Visual Field Tests: A Narrative Review of Different Perimetric Methods
Abstract
1. Introduction
2. Materials and Methods
3. Relevant Sections
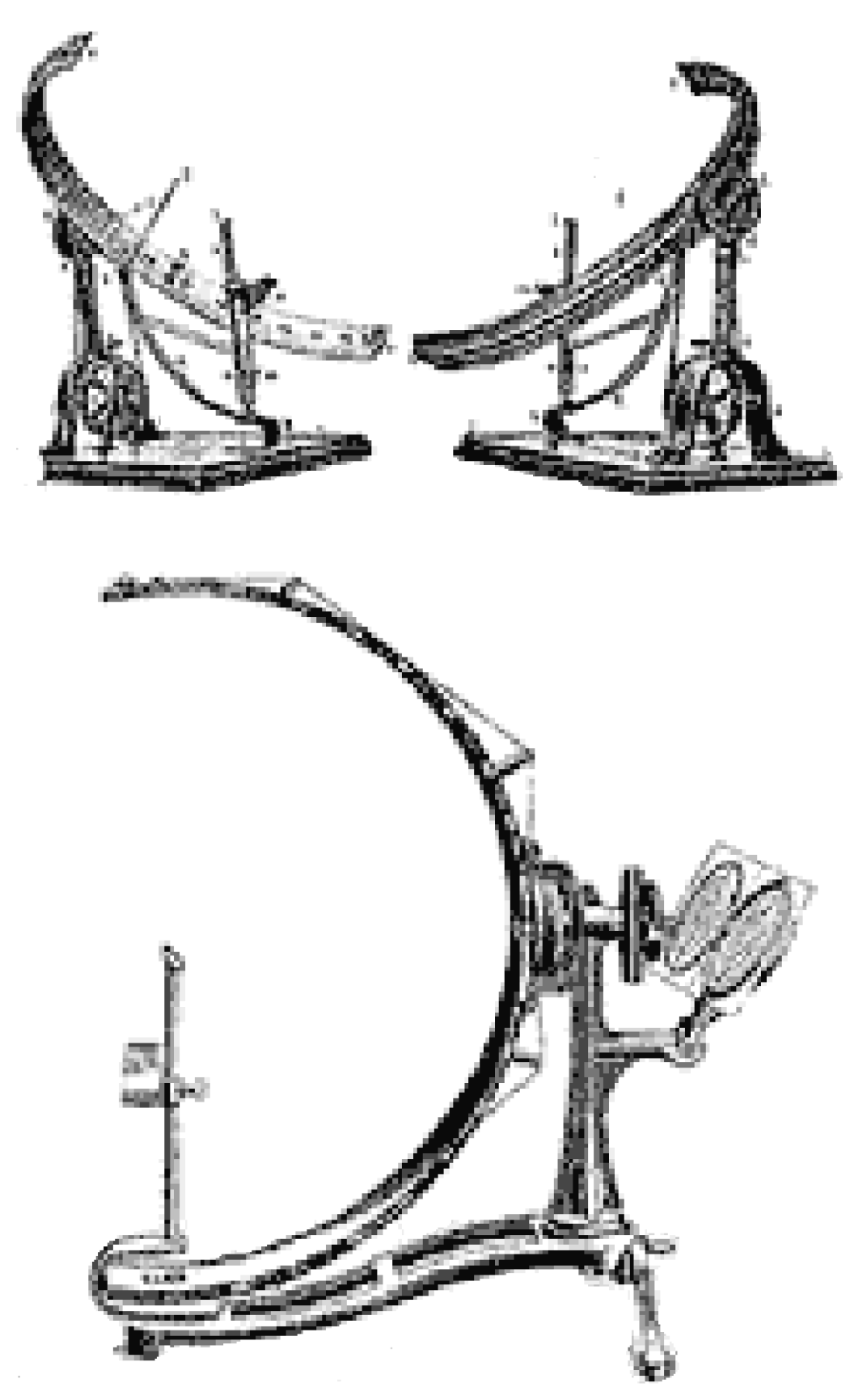
3.1. History and Evolution of Perimetry
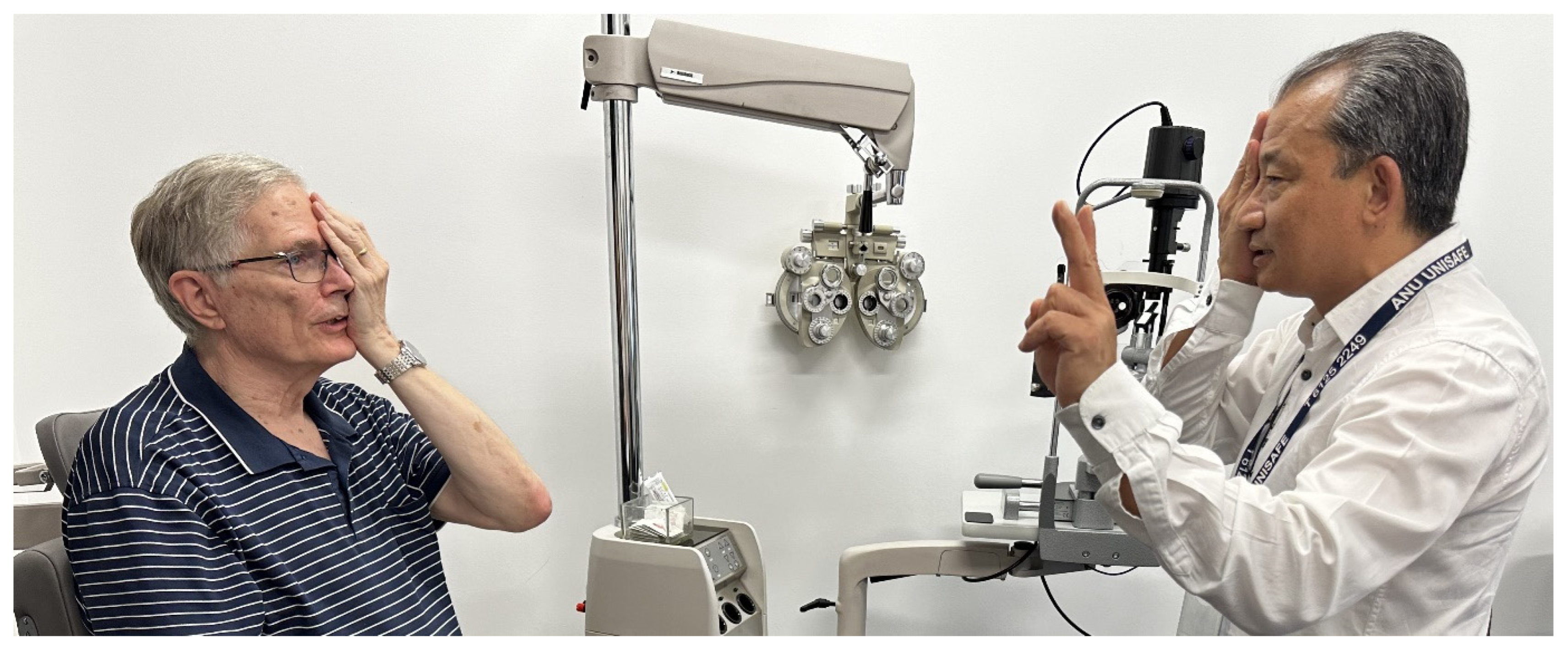
3.2. The Age of Automation: Goldmann Bowl Perimetry
4. Subjective Perimetric Methods
4.1. Standard Automated Perimetry (SAP)
4.2. Redundancy Limits SAP
4.3. Short Wavelength Automated Perimetry (SWAP)
4.4. Frequency Doubling Technology (FDT) Perimetry
4.5. High-Pass Resolution Perimetry (HRP)
4.6. Motion Automated Perimetry (MAP)
4.7. Moorfield’s Motion Displacement Test (MDT)
4.8. Rarebit Perimetry (RBP)
4.9. Microperimetry
5. Objective Perimetric Methods
5.1. Electroretinogram (ERG)
5.2. Visual Evoked Potential (VEP)
5.3. Multifocal Pupillographic Objective Perimetry (mfPOP)
6. Discussion
7. Conclusions
8. Key Messages
- Visual field testing provides critical information on eye, brain and neurological diseases and is an integral part of comprehensive ophthalmic evaluation.
- Subjective visual field tests, including standard automated perimetry, are limited by high test–retest variability, learning effects, variability due to under-sampling, and the principle of redundancy.
- Among the objective tests, electroretinograms and visually evoked potentials are limited by the inconvenience of applying electrodes and are time-consuming.
- Multifocal Pupillographic Objective Perimetry is an objective and reliable method which can test both eyes in less than 90 s and has the critical advantage of measuring response delay, which no other perimetric method provides. It has normative data.
- Some Multifocal Pupillographic Objective Perimetry stimulus regions are matched spatially to Early Treatment Diabetic Retinopathy Study 9 subfields for easy structural-functional correlation and recommended diagnostic effect size of >2, and thus Area under the Receiver Operating Characteristic Curve of >0.92 are better met by this method than any others.
9. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, C.A. Psychophysical factors that have been applied to clinical perimetry. Vis. Res. 2013, 90, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lascaratos, J.; Marketos, S. A historical outline of Greek ophthalmology from the Hellenistic period up to the establishment of the first universities. Doc. Ophthalmol. 1988, 68, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Wall, M.; Thompson, H.S. A History of Perimetry and Visual Field Testing. Optom. Vis. Sci. 2011, 88, E8–E15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W. Visual Field Test. In Primary Eye Examination: A Comprehensive Guide to Diagnosis; Lee, J.-S., Ed.; Springer: Singapore, 2019; pp. 179–207. [Google Scholar] [CrossRef]
- Wall, M.; May, D.R. Threshold Amsler Grid Testing in Maculopathies. Ophthalmology 1987, 94, 1126–1133. [Google Scholar] [CrossRef]
- Greenberg, A.; Su, D.; Simonson, J.; Park, S.C.; Tello, C.; Liebmann, J.; Ritch, R. Efficacy Of The Amsler Grid Test In Evaluating Glaucomatous Central Visual Field Defects. Investig. Ophthalmol. Vis. Sci. 2012, 53, 177. [Google Scholar]
- Augustin, A.J.; Offermann, I.; Lutz, J.; Schmidt-Erfurth, U.; Tornambe, P. Comparison of the original amsler grid with the modified Amsler grid: Result for Patients With Age-Related Macular Degeneration. Retina 2005, 25, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Bowling, B. Acquired Macular Disorders. In Kanski’s Clinical Ophthalmology: A Systematic Approach, 8th ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 580–640. [Google Scholar]
- Traquair, H.M. An Introduction to Clinical Perimetry; H. Kimpton: London, UK, 1927. [Google Scholar]
- Thompson, H.S.; Wall, M. The Age of Isopter Perimetry: Bjerrum and the Tangent Screen. Available online: https://www.perimetry.org/__static/c932446f936a5703745989b11e7c06a4/the-age-of-isopter-perimetry.pdf?dl=1 (accessed on 25 January 2023).
- Thompson, H.S.; Wall, M. Measurement of the Visual Field Limits: The Perimeter. Imaging and Perimetry Society. 1980. Available online: http://www.perimetry.org/index.php/measurement-of-the-visual-field-limits-the-perimeter (accessed on 1 May 2023).
- Gloor, B.R. Hans Goldmann (1899–1991). Eur. J. Ophthalmol. 2010, 20, 1–11. [Google Scholar] [CrossRef]
- Fankhauser, F.; Spahr, J.; Bebie, H. Some aspects of the automation of perimetry. Surv. Ophthalmol. 1977, 22, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Bebie, H.; Fankhauser, F.; Spahr, J. Static perimetry: Strategies. Acta Ophthalmol. 1976, 54, 325–338. [Google Scholar] [CrossRef]
- Gloor, B.P. Franz Fankhauser: The Father of the Automated Perimeter. Surv. Ophthalmol. 2009, 54, 417–425. [Google Scholar] [CrossRef]
- Heijl, A. The Field Analyzer Primer: Effective Perimetry; Carl Zeiss Meditec, Inc.: Dublin, CA, USA, 2012. [Google Scholar]
- Thompson, H.S.; Wall, M. The Age of Automation: Automating the Goldmann Bowl: 1980. Imaging and Perimetry Society. 1980. Available online: https://www.perimetry.org/__static/518956071152f7fc2a920d7ae70bba30/the-age-of-automation-automating-the-goldmann-bowl-1980.pdf?dl=1 (accessed on 26 January 2023).
- Sample, P.A.; Dannheim, F.; Artes, P.H.; Dietzsch, J.; Henson, D.; Johnson, C.A.; Ng, M.; Schiefer, U.; Wall, M.; Grp, I.S. Imaging and Perimetry Society Standards and Guidelines. Optom. Vis. Sci. 2011, 88, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.J.; Rowlands, A. Comparison of Diagnostic Accuracy between Octopus 900 and Goldmann Kinetic Visual Fields. Biomed. Res. Int. 2014, 2014, 214829. [Google Scholar] [CrossRef] [PubMed]
- Alencar, L.M.; Medeiros, F.A. The role of standard automated perimetry and newer functional methods for glaucoma diagnosis and follow-up. Indian J. Ophthalmol. 2011, 59 (Suppl. 1), S53–S58. [Google Scholar] [PubMed]
- Heijl, A.; Patella, V.M. The Essentials of Perimetry. In The Field Analyzer Primer, 3rd ed.; Haley, M.J., Ed.; Carl Zeiss Meditec, Inc.: Dublin, CA, USA, 2002; pp. 3–13. [Google Scholar]
- Heijl, A.; Patella, V.M.; Chong, L.X.; Iwase, A.; Leung, C.K.; Tuulonen, A.; Lee, G.C.; Callan, T.; Bengtsson, B. A New SITA Perimetric Threshold Testing Algorithm: Construction and a Multicenter Clinical Study. Am. J. Ophthalmol. 2019, 198, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Phu, J.; Khuu, S.K.; Agar, A.; Kalloniatis, M. Clinical Evaluation of Swedish Interactive Thresholding Algorithm-Faster Compared With Swedish Interactive Thresholding Algorithm-Standard in Normal Subjects, Glaucoma Suspects, and Patients With Glaucoma. Am. J. Ophthalmol. 2019, 208, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.X.; Chen, Q.; Cun, Q.; Tao, Y.J.; Yang, W.Y.; Yang, Y.; Hu, Z.Y.; Zhu, Y.T.; Zhong, H. Comparison of the SITA Faster-a new visual field strategy with SITA Fast strategy. Int. J. Ophthalmol. 2021, 14, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.K.; Cubbidge, R.P.; Heitmar, R. Comparative quantification of focal and diffuse visual field loss by the SPARK Precision threshold algorithm and SITA. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1983–1993. [Google Scholar] [CrossRef]
- Livingstone, M.S.; Hubel, D.H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J. Neurosci. 1987, 7, 3416–3468. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Anderson, R.S. The psychophysics of glaucoma: Improving the structure/function relationship. Prog. Retin. Eye Res. 2006, 25, 79–97. [Google Scholar] [CrossRef]
- Maddess, T.; Henry, G.H. Nonlinear visual responses and visual deficits in ocular hypertensive and glaucoma subjects. Clin. Vis. Sci. 1992, 7, 371–383. [Google Scholar]
- Maddess, T.; Goldberg, I.; Dobinson, J.; Wine, S.; Welsh, A.H.; James, A.C. Testing for glaucoma with the spatial frequency doubling illusion. Vision Res. 1999, 39, 4258–4273. [Google Scholar] [CrossRef] [PubMed]
- Sakata, L.M.; DeLeon-Ortega, J.; Girkin, C.A. Selective perimetry in glaucoma diagnosis. Curr. Opin. Ophthalmol. 2007, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Moss, I.D.; Wild, J.M.; Whitaker, D.J. The influence of age-related cataract on blue-on-yellow perimetry. Investig. Ophthalmol. Vis. Sci. 1995, 36, 764–773. [Google Scholar]
- Eiber, C.D.; Rahman, A.S.; Pietersen, A.N.J.; Zeater, N.; Dreher, B.; Solomon, S.G.; Martin, P.R. Receptive Field Properties of Koniocellular On/Off Neurons in the Lateral Geniculate Nucleus of Marmoset Monkeys. J. Neurosci. 2018, 38, 10384–10398. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.J.; Rodic, R.; Husted, R.; Stamper, R. Spectral sensitivity and color discrimination changes in glaucoma and glaucoma-suspect patients. Investig. Ophthalmol. Vis. Sci. 1982, 23, 516–524. [Google Scholar]
- Hamill, T.R.; Post, R.B.; Johnson, C.A.; Keltner, J.L. Correlation of color vision deficits and observable changes in the optic disc in a population of ocular hypertensives. Arch. Ophthalmol. 1984, 102, 1637–1639. [Google Scholar] [CrossRef]
- Sample, P.A.; Taylor, J.D.; Martinez, G.A.; Lusky, M.; Weinreb, R.N. Short-wavelength color visual fields in glaucoma suspects at risk. Am. J. Ophthalmol. 1993, 115, 225–233. [Google Scholar] [CrossRef]
- Johnson, C.A.; Adams, A.J.; Casson, E.J.; Brandt, J.D. Progression of early glaucomatous visual field loss as detected by blue-on-yellow and standard white-on-white automated perimetry. Arch. Ophthalmol. 1993, 111, 651–656. [Google Scholar] [CrossRef]
- Rai, B.B.; van Kleef, J.P.; Sabeti, F.; Vlieger, R.; Suominen, H.; Maddess, T. Early diabetic eye damage: Comparing detection methods using diagnostic power. Surv. Ophthalmol. 2023, 69, 24–33. [Google Scholar] [CrossRef]
- Sample, P.A.; Martinez, G.A.; Weinreb, R.N. Short-wavelength automated perimetry without lens density testing. Am. J. Ophthalmol. 1994, 118, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Maddess, T. Method and Apparatus for Use in Diagnosis of Glaucoma. Australia Patent 611585, 13 June 1991. [Google Scholar]
- Maddess, T. Method and Apparatus for Use in Diagnosis of Glaucoma. USA Patent 5065767, 19 November 1991. [Google Scholar]
- White, A.J.R.; Sun, H.; Swanson, W.H.; Lee, B.B. An Examination of Physiological Mechanisms Underlying the Frequency-Doubling Illusion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3590–3599. [Google Scholar]
- Medeiros, F.A.; Sample, P.A.; Weinreb, R.N. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am. J. Ophthalmol. 2004, 137, 863–871. [Google Scholar] [CrossRef]
- Landers, J.A.; Goldberg, I.; Graham, S.L. Detection of early visual field loss in glaucoma using frequency-doubling perimetry and short-wavelength automated perimetry. Arch. Ophthalmol. 2003, 121, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Wollstein, G.; Ishikawa, H.; Schuman, J.S. Comparison of Visual Field Defects Using Matrix Perimetry and Standard Achromatic Perimetry. Ophthalmology 2007, 114, 480–487. [Google Scholar] [CrossRef]
- Wall, M.; Chauhan, B.; Frisen, L.; House, P.H.; Brito, C. Visual field of high-pass resolution perimetry in normal subjects. J. Glaucoma 2004, 13, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C. The value of high-pass resolution perimetry in glaucoma. Curr. Opin. Ophthalmol. 2000, 11, 85–89. [Google Scholar] [CrossRef]
- McKendrick, A.M. Recent developments in perimetry: Test stimuli and procedures. Clin. Exp. Optom. 2005, 88, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.F.; Nguyen, N.T.; Cox, T.A.; Singh, K.; Lee, D.A.; Dueker, D.K.; Fechtner, R.D.; Juzych, M.S.; Lin, S.C.; Netland, P.A.; et al. Automated perimetry: A report by the American Academy of Ophthalmology. Ophthalmology 2002, 109, 2362–2374. [Google Scholar] [CrossRef]
- McKee, S.P.; Nakayama, K. The detection of motion in the peripheral visual field. Vis. Res. 1984, 24, 25–32. [Google Scholar] [CrossRef]
- Wall, M.; Brito, C.; Kutzko, K. Motion Perimetry: Properties and results. Perimetry Update 1996/1997. In International Perimetric Society; Wall, M., Ed.; Kugler Publications: Amsterdam, The Netherlands, 1997; pp. 21–33. [Google Scholar]
- Bosworth, C.F.; Sample, P.A.; Weinreb, R.N. Perimetric motion thresholds are elevated in glaucoma suspects and glaucoma patients. Vis. Res. 1997, 37, 1989–1997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruben, S.; Fitzke, F. Correlation of peripheral displacement thresholds and optic disc parameters in ocular hypertension. Br. J. Ophthalmol. 1994, 78, 291–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verdon-Roe, G.M.; Westcott, M.C.; Viswanathan, A.C.; Fitzke, F.W.; Garway-Heath, D.F. Exploration of the Psychophysics of a Motion Displacement Hyperacuity Stimulus. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Membrey, L.; Fitzke, F.W. Effect of lens opacity on white-on-white perimetry, frequency doubling perimetry, and motion detection perimetry. In Perimetry Update 2000/2001; Wall, M.W., Ed.; Kugler Publications: Amsterdam, The Netherland, 2000; pp. 259–266. [Google Scholar]
- Ong, E.-L.; Zheng, Y.; Aung, T.; Tan, L.; Cheng, C.-Y.; Wong, T.-Y.; How, A. Performance of the Moorfields Motion Displacement Test for Identifying Eyes with Glaucoma. Ophthalmology 2014, 121, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Frisen, L. New, sensitive window on abnormal spatial vision: Rarebit probing. Vis. Res. 2002, 42, 1931–1939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frisen, L. Spatial vision in visually asymptomatic subjects at high risk for multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1145–1147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brusini, P.; Salvetat, M.L.; Parisi, L.; Zeppieri, M. Probing glaucoma visual damage by rarebit perimetry. Br. J. Ophthalmol. 2005, 89, 180–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salvetat, M.L.; Zeppieri, M.; Parisi, L.; Brusini, P. Rarebit Perimetry in Normal Subjects: Test–Retest Variability, Learning Effect, Normative Range, Influence of Optical Defocus, and Cataract Extraction. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5320–5331. [Google Scholar] [CrossRef][Green Version]
- Wong, E.N.; Morgan, W.H.; Chen, F.K. Intersession test-retest variability of 10-2 MAIA microperimetry in fixation-threatening glaucoma. Clin. Ophthalmol. 2017, 11, 745–752. [Google Scholar] [CrossRef]
- Springer, C.; Bultmann, S.; Volcker, H.E.; Rohrschneider, K. Fundus perimetry with the Micro Perimeter 1 in normal individuals: Comparison with conventional threshold perimetry. Ophthalmology 2005, 112, 848–854. [Google Scholar] [CrossRef]
- Markowitz, S.N.; Reyes, S.V. Microperimetry and clinical practice: An evidence-based review. Can. J. Ophthalmol. J. Can. D’ophtalmol. 2013, 48, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A.; Timberlake, G.T.; Webb, R.H.; Hughes, G.W. Scanning laser ophthalmoscopy. Clinical applications. Ophthalmology 1982, 89, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.K.; Patel, P.J.; Xing, W.; Bunce, C.; Egan, C.; Tufail, A.T.; Coffey, P.J.; Rubin, G.S.; Da Cruz, L. Test-Retest Variability of Microperimetry Using the Nidek MP1 in Patients with Macular Disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3464–3472. [Google Scholar] [CrossRef] [PubMed]
- Liegl, R.; Liegl, K.; Ceklic, L.; Haritoglou, C.; Kampik, A.; Ulbig, M.W.; Kernt, M.; Neubauer, A.S. Nonmydriatic ultra-wide-field scanning laser ophthalmoscopy (Optomap) versus two-field fundus photography in diabetic retinopathy. Ophthalmologica 2014, 231, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, S.; Hirnschall, N.; Georgiev, S.; Leisser, C.; Findl, O. Test-Retest Reproducibility of the Microperimeter MP3 with Fundus Image Tracking in Healthy Subjects and Patients With Macular Disease. Transl. Vis. Sci. Technol. 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Fishman, G.A. Basic Principles of Clinical Electroretinogram. Retina 1985, 5, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Fulton, A.B.; Holder, G.E.; Miyake, Y.; Brigell, M.; Bach, M. International Society for Clinical Electrophysiology of Vision. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc. Ophthalmol. 2009, 118, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Zrenner, E. Standard for clinical electroretinography (1999 update). Doc. Ophthalmol. 1998, 97, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.E.; Tran, D. The field topography of ERG components in man--I. The photopic luminance response. Vis. Res. 1992, 32, 433–446. [Google Scholar] [CrossRef]
- Creel, D.J. Multifocal Electroretinograms. J. Vis. Exp. JoVE 2011, 58, 3176. [Google Scholar]
- Sutter, E.E. Noninvasive Testing Methods: Multifocal Electrophysiology. In Encyclopedia of the Eye; Dartt, D.A., Ed.; Academic Press: Oxford, UK, 2010; Volume 3, pp. 142–160. [Google Scholar]
- Lee, Y.W.; Schetzen, M. Measurement of the Wiener Kernels of a Non-linear System by Cross-correlation. Int. J. Control 1965, 2, 237–254. [Google Scholar] [CrossRef]
- Hood, D.C.; Bach, M.; Brigell, M.; Keating, D.; Kondo, M.; Lyons, J.S.; Marmor, M.F.; McCulloch, D.L.; Palmowski-Wolfe, A.M. International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc. Ophthalmol. 2012, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dolan, F.M.; Parks, S.; Hammer, H.; Keating, D. The wide field multifocal electroretinogram reveals retinal dysfunction in early retinitis pigmentosa. Br. J. Ophthalmol. 2002, 86, 480–481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, T.Y.; Chan, W.M.; Li, H.; Lai, R.Y.; Lam, D.S. Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am. J. Ophthalmol. 2005, 140, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B.; Brown, B.; Lovie-Kitchin, J.; Swann, P. Adaptation responses in early age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4722–4727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miguel-Jimenez, J.M.; Ortega, S.; Boquete, L.; Rodriguez-Ascariz, J.M.; Blanco, R. Multifocal ERG wavelet packet decomposition applied to glaucoma diagnosis. Biomed. Eng. Online 2011, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Williams, P.D.; Callanan, D.; Wang, R.; Locke, K.G.; Hood, D.C. Macular atrophy in birdshot retinochoroidopathy: An optical coherence tomography and multifocal electroretinography analysis. Retina 2010, 30, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, V.C.; Holopigian, K.; Seiple, W.; Carr, R.E.; Hood, D.C. Atypical multifocal ERG responses in patients with diseases affecting the photoreceptors. Vis. Res. 2004, 44, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.M.; Moskowitz, A.; Fulton, A.B. Multifocal ERG Responses in Infants. Investig. Ophthalmol. Vis. Sci. 2009, 50, 470–475. [Google Scholar] [CrossRef][Green Version]
- Hood, D.C.; Odel, J.G.; Chen, C.S.; Winn, B.J. The mfERG: Applications and Limitations in Neuro-ophthalmology. In Proceedings of the North American Neuro-Ophthalmological Society (NANOS), Snowbird, UT, USA, 13 February 2003. [Google Scholar]
- Berninger, T.A.; Arden, G.B. The pattern electroretinogram. Eye 1988, 2, S257–S283. [Google Scholar] [CrossRef]
- Bode, S.F.N.; Jehle, T.; Bach, M. Pattern Electroretinogram in Glaucoma Suspects: New Findings from a Longitudinal Study. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Sorokac, N.; Buchser, W. Habituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjects. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.E. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog. Retin. Eye Res. 2001, 20, 531–561. [Google Scholar] [CrossRef] [PubMed]
- Creel, D.J. Visually Evoked Potentials. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Jasper, H.H. Reports of Committee on Methods of Clinical Examination Electroencephalography. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Blumhardt, L.D.; Barrett, G.; Halliday, A.M. The asymmetrical visual evoked potential to pattern reversal in one half field and its significance for the analysis of visual field defects. Br. J. Ophthalmol. 1977, 61, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Klistorner, A.I.; Graham, S.L.; Grigg, J.R.; Billson, F.A. Multifocal topographic visual evoked potential: Improving objective detection of local visual field defects. Investig. Ophthalmol. Vis. Sci. 1998, 39, 937–950. [Google Scholar]
- Slotnick, S.D.; Klein, S.A.; Carney, T.; Sutter, E.; Dastmalchi, S. Using multi-stimulus VEP source localization to obtain a retinotopic map of human primary visual cortex. Clin. Neurophysiol. 1999, 110, 1793–1800. [Google Scholar] [CrossRef]
- Fortune, B.; Hood, D.C. Conventional Pattern-Reversal VEPs Are Not Equivalent to Summed Multifocal VEPs. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1364–1375. [Google Scholar] [CrossRef]
- James, A.C. The pattern-pulse multifocal visual evoked potential. Investig. Ophthalmol. Vis. Sci. 2003, 44, 879–890. [Google Scholar] [CrossRef][Green Version]
- Ruseckaite, R.; Maddess, T.; Danta, G.; Lueck, C.J.; James, A.C. Sparse multifocal stimuli for the detection of multiple sclerosis. Ann. Neurol. 2005, 57, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.; Graham, S.L.; Klistorner, A.I. Multifocal objective perimetry in the detection of glaucomatous field loss11Drs Graham, and Klistorner have patents pending for techniques used by the ObjectiVision system and stock in ObjectiVision. Klistorner is a Sydney Medical Foundation research fellow. Am. J. Ophthalmol. 2002, 133, 29–39. [Google Scholar] [PubMed]
- Baseler, H.A.; Sutter, E.E.; Klein, S.A.; Carney, T. The topography of visual evoked response properties across the visual field. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 65–81. [Google Scholar] [CrossRef]
- Graham, S.L.; Klistorner, A.I.; Grigg, J.R.; Billson, F.A. Objective VEP perimetry in glaucoma: Asymmetry analysis to identify early deficits. J. Glaucoma 2000, 9, 10–19. [Google Scholar] [CrossRef]
- Brindley, G.S. The variability of the human striate cortex. J. Physiol. 1972, 225, 1–3. [Google Scholar]
- Rademacher, J.; Caviness, J.V.S.; Steinmetz, H.; Galaburda, A.M. Topographical Variation of the Human Primary Cortices: Implications for Neuroimaging, Brain Mapping, and Neurobiology. Cereb. Cortex 1993, 3, 313–329. [Google Scholar] [CrossRef]
- Kardon, R.H.; Kirkali, P.A.; Thompson, H.S. Automated pupil perimetry. Pupil field mapping in patients and normal subjects. Ophthalmology 1991, 98, 485–495; discussion 486–495. [Google Scholar] [CrossRef]
- Chang, L.Y.-L.; Turuwhenua, J.; Qu, T.Y.; Black, J.M.; Acosta, M.L. Infrared Video Pupillography Coupled with Smart Phone LED for Measurement of Pupillary Light Reflex. Front. Integr. Neurosci. 2017, 11, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maddess, T.; Bedford, S.M.; Goh, X.L.; James, A.C. Multifocal pupillographic visual field testing in glaucoma. Clin. Exp. Ophthalmol. 2009, 30, 678–686. [Google Scholar] [CrossRef]
- Carle, C.F.; James, A.C.; Kolic, M.; Essex, R.W.; Maddess, T. Blue Multifocal Pupillographic Objective Perimetry in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6394–6403. [Google Scholar] [CrossRef]
- Tan, L.; Kondo, M.; Sato, M.; Kondo, N.; Miyake, Y. Multifocal pupillary light response fields in normal subjects and patients with visual field defects. Vis. Res. 2001, 41, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Smith, S.E. Contraction anisocoria: Nasal versus temporal illumination. Br. J. Ophthalmol. 1980, 64, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.A.; Drewes, C.P. Contraction Anisocoria Resulting from Half-Field Illumination. Am. J. Ophthalmol. 1984, 97, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Wilhelm, H.; Wilhelm, B.; Kriegbaum, C.; Miliczek, K.; Wannek, U. Naso-temporal differences in pupillomotor sensitivity. IOVS 1995, 37, 159. [Google Scholar]
- Rai, B.B.; Sabeti, F.; Carle, C.F.; Rohan, E.M.F.; Sarac, O.; van Kleef, J.; Maddess, T. Recovery dynamics of multifocal pupillographic objective perimetry from tropicamide dilation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 191–200. [Google Scholar] [CrossRef]
- Gamlin, P.D.; McDougal, D.H.; Pokorny, J.; Smith, V.C.; Yau, K.-W.; Dacey, D.M. Human and Macaque Pupil Responses Driven by Melanopsin-Containing Retinal Ganglion Cells. Vis. Res. 2007, 47, 946–954. [Google Scholar] [CrossRef]
- Kawasaki, A.; Kardon, R.H. Intrinsically photosensitive retinal ganglion cells. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2007, 27, 195–204. [Google Scholar] [CrossRef]
- Carle, C.F.; James, A.C.; Maddess, T. The Pupillary Response to Color and Luminance Variant Multifocal Stimuli. Investig. Ophthalmol. Vis. Sci. 2013, 54, 467–475. [Google Scholar] [CrossRef]
- Kimura, E.; Abe, S.; Goryo, K. Attenuation of the pupillary response to luminance and color changes during interocular suppression. J. Vis. 2014, 14, 14. [Google Scholar] [CrossRef]
- Barbur, J.L.; Harlow, A.J.; Sahraie, A. Pupillary responses to stimulus structure, colour and movement. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. (Optom.) 1992, 12, 137–141. [Google Scholar] [CrossRef]
- Ukai, K. Spatial pattern as a stimulus to the pupillary system. J. Opt. Soc. Am. 1985, 2, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.N.; Maddess, T.; James, A.C.; Voicu, C.; Lueck, C.J. Pupillary response to sparse multifocal stimuli in multiple sclerosis patients. Mult. Scler. 2014, 20, 854–861. [Google Scholar] [CrossRef]
- Maddess, T.; Ho, Y.L.; Wong, S.S.; Kolic, M.; Goh, X.L.; Carle, C.F.; James, A.C. Multifocal pupillographic perimetry with white and colored stimuli. J. Glaucoma 2011, 20, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, F.; James, A.C.; Carle, C.F.; Essex, R.W.; Bell, A.; Maddess, T. Comparing multifocal pupillographic objective perimetry (mfPOP) and multifocal visual evoked potentials (mfVEP) in retinal diseases. Sci. Rep. 2017, 7, 45847. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.B.; Maddess, T.; Carle, C.F.; Rohan, E.M.F.; van Kleef, J.P.; Barry, R.C.; Essex, R.W.; Nolan, C.J.; Sabeti, F. Comparing objective perimetry, matrix perimetry, and regional retinal thickness in early diabetic macular oedema. Transl. Vis. Sci. Technol. 2021, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, F.; Rai, B.B.; van Kleef, J.P.; Rohan, E.M.F.; Carle, C.F.; Barry, R.C.; Essex, R.W.; Nolan, C.J.; Maddess, T. Objective perimetry identifies regional functional progression and recovery in mild Diabetic Macular Oedema. PLoS ONE 2023, 18, e0287319. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.B.; Carle, C.F.; Rohan, E.M.F.; van Kleef, J.P.; Sabeti, F.; Nolan, C.J.; Whitfield, A.; Maddess, T. Assessing functional damage with rapid and objective macular perimetry in Type 1 Diabetes. In Proceedings of the International Diabetes Federation Congress, Lisbon, Portugal, 5–8 December 2022. [Google Scholar]
- Maddess, T.; Rohan, E.M.F.; Rai, B.B.; Carle, C.F.; Nolan, C.J.; Sabeti, F.; Essex, R.W.; van Kleef, J.P. Diagnostic power of rapid objective perimetry in young people with Type 1 Diabetes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2666. [Google Scholar]
- Sabeti, F.; van Kleef, J.P.; Iyer, R.M.; Carle, C.F.; Nolan, C.J.; Chia, R.H.; Maddess, T. Discriminating early-stage diabetic retinopathy with subjective and objective perimetry. Front. Endo 2023, 14, 1333826. [Google Scholar] [CrossRef]
- Rai, B.B.; Essex, R.W.; Sabeti, F.; Maddess, T.; Rohan, E.M.F.; van Kleef, J.P.; Carle, C.F. An objective perimetry study of central versus peripheral sensitivities and delays in age-related macular degeneration. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef]
- Sabeti, F.; Lane, J.; Rohan, E.M.F.; Rai, B.B.; Essex, R.W.; McKone, E.; Maddess, T. Correlation of Central Versus Peripheral Macular Structure-Function With Acuity in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef]
- Maddess, T.; van Kleef, J.P.; Rohan, E.M.F.; Carle, C.F.; Baird-Gunning, J.; Rai, B.B.; Bruestle, A.; Lane, J.; Lueck, C.J. Rapid, non-contact multifocal visual assessment in multiple sclerosis. Neurol. Sci. 2023, 44, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.N.; Lueck, C.J.; Carle, C.F.; Martin, K.L.; Borbelj, A.; Maddess, T. Response characteristics of objective perimetry in persons living with epilepsy. J. Neurol. Sci. 2022, 436, 120237. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.B.; Flanagan, J. 3—Assessment of visual function. In Clinical Procedures in Primary Eye Care, 3rd ed.; Elliott, D.B., Ed.; Butterworth-Heinemann: Edinburgh, UK, 2007; pp. 29–81. [Google Scholar] [CrossRef]
- Achard, O.A.; Safran, A.B.; Duret, F.C.; Ragama, E. Role of the completion phenomenon in the evaluation of Amsler grid results. Am. J. Ophthalmol. 1995, 120, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Capilla, A.; Melcon, M.; Kessel, D.; Calderon, R.; Pazo-Alvarez, P.; Carretie, L. Retinotopic mapping of visual event-related potentials. Biol. Psychol. 2016, 118, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Schimiti, R.B.; Avelino, R.R.; Kara-Jose, N.; Costa, V.P. Full-threshold versus Swedish Interactive Threshold Algorithm (SITA) in normal individuals undergoing automated perimetry for the first time. Ophthalmology 2002, 109, 2084–2092; discussion 2092. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, T.M.; Sherwood, M.B.; Hitchins, R.A.; Crowston, J.G. Electrophysiology in Glaucoma Assessment. In Glaucoma: Medical Diagnosis and Therapy, Volume—I; Saunders & Elsevier: Exeter Devon, UK, 2009; pp. 151–171. [Google Scholar]
- Maddess, T. Modeling the relative influence of fixation and sampling errors on retest variability in perimetry. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1611–1619. [Google Scholar] [CrossRef]
- Pearce, J.G.; Maddess, T. Retest Variability in the Medmont M700 Automated Perimeter. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2016, 93, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Maddess, T.; Matsumoto, C.; Okuyama, S.; Hashimoto, S.; Nomoto, H.; Shinomura, Y. Exploring test-retest variability using high-resolution perimetry. Transl. Vis. Sci. Technol. 2017, 6, 8. [Google Scholar] [CrossRef][Green Version]
- Wall, M.; Woodward, K.R.; Doyle, C.K.; Zamba, G. The effective dynamic ranges of standard automated perimetry sizes III and V and motion and matrix perimetry. Arch. Ophthalmol. 2010, 128, 570–576. [Google Scholar] [CrossRef]
- Fredette, M.J.; Giguere, A.; Anderson, D.R.; Budenz, D.L.; McSoley, J. Comparison of Matrix with Humphrey Field Analyzer II with SITA. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2015, 92, 527–536. [Google Scholar] [CrossRef]
- Carle, C.F.; Chain, A.Y.H.; Kolic, M.; Maddess, T. The structure-function relationship between multifocal pupil perimetry and retinal nerve fibre layer in Glaucoma. BMC Ophthalmol. 2024, 24, 159. [Google Scholar] [CrossRef]
- Pineles, S.L.; Volpe, N.J.; Miller-Ellis, E.; Galetta, S.L.; Sankar, P.S.; Shindler, K.S.; Maguire, M.G. Automated combined kinetic and static perimetry: An alternative to standard perimetry in patients with neuro-ophthalmic disease and glaucoma. Arch. Ophthalmol. 2006, 124, 363–369. [Google Scholar] [CrossRef][Green Version]
- Wong, A.M.; Sharpe, J.A. A comparison of tangent screen, goldmann, and humphrey perimetry in the detection and localization of occipital lesions. Ophthalmology 2000, 107, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.B.; Sabeti, F.; Carle, C.F.; Rohan, E.M.; van Kleef, J.P.; Essex, R.W.; Barry, R.C.; Maddess, T. Rapid Objective Testing of Visual Function Matched to the ETDRS Grid and Its Diagnostic Power in Age-Related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100143. [Google Scholar] [CrossRef] [PubMed]
- Kolic, M.; Chain, A.; James, A.; Maddess, T.; Carle, C. Structure and function in multifocal pupillographic objective perimetry (mfPOP). Investig. Ophthalmol. Vis. Sci. 2013, 54, 2294. [Google Scholar]
- Sabeti, F.; James, A.C.; Maddess, T. Spatial and temporal stimulus variants for multifocal pupillography of the central visual field. Vis. Res. 2011, 51, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, F.; Maddess, T.; Essex, R.W.; James, A.C. Multifocal pupillography identifies ranibizumab-induced changes in retinal function for exudative age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 253–260. [Google Scholar] [CrossRef]
- Maddess, T.; Lueck, C.J.; Voicu, C.; James, A.C. Multifocal Objective Pupil Perimetry (mfpop) In Ms. Investig. Ophthalmol. Vis. Sci. 2011, 52, 267. [Google Scholar]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, B.B.; Sabeti, F.; Carle, C.F.; Maddess, T. Visual Field Tests: A Narrative Review of Different Perimetric Methods. J. Clin. Med. 2024, 13, 2458. https://doi.org/10.3390/jcm13092458
Rai BB, Sabeti F, Carle CF, Maddess T. Visual Field Tests: A Narrative Review of Different Perimetric Methods. Journal of Clinical Medicine. 2024; 13(9):2458. https://doi.org/10.3390/jcm13092458
Chicago/Turabian StyleRai, Bhim Bahadur, Faran Sabeti, Corinne Frances Carle, and Ted Maddess. 2024. "Visual Field Tests: A Narrative Review of Different Perimetric Methods" Journal of Clinical Medicine 13, no. 9: 2458. https://doi.org/10.3390/jcm13092458
APA StyleRai, B. B., Sabeti, F., Carle, C. F., & Maddess, T. (2024). Visual Field Tests: A Narrative Review of Different Perimetric Methods. Journal of Clinical Medicine, 13(9), 2458. https://doi.org/10.3390/jcm13092458







