Advancing the Clinical and Molecular Understanding of Cornelia de Lange Syndrome: A Multidisciplinary Pediatric Case Series and Review of the Literature
Abstract
1. Introduction
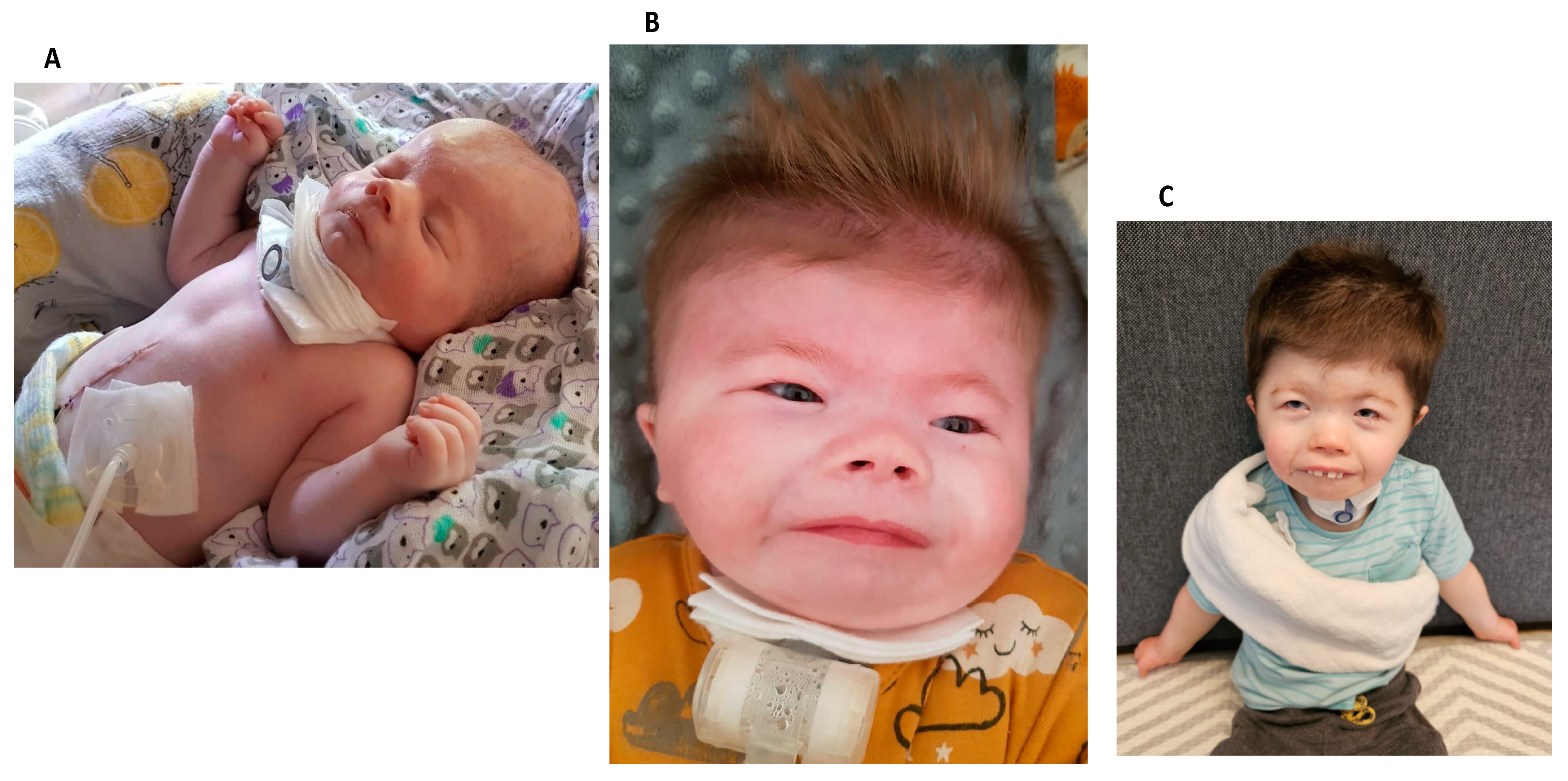
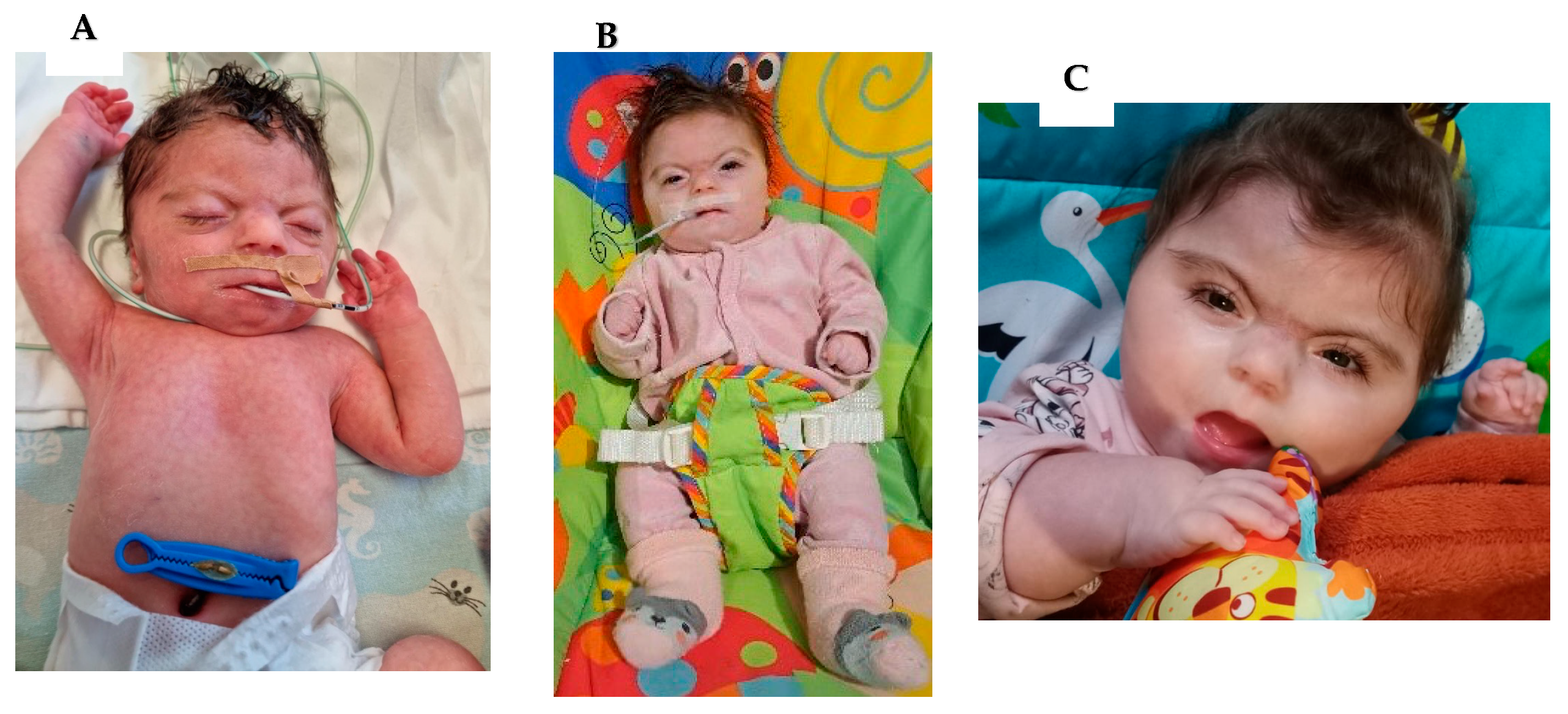
2. Case Series
- Case 1:
- Case 2:
- Case 3:
- Case 4:
3. Discussion
3.1. Clinical Aspects of Cornelia de Lange Syndrome
3.1.1. Facial Gestalt, Malformations, Behavioral Problems
3.1.2. Perioperative Care
3.1.3. Adolescent and Adult CdLS Problems
3.1.4. Carer and Family of a CDLS Patient
3.2. Deciphering the Genetic Puzzle: Advancements in Understanding Cornelia de Lange Syndrome
3.2.1. Cohesin Complex
3.2.2. Genotype–Phenotype Correlation
3.2.3. Molecular Diagnostics
3.2.4. Mosaicism in CDLs
3.2.5. Epigenetics
3.3. Prenatal Diagnosis
3.4. Differential Diagnosis
3.5. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CdLS | Cornelia de Lange syndrome |
| FGR | fetal growth restriction |
| PEG-PEJ | percutaneous endoscopic gastrostomy–jejunostomy |
| GERD | gastroesophageal reflux |
| VSD | ventricular septal defect |
| aCGH | array comparative genomic hybridization |
| NGS | next-generation sequencing |
| MLPA | multiplex ligation-dependent probe amplification |
| WES | whole-exome sequencing |
| WGS | whole-genome sequencing |
| TFU | transfontanelle ultrasound |
| TMJ | temporomandibular joint |
| TTE | transthoracic echocardiography |
| PEG | percutaneous endoscopic gastrostomy |
| SIB | self-injurious behavior |
| ADHD | attention deficit hyperactivity disorder |
| PACU | post-anesthesia care unit |
| CNV | copy number variation |
| SNPs | single nucleotide polymorphisms |
| CPs | chromatinopathies |
References
- Kline, A.D.; Moss, J.F.; Selicorni, A.; Bisgaard, A.-M.; Deardorff, M.A.; Gillett, P.M.; Ishman, S.L.; Kerr, L.M.; Levin, A.V.; Mulder, P.A.; et al. Diagnosis and management of Cornelia de Lange syndrome: First international consensus statement. Nat. Rev. Genet. 2018, 19, 649–666. [Google Scholar] [CrossRef]
- de Lange, C. Syndrome 1; CDLS1. Available online: https://www.omim.org/entry/122470 (accessed on 20 December 2023).
- Sarogni, P.; Pallotta, M.M.; Musio, A. Cornelia de Lange syndrome: From molecular diagnosis to therapeutic approach. J. Med. Genet. 2020, 57, 289–295. [Google Scholar] [CrossRef]
- Selicorni, A.; Mariani, M.; Lettieri, A.; Massa, V. Cornelia de Lange Syndrome: From a Disease to a Broader Spectrum. Genes. 2021, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Gerton, J.L. Cohesinopathies, gene expression, and chromatin organization. J. Cell Biol. 2010, 189, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mfarej, M.G.; Hyland, C.A.; Sanchez, A.C.; Falk, M.M.; Iovine, M.K.; Skibbens, R.V. Cohesin: An emerging master regulator at the heart of cardiac development. Mol. Biol. Cell 2023, 34, rs2. [Google Scholar] [CrossRef]
- Kaur, M.; Blair, J.; Devkota, B.; Fortunato, S.; Clark, D.; Lawrence, A.; Kim, J.; Do, W.; Semeo, B.; Katz, O.; et al. Genomic analyses in Cornelia de Lange Syndrome and related diagnoses: Novel candidate genes, genotype–phenotype correlations and common mechanisms. Am. J. Med. Genet. Part A 2023, 191, 2113–2131. [Google Scholar] [CrossRef]
- Izumi, K. Disorders of Transcriptional Regulation: An Emerging Category of Multiple Malformation Syndromes. Mol. Syndr. 2016, 7, 262–273. [Google Scholar] [CrossRef]
- Rohatgi, S.; Clark, D.; Kline, A.D.; Jackson, L.G.; Pie, J.; Siu, V.; Ramos, F.J.; Krantz, I.D.; Deardorff, M.A. Facial diagnosis of mild and variant CdLS: Insights from a dysmorphologist survey. Am. J. Med. Genet. Part A 2010, 152, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Kline, A.D.; Barr, M.A.; Koch, S. de Lange syndrome: A clinical review of 310 individuals. Am. J. Med. Genet. 1993, 47, 940–946. [Google Scholar] [CrossRef]
- Collis, L.; Moss, J.; Jutley, J.; Cornish, K.; Oliver, C. Facial expression of affect in children with Cornelia de Lange syndrome. J. Intellect. Disabil. Res. 2008, 52, 207–215. [Google Scholar] [CrossRef]
- Cereda, A.; Mariani, M.; Rebora, P.; Sajeva, A.; Ajmone, P.F.; Gervasini, C.; Russo, S.; Kullmann, G.; Valsecchi, G.; Selicorni, A. A new prognostic index of severity of intellectual disabilities in Cornelia de Lange syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, L.; Porras, A.R.; Kruszka, P.; Davis, B.; Hu, T.; Honey, E.; Badoe, E.; Thong, M.; Leon, E.; Girisha, K.M.; et al. Cornelia de Lange syndrome in diverse populations. Am. J. Med. Genet. Part A 2019, 179, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Vergano, S.A.S.; Deardorff, M.; Aggarwal, S.; Barot, A.; Johnson, D.M.; Miller, N.F.; Noon, S.E.; Kaur, M.; Jackson, L.; et al. Characterization of limb differences in children with Cornelia de Lange Syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, M.A.; Noon, S.E.; Krantz, I.D. Cornelia de Lange Syndrome. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1104/ (accessed on 10 December 2023).
- Luzzani, S.; Macchini, F.; Valadè, A.; Milani, D.; Selicorni, A. Gastroesophageal reflux and Cornelia de Lange syndrome: Typical and atypical symptoms. Am. J. Med. Genet. Part A 2003, 119, 283–287. [Google Scholar] [CrossRef]
- Gillis, L.A.; McCallum, J.; Kaur, M.; DeScipio, C.; Yaeger, D.; Mariani, A.; Kline, A.D.; Li, H.-H.; Devoto, M.; Jackson, L.G.; et al. NIPBL Mutational Analysis in 120 Individuals with Cornelia de Lange Syndrome and Evaluation of Genotype-Phenotype Correlations. Am. J. Hum. Genet. 2004, 75, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Clermidi, P.; Abadie, V.; Campeotto, F.; Irtan, S. Sigmoid Volvulus: An Underestimated Cause of Intestinal Obstruction in Cornelia de Lange Syndrome. J. Pediatr. 2015, 167, 941. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Oliver, C.; Wittkowski, A.; Moss, J.; Hare, D. Attenuated behaviour in Cornelia de Lange and fragile X syndromes. J. Intellect. Disabil. Res. 2018, 62, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Penhallow, J.; Ansari, M.; Barton, S.; Bourn, D.; FitzPatrick, D.R.; Goodship, J.; Hammond, P.; Roberts, C.; Welham, A.; et al. Genotype–phenotype correlations in Cornelia de Lange syndrome: Behavioral characteristics and changes with age. Am. J. Med. Genet. Part A 2017, 173, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Grados, M.A.; Alvi, M.H.; Srivastava, S. Behavioral and psychiatric manifestations in Cornelia de Lange syndrome. Curr. Opin. Psychiatry 2017, 30, 92–96. [Google Scholar] [CrossRef]
- Moss, J.; Nelson, L.; Powis, L.; Waite, J.; Richards, C.; Oliver, C. A Comparative Study of Sociability in Angelman, Cornelia de Lange, Fragile X, Down and Rubinstein Taybi Syndromes and Autism Spectrum Disorder. Am. J. Intellect. Dev. Disabil. 2016, 121, 465–486. [Google Scholar] [CrossRef]
- Stevic, M.; Milojevic, I.; Bokun, Z.; Simic, D. Unpredictable drug reaction in a child with Cornelia de Lange syndrome. Int. J. Clin. Pharm. 2015, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A.; Scaravilli, V.; Ciceri, V.; Bosatra, M.; Giannatelli, F.; Ateniese, B.; Mariani, M.; Cereda, A.; Sosio, S.; Zanella, A.; et al. Sedation and general anesthesia for patients with Cornelia De Lange syndrome: A case series. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.orphananesthesia.eu/en/rare-diseases/published-guidelines/cornelia-de-lange-syndrome/285-cornelia-de-lange-syndrome/file.html (accessed on 22 December 2023).
- Available online: https://www.cdlsworld.org/xwiki/bin/download/cdlsPublications/medicalPassport/worldCarecard2018.pdf?rev=1.1 (accessed on 22 December 2023).
- Nelson, L.; Moss, J.; Oliver, C. A Longitudinal Follow-Up Study of Affect in Children and Adults With Cornelia de Lange Syndrome. Am. J. Intellect. Dev. Disabil. 2014, 119, 235–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wierzba, J.; Wierzba, T.; Mazurkiewicz-Bełdzińska, M.; Szyca, R.; Kozłowski, J.; Banach, P.; Potaż, P.; Limon, J. Dorosły z Rzadkim Schorzeniem Genetycznym—Diagnostyka i Terapia Zespołu Cornelii de Lange. Forum Med. Rodz. 2010, 4, 273–280. [Google Scholar]
- Mariani, M.; Decimi, V.; Bettini, L.R.; Maitz, S.; Gervasini, C.; Masciadri, M.; Ajmone, P.; Kullman, G.; Dinelli, M.; Panceri, R.; et al. Adolescents and adults affected by Cornelia de Lange syndrome: A report of 73 Italian patients. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, M.M.; Di Nardo, M.; Hennekam, R.C.M.; Kaiser, F.J.; Parenti, I.; Pié, J.; Ramos, F.J.; Kline, A.D.; Musio, A. Cornelia de Lange syndrome and cancer: An open question. Am. J. Med. Genet. Part A 2023, 191, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Janek, K.C.; Smith, D.F.; Kline, A.D.; Benke, J.R.; Chen, M.-L.; Kimball, A.; Ishman, S.L. Improvement in hearing loss over time in Cornelia de Lange syndrome. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Levin, A.V. Ophthalmologic findings in the Cornelia de Lange syndrome. Ophthalmic Genet. 2019, 40, 1–6. [Google Scholar] [CrossRef]
- Avgitidou, G.; Cursiefen, C.; Heindl, L. OOphthalmological manifestations of Cornelia de Lange syndrome: Case report and review of the literature. Ophthalmologe. 2015, 112, 455–458. [Google Scholar] [CrossRef]
- Zambrelli, E.; Fossati, C.; Turner, K.; Taiana, M.; Vignoli, A.; Gervasini, C.; Russo, S.; Furia, F.; Masciadri, M.; Ajmone, P.; et al. Sleep disorders in Cornelia de Lange syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 214–221. [Google Scholar] [CrossRef]
- Kline, A.D.; Grados, M.; Sponseller, P.; Levy, H.P.; Blagowidow, N.; Schoedel, C.; Rampolla, J.; Clemens, D.K.; Krantz, I.; Kimball, A.; et al. Natural history of aging in Cornelia de Lange syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2007, 145, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Wierzba, J.; Mazurkiewicz-Bełdzińska, M.; Jabłońska-Brudło, J.; Potaż, P.; Banach, P. Challenges of caring for a patient with a rare disease--as demonstrated by Cornelia de Lange Syndrome. Dev. Period. Med. 2015, 19, 511–515. [Google Scholar] [PubMed]
- Cacioppo, C.N.; Conway, L.J.; Mehta, D.; Krantz, I.D.; Noon, S.E. Attitudes about the use of internet support groups and the impact among parents of children with Cornelia de Lange syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdlsworld.org/xwiki/bin/view/Main/ (accessed on 22 December 2023).
- January, K.; Conway, L.J.; Deardorff, M.; Harrington, A.; Krantz, I.D.; Loomes, K.; Pipan, M.; Noon, S.E. Benefits and limitations of a multidisciplinary approach to individualized management of Cornelia de Lange syndrome and related diagnoses. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wulffaert, J.; van Berckelaer-Onnes, I.; Kroonenberg, P.; Scholte, E.; Bhuiyan, Z.; Hennekam, R. Simultaneous analysis of the behavioural phenotype, physical factors, and parenting stress in people with Cornelia de Lange syndrome. J. Intellect. Disabil. Res. 2009, 53, 604–619. [Google Scholar] [CrossRef]
- Avagliano, L.; Parenti, I.; Grazioli, P.; Di Fede, E.; Parodi, C.; Mariani, M.; Kaiser, F.J.; Selicorni, A.; Gervasini, C.; Massa, V. Chromatinopathies: A focus on Cornelia de Lange syndrome. Clin. Genet. 2020, 97, 3–11. [Google Scholar] [CrossRef]
- Meisenberg, C.; Pinder, S.I.; Hopkins, S.R.; Wooller, S.K.; Benstead-Hume, G.; Pearl, F.M.G.; Jeggo, P.A.; Downs, J.A. Repression of Transcription at DNA Breaks Requires Cohesin throughout Interphase and Prevents Genome Instability. Mol. Cell 2019, 73, 212–223.e7. [Google Scholar] [CrossRef]
- Litwin, I.; Pilarczyk, E.; Wysocki, R. The Emerging Role of Cohesin in the DNA Damage Response. Genes 2018, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, W.; Abdennur, N.; Goloborodko, A.; Pekowska, A.; Fudenberg, G.; Loe-Mie, Y.; Fonseca, N.A.; Huber, W.; Haering, C.H.; Mirny, L.; et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature 2017, 551, 51–56. [Google Scholar] [CrossRef]
- Bose, T.; Lee, K.K.; Lu, S.; Xu, B.; Harris, B.; Slaughter, B.; Unruh, J.; Garrett, A.; McDowell, W.; Box, A.; et al. Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells. PLoS Genet. 2012, 8, e1002749. [Google Scholar] [CrossRef]
- Makrantoni, V.; Marston, A.L. Cohesin and chromosome segregation. Curr. Biol. 2018, 28, R688–R693. [Google Scholar] [CrossRef] [PubMed]
- Piché, J.; Van Vliet, P.P.; Pucéat, M.; Andelfinger, G. The expanding phenotypes of cohesinopathies: One ring to rule them all! Cell Cycle 2019, 18, 2828–2848. [Google Scholar] [CrossRef] [PubMed]
- Olley, G.; Ansari, M.; Bengani, H.; Grimes, G.R.; Rhodes, J.; von Kriegsheim, A.; Blatnik, A.; Stewart, F.J.; Wakeling, E.; Carroll, N.; et al. BRD4 interacts with NIPBL and BRD4 is mutated in a Cornelia de Lange–like syndrome. Nat. Genet. 2018, 50, 329–332. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Wilde, J.J.; Albrecht, M.; Dickinson, E.; Tennstedt, S.; Braunholz, D.; Mönnich, M.; Yan, Y.; Xu, W.; Gil-Rodríguez, M.C.; et al. RAD21 Mutations Cause a Human Cohesinopathy. Am. J. Hum. Genet. 2012, 90, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Musio, A. The multiple facets of the SMC1A gene. Gene 2020, 743, 144612. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, H.; Chen, R. Phenotypes of Cornelia de Lange syndrome caused by non-cohesion genes: Novel variants and literature review. Front. Pediatr. 2022, 10, 940294. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Gervasini, C.; Pozojevic, J.; Graul-Neumann, L.; Azzollini, J.; Braunholz, D.; Watrin, E.; Wendt, K.S.; Cereda, A.; Cittaro, D.; et al. Broadening of cohesinopathies: Exome sequencing identifies mutations in ANKRD11 in two patients with Cornelia de Lange-overlapping phenotype. Clin. Genet. 2016, 89, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Pehlivan, D.; Karaca, E.; Patel, N.; Charng, W.-L.; Gambin, T.; Gonzaga-Jauregui, C.; Sutton, V.R.; Yesil, G.; Bozdogan, S.T.; et al. Global transcriptional disturbances underlie Cornelia de Lange syndrome and related phenotypes. J. Clin. Investig. 2015, 125, 636–651. [Google Scholar] [CrossRef]
- Mannini, L.; Lamaze, F.C.; Cucco, F.; Amato, C.; Quarantotti, V.; Rizzo, I.M.; Krantz, I.D.; Bilodeau, S.; Musio, A. Mutant cohesin affects RNA polymerase II regulation in Cornelia de Lange syndrome. Sci. Rep. 2015, 5, 16803. [Google Scholar] [CrossRef]
- Musio, A.; Selicorni, A.; Focarelli, M.L.; Gervasini, C.; Milani, D.; Russo, S.; Vezzoni, P.; Larizza, L. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat. Genet. 2006, 38, 528–530. [Google Scholar] [CrossRef]
- Huisman, S.; Mulder, P.A.; Redeker, E.; Bader, I.; Bisgaard, A.-M.; Brooks, A.; Cereda, A.; Cinca, C.; Clark, D.; Cormier-Daire, V.; et al. Phenotypes and genotypes in individuals with SMC1A variants. Am. J. Med. Genet. Part A 2017, 173, 2108–2125. [Google Scholar] [CrossRef] [PubMed]
- Krantz, I.D.; McCallum, J.; DeScipio, C.; Kaur, M.; Gillis, L.A.; Yaeger, D.; Jukofsky, L.; Wasserman, N.; Bottani, A.; Morris, C.A.; et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 2004, 36, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Selicorni, A.; Russo, S.; Gervasini, C.; Castronovo, P.; Milani, D.; Cavalleri, F.; Bentivegna, A.; Masciadri, M.; Domi, A.; Divizia, M.; et al. Clinical score of 62 Italian patients with Cornelia de Lange syndrome and correlations with the presence and type of NIPBL mutation. Clin. Genet. 2007, 72, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Nizon, M.; Henry, M.; Michot, C.; Baumann, C.; Bazin, A.; Bessières, B.; Blesson, S.; Cordier-Alex, M.-P.; David, A.; Delahaye-Duriez, A.; et al. A series of 38 novel germline and somatic mutations of NIPBL in Cornelia de Lange syndrome. Clin. Genet. 2016, 89, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Symonds, J.D.; Joss, S.; Metcalfe, K.A.; Somarathi, S.; Cruden, J.; Devlin, A.M.; Donaldson, A.; DiDonato, N.; Fitzpatrick, D.; Kaiser, F.J.; et al. Heterozygous truncation mutations of the SMC1A gene cause a severe early onset epilepsy with cluster seizures in females: Detailed phenotyping of 10 new cases. Epilepsia 2017, 58, 565–575. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Kaur, M.; Yaeger, D.; Rampuria, A.; Korolev, S.; Pie, J.; Gil-Rodríguez, C.; Arnedo, M.; Loeys, B.; Kline, A.D.; et al. Mutations in Cohesin Complex Members SMC3 and SMC1A Cause a Mild Variant of Cornelia de Lange Syndrome with Predominant Mental Retardation. Am. J. Hum. Genet. 2007, 80, 485–494. [Google Scholar] [CrossRef]
- Gil-Rodríguez, M.C.; Deardorff, M.A.; Ansari, M.; Tan, C.A.; Parenti, I.; Baquero-Montoya, C.; Ousager, L.B.; Puisac, B.; Hernández-Marcos, M.; Teresa-Rodrigo, M.E.; et al. De Novo Heterozygous Mutations in SMC3 Cause a Range of Cornelia de Lange Syndrome-Overlapping Phenotypes. Hum. Mutat. 2015, 36, 454–462. [Google Scholar] [CrossRef]
- De Falco, A.; De Brasi, D.; Della Monica, M.; Cesario, C.; Petrocchi, S.; Novelli, A.; D’alterio, G.; Iolascon, A.; Capasso, M.; Piscopo, C. A Novel Variant in RAD21 in Cornelia De Lange Syndrome Type 4: Case Report and Bioinformatic Analysis. Genes 2023, 14, 119. [Google Scholar] [CrossRef]
- Kaiser, F.J.; Ansari, M.; Braunholz, D.; Decroos, C.; Wilde, J.J.; Fincher, C.T.; Kaur, M.; Bando, M.; Amor, D.J.; Atwal, P.S.; et al. Loss-of-function HDAC8 mutations cause a phenotypic spectrum of Cornelia de Lange syndrome-like features, ocular hypertelorism, large fontanelle and X-linked inheritance. Hum. Mol. Genet. 2014, 23, 2888–2900. [Google Scholar] [CrossRef]
- Parenti, I.; Gervasini, C.; Pozojevic, J.; Wendt, K.; Watrin, E.; Azzollini, J.; Braunholz, D.; Buiting, K.; Cereda, A.; Engels, H.; et al. Expanding the clinical spectrum of the ‘HDAC8-phenotype’—Implications for molecular diagnostics, counseling and risk prediction. Clin. Genet. 2016, 89, 564–573. [Google Scholar] [CrossRef]
- Whitehead, M.T.; Nagaraj, U.D.; Pearl, P.L. Neuroimaging features of Cornelia de Lange syndrome. Pediatr. Radiol. 2015, 45, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Mallozzi, M.B.; Hüning, I.; Gervasini, C.; Kuechler, A.; Agolini, E.; Albrecht, B.; Baquero-Montoya, C.; Bohring, A.; Bramswig, N.C.; et al. ANKRD11 variants: KBG syndrome and beyond. Clin. Genet. 2021, 100, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Diab, F.; Gil, S.R.; Mulugeta, E.; Casa, V.; Berutti, R.; Brouwer, R.W.; Dupé, V.; Eckhold, J.; Graf, E.; et al. MAU2 and NIPBL Variants Impair the Heterodimerization of the Cohesin Loader Subunits and Cause Cornelia de Lange Syndrome. Cell Rep. 2020, 31, 107647. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhu, Y.; Wu, L.; Deng, F. Clinical study and genetic analysis of Cornelia de Lange syndrome caused by a novel MAU2 gene variant in a Chinese boy. Mol. Genet. Genom. Med. 2024, 12, e2318. [Google Scholar] [CrossRef]
- Huisman, S.A.; Redeker, E.J.W.; Maas, S.M.; Mannens, M.M.; Hennekam, R.C.M. High rate of mosaicism in individuals with Cornelia de Lange syndrome. J. Med. Genet. 2013, 50, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Poke, G.; Ferry, Q.; Williamson, K.; Aldridge, R.; Meynert, A.M.; Bengani, H.; Chan, C.Y.; Kayserili, H.; Avci, Ş.; et al. Genetic heterogeneity in Cornelia de Lange syndrome (CdLS) and CdLS-like phenotypes with observed and predicted levels of mosaicism. J. Med. Genet. 2014, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Pozojevic, J.; Parenti, I.; Graul-Neumann, L.; Ruiz Gil, S.; Watrin, E.; Wendt, K.S.; Werner, R.; Strom, T.M.; Gillessen-Kaesbach, G.; Kaiser, F.J. Novel mosaic variants in two patients with Cornelia de Lange syndrome. Eur. J. Med. Genet. 2018, 61, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Pellicer, A.; Gil-Salvador, M.; Parenti, I.; Lucia-Campos, C.; Trujillano, L.; Marcos-Alcalde, I.; Arnedo, M.; Ascaso, Á.; Ayerza-Casas, A.; Antoñanzas-Pérez, R.; et al. Clinical relevance of postzygotic mosaicism in Cornelia de Lange syndrome and purifying selection of NIPBL variants in blood. Sci. Rep. 2021, 11, 15459. [Google Scholar] [CrossRef]
- Slavin, T.P.; Lazebnik, N.; Clark, D.M.; Vengoechea, J.; Cohen, L.; Kaur, M.; Konczal, L.; Crowe, C.A.; Corteville, J.E.; Nowaczyk, M.J.; et al. Germline mosaicism in Cornelia de Lange syndrome. Am. J. Med. Genet. Part A 2012, 158, 1481–1485. [Google Scholar] [CrossRef]
- Krawczynska, N.; Wierzba, J.; Wasag, B. Genetic Mosaicism in a Group of Patients With Cornelia de Lange Syndrome. Front. Pediatr. 2019, 7, 203. [Google Scholar] [CrossRef]
- Mills, J.A.; Herrera, P.S.; Kaur, M.; Leo, L.; McEldrew, D.; Tintos-Hernandez, J.A.; Rajagopalan, R.; Gagne, A.; Zhang, Z.; Ortiz-Gonzalez, X.R.; et al. NIPBL+/− haploinsufficiency reveals a constellation of transcriptome disruptions in the pluripotent and cardiac states. Sci. Rep. 2018, 8, 1056. [Google Scholar] [CrossRef]
- Sadikovic, B.; Levy, M.A.; Aref-Eshghi, E. Functional annotation of genomic variation: DNA methylation episignatures in neurodevelopmental Mendelian disorders. Hum. Mol. Genet. 2020, 29, R27–R32. [Google Scholar] [CrossRef] [PubMed]
- Turinsky, A.L.; Choufani, S.; Lu, K.; Liu, D.; Mashouri, P.; Min, D.; Weksberg, R.; Brudno, M. EpigenCentral: Portal for DNA methylation data analysis and classification in rare diseases. Hum. Mutat. 2020, 41, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B.; Levy, M.A.; Kerkhof, J.; Aref-Eshghi, E.; Schenkel, L.; Stuart, A.; McConkey, H.; Henneman, P.; Venema, A.; Schwartz, C.E.; et al. Clinical epigenomics: Genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet. Med. 2021, 23, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, F.; Sparago, A.; Ariani, F.; Brugnoletti, F.; Calzari, L.; Coppedè, F.; De Luca, A.; Gervasini, C.; Giardina, E.; Gurrieri, F.; et al. DNA Methylation in the Diagnosis of Monogenic Diseases. Genes 2020, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.-M.; Allis, C.D.; Li, H. Reading between the Lines: “ADD”-ing Histone and DNA Methylation Marks toward a New Epigenetic “Sum”. ACS Chem. Biol. 2016, 11, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, S.; Bhai, P.; Aref-Eshghi, E.; Sadikovic, B. Diagnostic Utility of Genome-Wide DNA Methylation Analysis in Mendelian Neurodevelopmental Disorders. Int. J. Mol. Sci. 2020, 21, 9303. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B. Available online: https://episign.lhsc.on.ca/can.html (accessed on 7 March 2024).
- Kerkhof, J.; Squeo, G.M.; McConkey, H.; Levy, M.A.; Piemontese, M.R.; Castori, M.; Accadia, M.; Biamino, E.; Della Monica, M.; Di Giacomo, M.C.; et al. DNA methylation episignature testing improves molecular diagnosis of Mendelian chromatinopathies. Genet. Med. 2022, 24, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Bulfamante, G.P.; Massa, V. Cornelia de Lange syndrome: To diagnose or not to diagnose in utero? Birth Defects Res. 2017, 109, 771–777. [Google Scholar] [CrossRef]
- Panaitescu, A.M.; Duta, S.; Gica, N.; Botezatu, R.; Nedelea, F.; Peltecu, G.; Veduta, A. A Broader Perspective on the Prenatal Diagnosis of Cornelia de Lange Syndrome: Review of the Literature and Case Presentation. Diagnostics 2021, 11, 142. [Google Scholar] [CrossRef]
- Hague, J.; Twiss, P.; Mead, Z.; Park, S.-M. Clinical Diagnosis of Classical Cornelia de Lange Syndrome Made From Postmortem Examination of Second Trimester Fetus With Novel NIPBL Pathogenic Variant. Pediatr. Dev. Pathol. 2019, 22, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Teresa-Rodrigo, M.E.; Pozojevic, J.; Ruiz Gil, S.; Bader, I.; Braunholz, D.; Bramswig, N.C.; Gervasini, C.; Larizza, L.; Pfeiffer, L.; et al. Mutations in chromatin regulators functionally link Cornelia de Lange syndrome and clinically overlapping phenotypes. Hum. Genet. 2017, 136, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, G.; Vergarajauregui, S.; Endele, S.; Popp, B.; Büttner, C.; Ekici, A.B.; Gerard, M.; Bramswig, N.C.; Albrecht, B.; Clayton-Smith, J.; et al. Mutations in the BAF-Complex Subunit DPF2 Are Associated with Coffin-Siris Syndrome. Am. J. Hum. Genet. 2018, 102, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Rinaldi, B.; Rimoldi, M.; Villa, R.; Iascone, M.; Gangi, S.; Porro, M.; Ajmone, P.F.; Colli, A.M.; Mosca, F.; et al. Chung–Jansen syndrome can mimic Cornelia de Lange syndrome: Another player among chromatinopathies? Am. J. Med. Genet. Part A 2023, 191, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sowa, N.; Cardenas, M.E.; Gerton, J.L. l-leucine partially rescues translational and developmental defects associated with zebrafish models of Cornelia de Lange syndrome. Hum. Mol. Genet. 2015, 24, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Pistocchi, A.; Fazio, G.; Cereda, A.; Ferrari, L.; Bettini, L.R.; Messina, G.; Cotelli, F.; Biondi, A.; Selicorni, A.; Massa, V. Cornelia de Lange Syndrome: NIPBL haploinsufficiency downregulates canonical Wnt pathway in zebrafish embryos and patients fibroblasts. Cell Death Dis. 2013, 4, e866. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Nguyen-McCarty, M.; Hexner, E.O.; Danet-Desnoyers, G.; Klein, P.S. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat. Med. 2012, 18, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Grazioli, P.; Mariani, M.; Bulfamante, G.P.; Selicorni, A.; Massa, V. Integrating molecular and structural findings: Wnt as a possible actor in shaping cognitive impairment in Cornelia de Lange syndrome. Orphanet J. Rare Dis. 2017, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, P.; Parodi, C.; Mariani, M.; Bottai, D.; Di Fede, E.; Zulueta, A.; Avagliano, L.; Cereda, A.; Tenconi, R.; Wierzba, J.; et al. Lithium as a possible therapeutic strategy for Cornelia de Lange syndrome. Cell Death Discov. 2021, 7, 34. [Google Scholar] [CrossRef]
- de Graaf, M.; Kant, S.G.; Wit, J.M.; Redeker, E.J.W.; Santen, G.W.E.; Verkerk, A.J.M.H.; Uitterlinden, A.G.; Losekoot, M.; Oostdijk, W. Successful Growth Hormone Therapy in Cornelia de Lange Syndrome. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 366–370. [Google Scholar] [CrossRef]
- Cukrov, D.; Newman, T.A.C.; Leask, M.; Leeke, B.; Sarogni, P.; Patimo, A.; Kline, A.D.; Krantz, I.D.; Horsfield, J.A.; Musio, A. Antioxidant treatment ameliorates phenotypic features of SMC1A-mutated Cornelia de Lange syndrome in vitro and in vivo. Hum. Mol. Genet. 2018, 27, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- NCT04381897. Available online: https://clinicaltrials.gov/study/NCT04381897 (accessed on 7 March 2024).
- NCT05829668. Available online: https://www.clinicaltrials.gov/study/NCT05829668 (accessed on 7 March 2024).
- Ganesan, A.; Arimondo, P.B.; Rots, M.G.; Jeronimo, C.; Berdasco, M. The timeline of epigenetic drug discovery: From reality to dreams. Clin. Epigenetics 2019, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, J.K.; Chen, J.; Pommier, G.C.; Cogan, J.Z.; Replogle, J.M.; Adriaens, C.; Ramadoss, G.N.; Shi, Q.; Hung, K.L.; Samelson, A.J.; et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 2021, 184, 2503–2519.e17. [Google Scholar] [CrossRef]
- Awamleh, Z.; Goodman, S.; Choufani, S.; Weksberg, R. DNA methylation signatures for chromatinopathies: Current challenges and future applications. Hum. Genet. 2023, 1–7. [Google Scholar] [CrossRef]
| Clinical Features of CdLS [1] | |
|---|---|
| Cardinal Features (2 Points Each if Present) | Suggestive Features (1 Point Each if Present) |
|
|
| ≥11 points, at least three features are cardinal: classic CdLS | |
| 9–10 points, at least two features are cardinal: non-classic CdLS | |
| 4–8 points and at least one feature is cardinal: indication for molecular testing for CdLS | |
| <4 points: insufficient to indicate molecular testing for CdLS | |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Prenatal ultrasound findings | FGR | FGR | FGR | FGR |
| upper limb defects | renal abnormality | upper limb defects | single umbilical artery | |
| brain defects | oligohydramnios | |||
| Cardinal features | synophrys | synophrys | synophrys | synophrys |
| short nose | flat and broad nasal bridge and short nose | short nose | short nose | |
| narrowed red lips | syndactyly of the 2nd and 3rd toes | thin upper lip | thin upper lip | |
| elongated philtrum | diaphragmatic hernia | long philtrum | ||
| upper limb defects | upper limb defects | |||
| Suggestive features | pre- and postnatal growth restriction | pre- and postnatal growth restriction | pre- and postnatal growth restriction | pre- and postnatal growth restriction |
| microcephaly | microcephaly | microcephaly | microcephaly | |
| hirsutism | hirsutism | hirsutism | hirsutism, | |
| small hands and feets | short fifth finger | small feet | ||
| global developmental delay | global developmental delay | global developmental delay | global developmental delay | |
| Cumulative score in clinical assesment | 12 points | 11 points | 11 points | 11 points |
| Genetics tests | aCGH (postnataly)—deletion 2q13 | aCGH, karyotype (prenatally)—normal findings | aCGH (postnataly)—normal findings | aCGH (prenatally)—normal findings |
| WES (postnataly)—pathogenic de novo variant in HDAC8 gene (c.883C>T) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruca-Stryjak, K.; Doda-Nowak, E.; Dzierla, J.; Wróbel, K.; Szymankiewicz-Bręborowicz, M.; Mazela, J. Advancing the Clinical and Molecular Understanding of Cornelia de Lange Syndrome: A Multidisciplinary Pediatric Case Series and Review of the Literature. J. Clin. Med. 2024, 13, 2423. https://doi.org/10.3390/jcm13082423
Gruca-Stryjak K, Doda-Nowak E, Dzierla J, Wróbel K, Szymankiewicz-Bręborowicz M, Mazela J. Advancing the Clinical and Molecular Understanding of Cornelia de Lange Syndrome: A Multidisciplinary Pediatric Case Series and Review of the Literature. Journal of Clinical Medicine. 2024; 13(8):2423. https://doi.org/10.3390/jcm13082423
Chicago/Turabian StyleGruca-Stryjak, Karolina, Emilia Doda-Nowak, Julia Dzierla, Karolina Wróbel, Marta Szymankiewicz-Bręborowicz, and Jan Mazela. 2024. "Advancing the Clinical and Molecular Understanding of Cornelia de Lange Syndrome: A Multidisciplinary Pediatric Case Series and Review of the Literature" Journal of Clinical Medicine 13, no. 8: 2423. https://doi.org/10.3390/jcm13082423
APA StyleGruca-Stryjak, K., Doda-Nowak, E., Dzierla, J., Wróbel, K., Szymankiewicz-Bręborowicz, M., & Mazela, J. (2024). Advancing the Clinical and Molecular Understanding of Cornelia de Lange Syndrome: A Multidisciplinary Pediatric Case Series and Review of the Literature. Journal of Clinical Medicine, 13(8), 2423. https://doi.org/10.3390/jcm13082423






