Acute Lung Injury after Cardiopulmonary Resuscitation: A Narrative Review
Abstract
:1. Introduction
2. CPR-Related Injuries
| Reference | Study Design | Rib Fractures | Sternal Fractures | Lung Contusions | Pneumo-Thorax | Effusion or Hemothorax | Hemo-Pericardium | Liver Lesions | Spleen Lesions |
|---|---|---|---|---|---|---|---|---|---|
| Ihnát Rudinská 2016 [4] | Prospective analysis of 80 autopsies after OHCA | 74% | 66% | 31% | - | 0.5% | 9% | - | - |
| Hoke 2004 [5] | Review study including 16 studies:
| 13–97% | 1–43% | 1.3–3% | 0.8–8.7% | 1.1–8.4% | 0.8–4.3% | 0.3–2.6% | |
| Smekal 2014 [6] | Prospective multicenter study of 222 autopsies after OHCA | CC 64.6% mCC 78.8% | CC 54.2% mCC 58.3% | - | - | - | - | - | - |
| Karasek 2022 [7] | Retrospective analysis of 628 autopsies after IHCA and OHCA | 94.6% | 62.4% | 9.9% | - | - | - | 2.5% | 1.8% |
| Ondruschka 2018 [8] | Retrospective analysis of 614 autopsies after IHCA and OHCA | CC 59.7% mCC 74.3% | CC 27.2% mCC 47.8% | CC 0.04% mCC 18.6% | CC 0.6% mCC 6.2% | CC 1.2% mCC 8.9% | CC 0.6% mCC 2.7% | CC 1.4% mCC 9.7% | - |
| Miller 2014 [10] | Systematic review with pooled data analysis from 27 studies. Injuries detected by chest radiographs and/or CT scan and/or ultrasound after IHCA and OHCA | 31.2% CC 25.9% mCC 32.7% | 15.1% CC 8.5% mCC 25.8% | 1.7% CC 0% mCC 2.8% | 2.5% CC 2.1% mCC 2.6% | 2.1% CC 3.9% mCC 6.3% | 7.5% | - | - |
| Ram 2018 [23] | Review including 23 studies with autopsies and/or chest radiographs and/or CT scans from > 53.000 non-traumatic and traumatic IHCA and OHCA | 27–90% | 4–21% | 1.7–41% | - | - | 7.5% | 0.6–3% | - |
| Lafuente-Lafuente 2013 [27] | Analysis of autopsy-documented injury caused by standard mCC vs. AD-mCC after 221 OHCA | 69% mCC 67% AD-mCC 72% | 46% mCC 44% AD-mCC 48% | 5% mCC 3% AD-mCC 8% | 18% mCC16% AD-mCC 20% | 2% mCC 0% AD-mCC 4% | 1% mCC 2% AD-mCC 0% |
| Lung Injury | Incidence |
|---|---|
| Consolidations only | 0–14% |
| Ground-glass opacities only | 0–11% |
| Concurrent consolidations and ground-glass opacities | 74–100% |
| Unilateral (only right or left lung) injuries | 0–46% |
| Bilateral (both right and left lung) injuries | 54–100% |
| Location in the dependent lung regions | 83–95% |
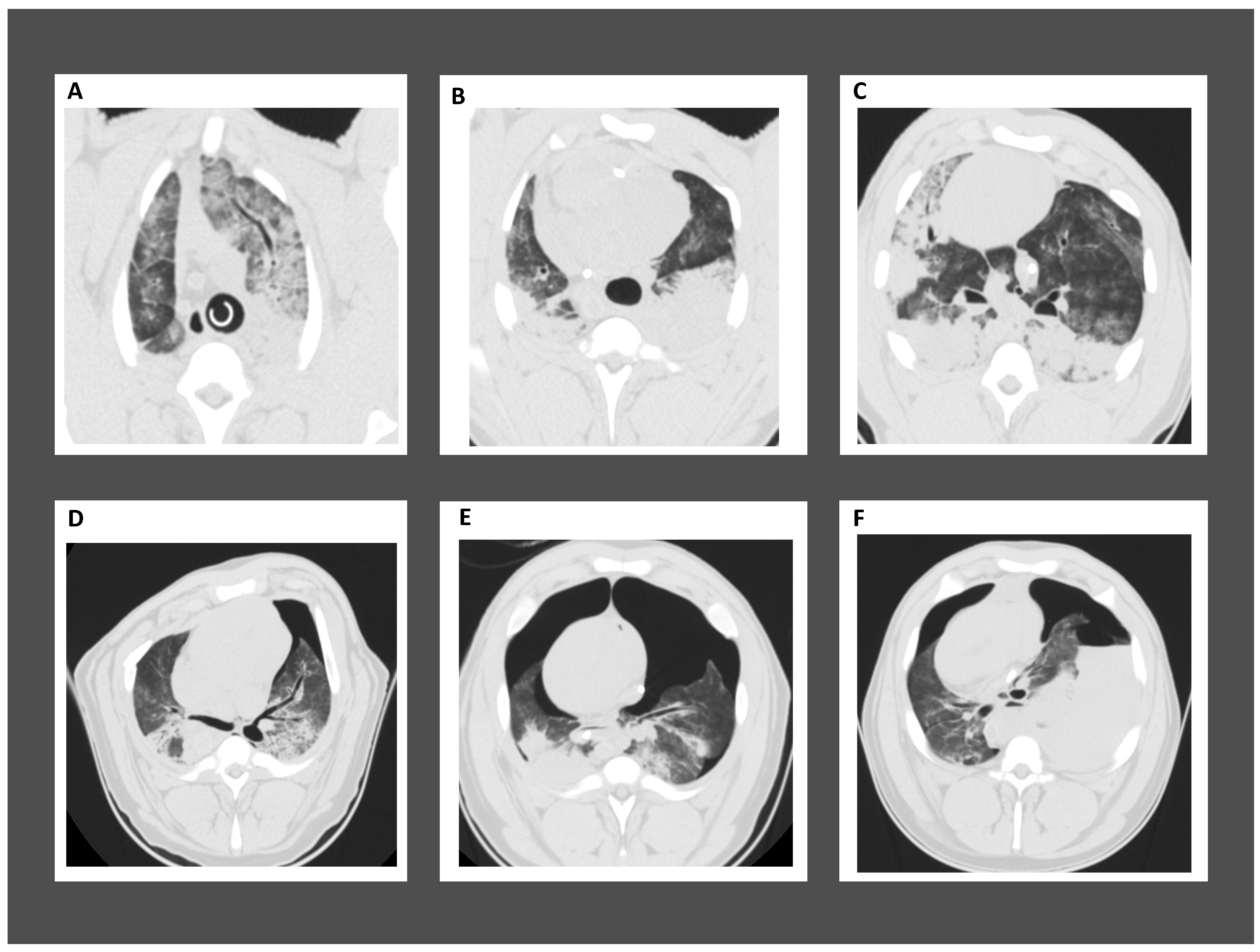
3. ALI in Post-Cardiac Arrest Patients
4. Cardiopulmonary Resuscitation-Associated Lung Edema (CRALE)
5. Ventilation Strategies and ALI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| AD-mCC | mechanical chest compression with active decompression |
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| CC | chest compression |
| CPR | cardiopulmonary resuscitation |
| CPV | cardiopulmonary ventilation |
| CRALE | cardiopulmonary resuscitation-induced lung edema |
| Crs | respiratory system compliance |
| CT | computed tomography |
| EELV | end-expiratory lung volume |
| ICU | intensive care unit |
| IHCA | in-hospital cardiac arrest |
| HU | Hounsfield unit |
| mCC | mechanical chest compression |
| OR | odds ratio |
| PaO2/FiO2 | oxygen arterial partial pressure/oxygen inspiratory fraction |
| PCAS | post-cardiac arrest syndrome |
| PEEP | positive end-expiratory pressure |
| OHCA | out-of-hospital cardiac arrest |
| Rrs | respiratory system resistance |
| ROSC | return of spontaneous circulation |
| VD/Vt | dead space |
| VILI | ventilator-induced lung injury |
References
- Penna, A.; Magliocca, A.; Merigo, G.; Stirparo, G.; Silvestri, I.; Fumagalli, F.; Ristagno, G. One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials. J. Clin. Med. 2023, 12, 2235. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.-T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Olds, K.; Byard, R.W.; Langlois, N.E. Injuries associated with resuscitation—An overview. J. Forensic Leg. Med. 2015, 33, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Rudinská, L.I.; Hejna, P.; Ihnát, P.; Tomášková, H.; Smatanová, M.; Dvořáček, I. Intra-thoracic injuries associated with cardiopulmonary resuscitation—Frequent and serious. Resuscitation 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hoke, R.S.; Chamberlain, D. Skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation 2004, 63, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Smekal, D.; Lindgren, E.; Sandler, H.; Johansson, J.; Rubertsson, S. CPR-related injuries after manual or mechanical chest compressions with the LUCAS™ device: A multicentre study of victims after unsuccessful resuscitation. Resuscitation 2014, 85, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Karasek, J.; Blankova, A.; Doubková, A.; Pitasova, T.; Nahalka, D.; Bartes, T.; Hladik, J.; Adamek, T.; Strycek, M.; Jirasek, T.; et al. Trauma associated with cardiopulmonary resuscitation based on autopsy reports after the 2015 ERC guidelines. Am. J. Emerg. Med. 2022, 61, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ondruschka, B.; Baier, C.; Bayer, R.; Hammer, N.; Dreßler, J.; Bernhard, M. Chest compression-associated injuries in cardiac arrest patients treated with manual chest compressions versus automated chest compression devices (LUCAS II)—A forensic autopsy-based comparison. Forensic Sci. Med. Pathol. 2018, 14, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, Y.; Sasakawa, T.; Tampo, A.; Kawata, D.; Nishiura, T.; Kokita, N.; Iwasaki, H.; Fujita, S. Computed tomography findings of complications resulting from cardiopulmonary resuscitation. Resuscitation 2015, 88, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Rosati, S.F.; Suffredini, A.F.; Schrump, D.S. A systematic review and pooled analysis of CPR-associated cardiovascular and thoracic injuries. Resuscitation 2014, 85, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, C.T.; Tsokos, M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009, 35, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Azeli, Y.; Lorente Olazabal, J.V.; Monge García, M.I.; Bardají, A. Understanding the Adverse Hemodynamic Effects of Serious Thoracic Injuries During Cardiopulmonary Resuscitation: A Review and Approach Based on the Campbell Diagram. Front. Physiol. 2019, 10, 1475. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.J.; Caldwell, E.; Carlbom, D.J.; Gaieski, D.F.; Prekker, M.E.; Rea, T.D.; Sayre, M.; Hough, C.L. The acute respiratory distress syndrome after out-of-hospital cardiac arrest: Incidence, risk factors, and outcomes. Resuscitation 2019, 135, 37–44. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.J.; Carlbom, D.J.; Gaieski, D.F. Ventilator Management and Respiratory Care After Cardiac Arrest: Oxygenation, Ventilation, Infection, and Injury. Chest 2018, 153, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zheng, H.; Lin, L.; Hou, J.; Wen, C.; Wang, Y.; Ling, Q.; Jiang, L.; Tang, W.; Chen, R. Alterations in Respiratory Mechanics and Neural Respiratory Drive After Restoration of Spontaneous Circulation in a Porcine Model Subjected to Different Downtimes of Cardiac Arrest. J. Am. Heart Assoc. 2019, 8, e012441. [Google Scholar] [CrossRef] [PubMed]
- Magliocca, A.; Rezoagli, E.; Zani, D.; Manfredi, M.; De Giorgio, D.; Olivari, D.; Fumagalli, F.; Langer, T.; Avalli, L.; Grasselli, G.; et al. Cardiopulmonary Resuscitation-associated Lung Edema (CRALE). A Translational Study. Am. J. Respir. Crit. Care Med. 2021, 203, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Beloncle, F.M.; Merdji, H.; Lesimple, A.; Pavlovsky, B.; Yvin, E.; Savary, D.; Mercat, A.; Meziani, F.; Richard, J.C. Gas Exchange and Respiratory Mechanics after a Cardiac Arrest: A Clinical Description of Cardiopulmonary Resuscitation-associated Lung Edema. Am. J. Respir. Crit. Care Med. 2022, 206, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.M.; Bray, J.E.; Ng, K.-C.; Liley, H.G.; Greif, R.; Carlson, J.N.; Morley, P.T.; Drennan, I.R.; Smyth, M.; Scholefield, B.R.; et al. 2023 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Resuscitation 2024, 195, 109992. [Google Scholar] [CrossRef]
- Ristagno, G.; Pellis, T.; Semeraro, F.; Italian Resuscitation Council. The nonsense paradigm of rethinking the second link of the chain of survival: “if shock is not advised, wait and do nothing!” Aren’t we condemning our cardiac arrest patients? Am. Heart J. 2016, 176, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.T. Complications following closed-chest cardiac massage. JAMA 1962, 181, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.; Menezes, R.G.; Sirinvaravong, N.; Luis, S.A.; Hussain, S.A.; Madadin, M.; Lasrado, S.; Eiger, G. Breaking your heart-A review on CPR-related injuries. Am. J. Emerg. Med. 2018, 36, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, E.Y.; Choi, S.J.; Kim, Y.K.; Sung, Y.M.; Choi, H.-Y.; Cho, J.; Yang, H.J. Multidetector CT and radiographic findings of lung injuries secondary to cardiopulmonary resuscitation. Injury 2013, 44, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Oya, S.; Shinjo, T.; Fujii, Y.; Kamo, J.; Teruya, H.; Kinoshita, H. CPR related thoracic injury: A comparison of CPR guidelines between 2005 and 2010. Acute Med. Surg. 2016, 3, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Handley, A.J.; Koster, R.W.; Castrén, M.; Smyth, M.A.; Olasveengen, T.; Monsieurs, K.G.; Raffay, V.; Gräsner, J.T.; Wenzel, V.; et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation 2010, 81, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Melero-Bascones, M. Active chest compression-decompression for cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 2013, 2013, CD002751. [Google Scholar] [CrossRef] [PubMed]
- Petrovich, P.; Berve, P.O.; Roald, B.B.-H.; Kongsgård, H.W.; Stray-Pedersen, A.; Kramer-Johansen, J.; Wik, L. Injuries associated with mechanical chest compressions and active decompressions after out-of-hospital cardiac arrest: A subgroup analysis of non-survivors from a randomized study. Resusc. Plus. 2023, 13, 100362. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.J.; Cha, Y.K.; Kim, J.S.; Do, H.H.; Bak, S.H.; Kwack, W.G. Computed tomographic findings of chest injuries following cardiopulmonary resuscitation: More complications for prolonged chest compressions? Medicine 2020, 99, e21685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Wu, G.; Li, H.; Wang, Y.; Chen, R.; Wen, C.; Ling, Q.; Yang, Z.; Tang, W. Quantitative CT assessment of lung injury after successful cardiopulmonary resuscitation in a porcine cardiac arrest model of different downtimes. Quant. Imaging Med. Surg. 2018, 8, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.; Jaeger, D.; Luo, Y.; Lardenois, E.; Badat, B.; Roquet, F.E.; Rigollot, M.; Kimmoun, A.; Tran, N.; Richard, J.-C.M.; et al. Impact of different ventilation strategies on gas exchanges and circulation during prolonged mechanical cardio-pulmonary resuscitation in a porcine model. Shock 2022, 58, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.W.; Kilgannon, J.H.; Chansky, M.E.; Mittal, N.; Wooden, J.; Parrillo, J.E.; Trzeciak, S. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit. Care Med. 2013, 41, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition of Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Ganie, F.A.; Lone, H.; Lone, G.N.; Wani, M.L.; Singh, S.; Dar, A.M.; Wani, N.-U.; Wani, S.N.; Nazeer, N.-U. Lung Contusion: A Clinico-Pathological Entity with Unpredictable Clinical Course. Bull. Emerg. Trauma. 2013, 1, 7–16. [Google Scholar] [PubMed]
- Bakowitz, M.; Bruns, B.; McCunn, M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Dohi, S. Postcardiopulmonary resuscitation pulmonary edema. Crit. Care Med. 1983, 11, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Böttiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Verin, A.D.; Black, S.M.; Catravas, J.D. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem. Pharmacol. 2009, 77, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.W.; Rea, T.D.; Becker, L.J.; Eisenberg, M.S. The incidence and significance of emesis associated with out-of-hospital cardiac arrest. Resuscitation 2007, 74, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Geurts, M.; Macleod, M.R.; Kollmar, R.; Kremer, P.H.; van der Worp, H.B. Therapeutic hypothermia and the risk of infection: A systematic review and meta-analysis. Crit. Care Med. 2014, 42, 231–242. [Google Scholar] [CrossRef]
- Behringer, W.; Böttiger, B.W.; Biasucci, D.G.; Chalkias, A.; Connolly, J.; Dodt, C.; Khoury, A.; Laribi, S.; Leach, R.; Ristagno, G. Temperature control after successful resuscitation from cardiac arrest in adults: A joint statement from the European Society for Emergency Medicine and the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2024, 41, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Mongardon, N.; Perbet, S.; Lemiale, V.; Dumas, F.; Poupet, H.; Charpentier, J.; Péne, F.; Chiche, J.-D.; Mira, J.-P.; Cariou, A. Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit. Care Med. 2011, 39, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Perbet, S.; Mongardon, N.; Dumas, F.; Bruel, C.; Lemiale, V.; Mourvillier, B.; Carli, P.; Varenne, O.; Mira, J.-P.; Wolff, M.; et al. Early-onset pneumonia after cardiac arrest: Characteristics, risk factors and influence on prognosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.C.; Kim, H.I.; Kim, O.H.; Cha, Y.S.; Kim, H.; Lee, K.H.; Hwang, S.O. Echocardiographic patterns of postresuscitation myocardial dysfunction. Resuscitation 2018, 124, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Chonde, M.D.; Shafton, A.; Abu-Daya, H.; Chalhoub, D.; Althouse, A.D.; Rittenberger, J.C. Echocardiographic left ventricular systolic dysfunction early after resuscitation from cardiac arrest does not predict mortality or vasopressor requirements. Resuscitation 2016, 106, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Laurent, I.; Monchi, M.; Chiche, J.-D.; Joly, L.-M.; Spaulding, C.; Bourgeois, B.; Cariou, A.; Rozenberg, A.; Carli, P.; Weber, S.; et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2002, 40, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Ornato, J.P.; Ryschon, T.W.; Gonzalez, E.R.; Bredthauer, J.L. Rapid change in pulmonary vascular hemodynamics with pulmonary edema during cardiopulmonary resuscitation. Am. J. Emerg. Med. 1985, 3, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.G.; Abella, B.S.; Amorim, E.; Bader, M.K.; Barletta, J.F.; Berg, K.; Callaway, C.W.; Friberg, H.; Gilmore, E.J.; Greer, D.M.; et al. Critical Care Management of Patients After Cardiac Arrest: A Scientific Statement from the American Heart Association and Neurocritical Care Society. Circulation 2024, 149, e168–e200. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; Pelosi, P.; Robba, C. Ten rules for optimizing ventilatory settings and targets in post-cardiac arrest patients. Crit. Care 2022, 26, 390. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, R.L.; Brochard, L.; Suppan, L.; Lyazidi, A.; Templier, F.; Khoury, A.; Delisle, S.; Savary, D.; Richard, J.-C. How Ventilation Is Delivered During Cardiopulmonary Resuscitation: An International Survey. Respir. Care 2018, 63, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- McDannold, R.; Bobrow, B.J.; Chikani, V.; Silver, A.; Spaite, D.W.; Vadeboncoeur, T. Quantification of ventilation volumes produced by compressions during emergency department cardiopulmonary resuscitation. Am. J. Emerg. Med. 2018, 36, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, R.L.; Grieco, D.L.; Charbonney, E.; Richard, J.C.; Savary, D. New physiological insights in ventilation during cardiopulmonary resuscitation. Curr. Opin. Crit. Care 2019, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ayres, S.M.; Grace, W.J. Inappropriate ventilation and hypoxemia as causes of cardiac arrhythmias. The control of arrhythmias without antiarrhythmic drugs. Am. J. Med. 1969, 46, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kerber, R.E.; Sarnat, W. Factors influencing the success of ventricular defibrillation in man. Circulation 1979, 60, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, T.P.; Lurie, K.G. Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit. Care Med. 2004, 32 (Suppl. S9), S345–S351. [Google Scholar] [CrossRef] [PubMed]
- Muizelaar, J.P.; Marmarou, A.; Ward, J.D.; Kontos, H.A.; Choi, S.C.; Becker, D.P.; Gruemer, H.; Young, H.F. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized clinical trial. J. Neurosurg. 1991, 75, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Orso, D.; Vetrugno, L.; Federici, N.; Borselli, M.; Spadaro, S.; Cammarota, G.; Bove, T. Mechanical Ventilation Management During Mechanical Chest Compressions. Respir. Care 2021, 66, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhu, A.; Tang, Y. Continuous compression with asynchronous ventilation improves CPR prognosis? A meta-analysis from human and animal studies. Am. J. Emerg. Med. 2023, 64, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Leroux, B.; Wang, H.; Callaway, C.W.; Sopko, G.; Weisfeldt, M.; Stiell, I.; Morrison, L.J.; Aufderheide, T.P.; Cheskes, S.; et al. Trial of Continuous or Interrupted Chest Compressions during CPR. N. Engl. J. Med. 2015, 373, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Tangpaisarn, T.; Tosibphanom, J.; Sata, R.; Kotruchin, P.; Drumheller, B.; Phungoen, P. The effects of mechanical versus bag-valve ventilation on gas exchange during cardiopulmonary resuscitation in emergency department patients: A randomized controlled trial (CPR-VENT). Resuscitation 2023, 193, 109966. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Tejedor, A.; Puebla, V.G.; Torres, E.C.; Sánchez, A.B.; López, R.P.; Calategui, M.D.G. Ventilatory improvement with mechanical ventilator versus bag in non-traumatic out-of-hospital cardiac arrest: SYMEVECA study, phase 1. Resuscitation 2023, 192, 109965. [Google Scholar] [CrossRef] [PubMed]
- Malinverni, S.; Wilmin, S.; de Longueville, D.; Sarnelli, M.; Vermeulen, G.; Kaabour, M.; Van Nuffelen, M.; Hubloue, I.; Scheyltjens, S.; Manara, A.; et al. A retrospective comparison of mechanical cardio-pulmonary ventilation and manual bag valve ventilation in non-traumatic out-of-hospital cardiac arrests: A study from the Belgian cardiac arrest registry. Resuscitation 2024, 4, 110203. [Google Scholar] [CrossRef] [PubMed]
- Beitler, J.R.; Ghafouri, T.B.; Jinadasa, S.P.; Mueller, A.; Hsu, L.; Anderson, R.J.; Joshua, J.; Tyagi, S.; Malhotra, A.; Sell, R.E.; et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am. J. Respir. Crit. Care Med. 2017, 195, 1198–1206. [Google Scholar] [CrossRef]
- Tiruvoipati, R.; Pilcher, D.; Botha, J.; Buscher, H.; Simister, R.; Bailey, M. Association of Hypercapnia and Hypercapnic Acidosis with Clinical Outcomes in Mechanically Ventilated Patients With Cerebral Injury. JAMA Neurol. 2018, 75, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, G.; Nichol, A.D.; Hodgson, C.; Parke, R.L.; McGuinness, S.; Nielsen, N.; Bernard, S.; Skrifvars, M.B.; Stub, D.; Taccone, F.S.; et al. Mild Hypercapnia or Normocapnia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2023, 389, 45–57. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchese, G.; Bungaro, E.; Magliocca, A.; Fumagalli, F.; Merigo, G.; Semeraro, F.; Mereto, E.; Babini, G.; Roman-Pognuz, E.; Stirparo, G.; et al. Acute Lung Injury after Cardiopulmonary Resuscitation: A Narrative Review. J. Clin. Med. 2024, 13, 2498. https://doi.org/10.3390/jcm13092498
Marchese G, Bungaro E, Magliocca A, Fumagalli F, Merigo G, Semeraro F, Mereto E, Babini G, Roman-Pognuz E, Stirparo G, et al. Acute Lung Injury after Cardiopulmonary Resuscitation: A Narrative Review. Journal of Clinical Medicine. 2024; 13(9):2498. https://doi.org/10.3390/jcm13092498
Chicago/Turabian StyleMarchese, Giuseppe, Elisabetta Bungaro, Aurora Magliocca, Francesca Fumagalli, Giulia Merigo, Federico Semeraro, Elisa Mereto, Giovanni Babini, Erik Roman-Pognuz, Giuseppe Stirparo, and et al. 2024. "Acute Lung Injury after Cardiopulmonary Resuscitation: A Narrative Review" Journal of Clinical Medicine 13, no. 9: 2498. https://doi.org/10.3390/jcm13092498
APA StyleMarchese, G., Bungaro, E., Magliocca, A., Fumagalli, F., Merigo, G., Semeraro, F., Mereto, E., Babini, G., Roman-Pognuz, E., Stirparo, G., Cucino, A., & Ristagno, G. (2024). Acute Lung Injury after Cardiopulmonary Resuscitation: A Narrative Review. Journal of Clinical Medicine, 13(9), 2498. https://doi.org/10.3390/jcm13092498






