engl. J. Med. 2020, 382, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Louttit, C.; Fry, C.; Najafabadi, A.H.; Han, K.; Nemzek, J.; Moon, J.J. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 2020, 238, 119836. [Google Scholar] [CrossRef]
- Elborn, J.S.; Perrett, J.; Forsman-Semb, K.; Marks-Konczalik, J.; Gunawardena, K.; Entwistle, N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur. Respir. J. 2012, 40, 969–976. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Aquino, T.O.; O’Brien, C.D.; Perrett, J.; Gunawardena, K.A. A randomised, placebo-controlled, dose-finding study of AZD9668, an oral inhibitor of neutrophil elastase, in patients with chronic obstructive pulmonary disease treated with tiotropium. COPD 2012, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Stockley, R.; De Soyza, A.; Gunawardena, K.; Perrett, J.; Forsman-Semb, K.; Entwistle, N.; Snell, N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 2013, 107, 524–533. [Google Scholar] [CrossRef]
- Watz, H.; Nagelschmitz, J.; Kirsten, A.; Pedersen, F.; van der Mey, D.; Schwers, S.; Bandel, T.J.; Rabe, K.F. Safety and efficacy of the human neutrophil elastase inhibitor BAY 85-8501 for the treatment of non-cystic fibrosis bronchiectasis: A randomized controlled trial. Pulm. Pharmacol. Ther. 2019, 56, 86–93. [Google Scholar] [CrossRef]
- Palmer, R.; Maenpaa, J.; Jauhiainen, A.; Larsson, B.; Mo, J.; Russell, M.; Root, J.; Prothon, S.; Chialda, L.; Forte, P.; et al. Dipeptidyl Peptidase 1 Inhibitor AZD7986 Induces a Sustained, Exposure-Dependent Reduction in Neutrophil Elastase Activity in Healthy Subjects. Clin. Pharmacol. Ther. 2018, 104, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Haworth, C.S.; Metersky, M.L.; Loebinger, M.R.; Blasi, F.; Sibila, O.; O’Donnell, A.E.; Sullivan, E.J.; Mange, K.C.; Fernandez, C.; et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N. Engl. J. Med. 2020, 383, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Gompertz, S.; Stockley, R.A. A randomized, placebo-controlled trial of a leukotriene synthesis inhibitor in patients with COPD. Chest 2002, 122, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Doring, G.; Heltshe, S.L.; Lands, L.C.; Hilliard, K.A.; Koker, P.; Bhattacharya, S.; Staab, A.; Hamilton, A. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Waschki, B.; Marwitz, S.; Goldmann, T.; Kirsten, A.; Malmgren, A.; Rabe, K.F.; Uddin, M.; Watz, H. Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur. Respir. J. 2018, 51, 1700970. [Google Scholar] [CrossRef]
- De Soyza, A.; Pavord, I.; Elborn, J.S.; Smith, D.; Wray, H.; Puu, M.; Larsson, B.; Stockley, R. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur. Respir. J. 2015, 46, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Allegretti, M.; Ley, K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br. J. Pharmacol. 2008, 155, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Leunig, A.; Pekayvaz, K.; Popp, O.; Joppich, M.; Polewka, V.; Escaig, R.; Anjum, A.; Hoffknecht, M.L.; Gold, C.; et al. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight 2021, 6, e150862. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Lazaar, A.L.; Miller, B.E.; Tabberer, M.; Yonchuk, J.; Leidy, N.; Ambery, C.; Bloomer, J.; Watz, H.; Tal-Singer, R. Effect of the CXCR2 antagonist danirixin on symptoms and health status in COPD. Eur. Respir. J. 2018, 52, 1801020. [Google Scholar] [CrossRef]
- Bystrzycka, W.; Manda-Handzlik, A.; Sieczkowska, S.; Moskalik, A.; Demkow, U.; Ciepiela, O. Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. Int. J. Mol. Sci. 2017, 18, 2666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiu, S.L.; Tang, Q.Y.; Zhou, X.; Zhang, J.Q.; He, Z.Y.; Bai, J.; Li, M.H.; Deng, J.M.; Liang, Y.; et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis. 2019, 10, 678. [Google Scholar] [CrossRef]
- Shukla, S.D.; Taylor, S.L.; Gibson, P.G.; Barker, D.; Upham, J.W.; Yang, I.A.; Reynolds, P.N.; Hodge, S.; James, A.L.; Rogers, G.B.; et al. Add-on azithromycin reduces sputum cytokines in non-eosinophilic asthma: An AMAZES substudy. Thorax 2021, 76, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, Z.; Han, Z.; Wang, J.; Zhang, X.; Wang, Y.; Liu, Q.; Yang, Z. Neutrophil extracellular traps promote cadmium chloride-induced lung injury in mice. Environ. Pollut. 2019, 254, 113021. [Google Scholar] [CrossRef]
- Bonilla, M.C.; Quiros, O.N.; Wendt, M.; Hennig-Pauka, I.; Morgelin, M.; von Kockritz-Blickwede, M.; de Buhr, N. New Insights into Neutrophil Extracellular Trap (NETs) Formation from Porcine Neutrophils in Response to Bacterial Infections. Int. J. Mol. Sci. 2022, 23, 8953. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Ailiyaer, Y.; Liu, R.; Zhang, Y.; Li, C.; Liu, M.; Wang, X.; Jing, L.; Li, Y. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): A randomized controlled trial. Respir. Res. 2019, 20, 73. [Google Scholar] [CrossRef]
- Jayaram, L.; King, P.T.; Hunt, J.; Lim, M.; Park, C.; Hu, E.; Dousha, L.; Ha, P.; Bartlett, J.B.; Southcott, A.M.; et al. Evaluation of high dose N- Acetylcysteine on airway inflammation and quality of life outcomes in adults with bronchiectasis: A randomised placebo-controlled pilot study. Pulm. Pharmacol. Ther. 2023, 84, 102283. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef]
- Usman, A.; Bliden, K.P.; Cho, A.; Walia, N.; Jerjian, C.; Singh, A.; Kundan, P.; Duhan, S.; Tantry, U.S.; Gurbel, P.A. Metformin use in patients hospitalized with COVID-19: Lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. J. Thromb. Thrombolysis 2022, 53, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Biron, B.M.; Chung, C.S.; O’Brien, X.M.; Chen, Y.; Reichner, J.S.; Ayala, A. Cl-Amidine Prevents Histone 3 Citrullination and Neutrophil Extracellular Trap Formation, and Improves Survival in a Murine Sepsis Model. J. Innate Immun. 2017, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, Y.; Li, J.; Huang, J.; Zhang, L.; Feng, J.; Li, J.; Xia, Q.; Zhao, Q.; Huang, L.; et al. Eosinophil extracellular traps drive asthma progression through neuro-immune signals. Nat. Cell Biol. 2021, 23, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; Wang, F.; Guo, X.; Liu, T.; Zhao, Y.; Gu, B.; Chen, H.; Li, Y. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin. Immunol. 2020, 216, 108461. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Bobba, R.; Su, J.; Shariati-Sarabi, Z.; Gladman, D.D.; Urowitz, M.; Lou, W.; Fortin, P.R. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Li, H.; Qiao, C.; Zhao, L.; Jing, Q.; Xue, D.; Zhang, Y. Epigallocatechin-3-gallate reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J. Leukoc. Biol. 2022, 112, 1427–1443. [Google Scholar] [CrossRef]
- Ali, R.A.; Gandhi, A.A.; Dai, L.; Weiner, J.; Estes, S.K.; Yalavarthi, S.; Gockman, K.; Sun, D.; Knight, J.S. Antineutrophil properties of natural gingerols in models of lupus. JCI Insight 2021, 6, e138385. [Google Scholar] [CrossRef]

| Infectious | Non-Infectious |
|---|---|
| Bacterial S. aureus, H. influenzae, Strep spp., P. aeruginosa, M. tuberculosis Viral Influenza, RV, RSV, SARS-CoV-2 Fungal A. Fumigatus, C. Albicans | Chemical Mitogens like PMA Autoimmunity/antibodies SLE, rheumatoid arthritis, vasculitis Cancer Other Gout crystals, cigarette smoke |
| Target | Therapy | Effect |
|---|---|---|
| DNA | DNase |
|
| Neutrophil elastase | AAT |
|
| Other direct NE inhibitors (GW311616A) |
| |
| DDP1 inhibition |
| |
| Oxidative burst/ROS | NAC |
|
| PAD 4 | PAD4 knockout mice |
|
| PAD4 inhibitors |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, P.T.; Dousha, L. Neutrophil Extracellular Traps and Respiratory Disease. J. Clin. Med. 2024, 13, 2390. https://doi.org/10.3390/jcm13082390
King PT, Dousha L. Neutrophil Extracellular Traps and Respiratory Disease. Journal of Clinical Medicine. 2024; 13(8):2390. https://doi.org/10.3390/jcm13082390
Chicago/Turabian StyleKing, Paul T., and Lovisa Dousha. 2024. "Neutrophil Extracellular Traps and Respiratory Disease" Journal of Clinical Medicine 13, no. 8: 2390. https://doi.org/10.3390/jcm13082390
APA StyleKing, P. T., & Dousha, L. (2024). Neutrophil Extracellular Traps and Respiratory Disease. Journal of Clinical Medicine, 13(8), 2390. https://doi.org/10.3390/jcm13082390





