Associations between Corneal Nerve Structure and Function in a Veteran Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Ocular Examination
- Inflammation assessed with a qualitative measure of matrix metalloproteinase 9 (MMP-9) (InflammaDry, Quidel, San Diego, CA, USA) [23] graded as 0 = none, 1 = mild, 2 = moderate, and 3 = severe;
- Corneal sensation assessed with a cotton-tipped swap and qualitatively graded as 0 = absent, 1 = reduced, 2 = normal, and 3 = increased;
- Tear stability measured via tear break-up time (TBUT), 5 μL of fluorescein placed, and 3 measurements recorded and averaged. A TBUT value > 8–10 s is usually considered healthy. Lower scores indicate faster tear evaporation and potential dry eye disease;
- Fluorescein corneal staining graded using the National Eye Institute (NEI) scale [24]; graded in 5 areas on a scale of 0 to 3 and scores summed (total range of 0–15). 0 = no staining or damage, 1 = mild staining (sparse or superficial spots), 2 = moderate staining (confluent spots), 3 = severe staining (intense, confluent staining or large areas covered). A healthy result on this test would be a score of 0 in all zones, indicating no observable damage or dye uptake;
- Pain intensity using a 0–10 NRS assessed before and 30 s after application of 10 µL of proparacaine hydrochloride 0.5% (one drop in each eye). A score of 0 indicates no pain and 10 represents the worst possible pain;
- Tear production using the Schirmer test at 5 min, measured in mm with anesthesia for measurement of basal tear secretion. We recognize that this test does not assess reflex tear secretion but was selected to optimize comfort. Results range from 0 to 35 mm; a score below 10 mm indicates reduced tear production;
- Intraocular pressure was measured using the Tonopen XL® applanation tonometer (Reichert, Depew, NY, USA). Healthy values range from 10 to 21 mmHg.
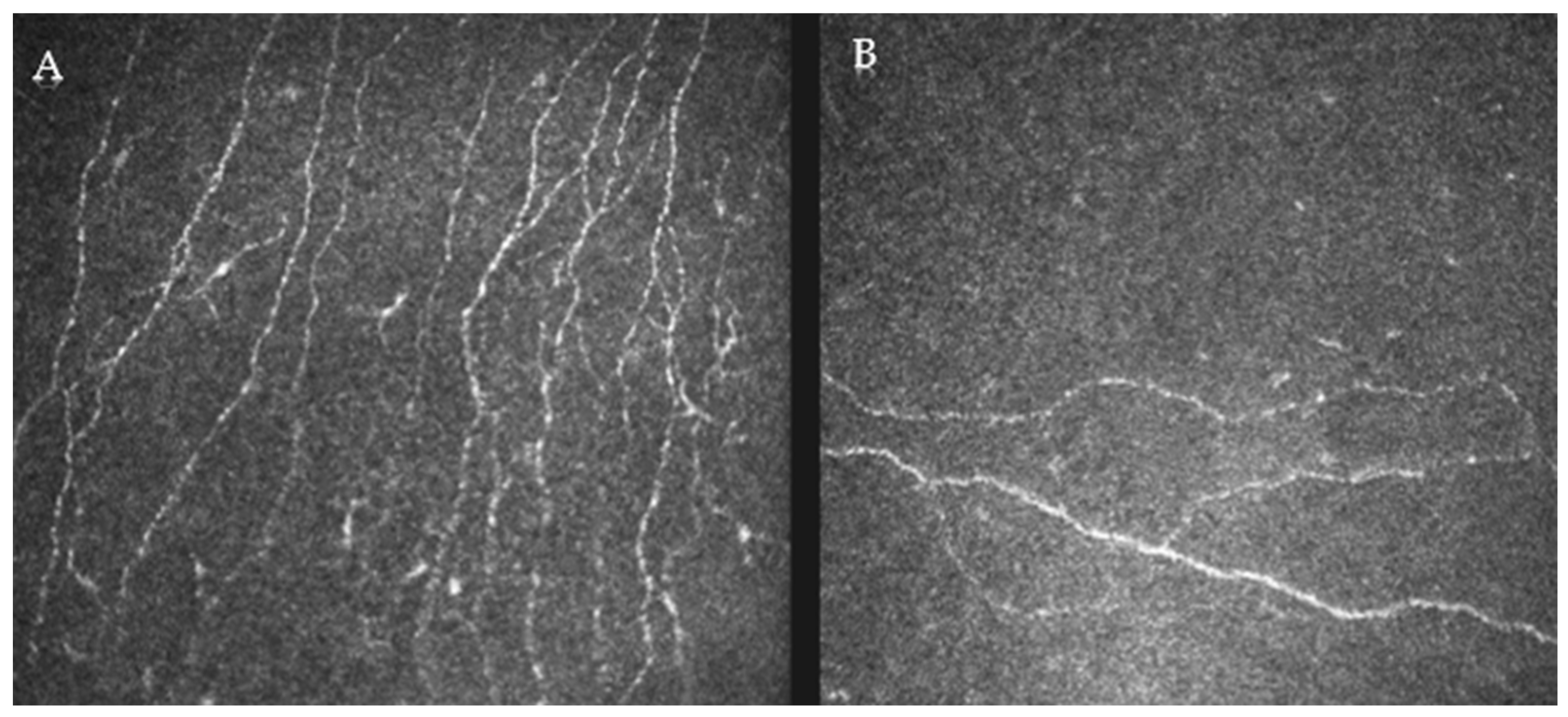
2.4. Confocal Analysis
2.5. Data Analysis
3. Results
3.1. Study Population
3.2. Corneal Structure and Function Correlations
3.3. Multivariable Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Yang, A.Y.; Chow, J.; Liu, J. Corneal Innervation and Sensation: The Eye and Beyond. Yale J. Biol. Med. 2018, 91, 13–21. [Google Scholar] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Di Cello, L.; Pellegrini, M.; Vagge, A.; Borselli, M.; Ferro Desideri, L.; Scorcia, V.; Giannaccare, G. Advances in the Noninvasive Diagnosis of Dry Eye Disease. Appl. Sci. 2021, 11, 10384. [Google Scholar] [CrossRef]
- Kundu, G.; Shetty, R.; D’Souza, S.; Khamar, P.; Nuijts, R.; Sethu, S.; Roy, A.S. A novel combination of corneal confocal microscopy, clinical features and artificial intelligence for evaluation of ocular surface pain. PLoS ONE 2022, 17, e0277086. [Google Scholar] [CrossRef]
- Labbe, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hwang, J.; Mehra, D.; Galor, A. Corneal Nerve Abnormalities in Ocular and Systemic Diseases. Exp. Eye Res. 2021, 202, 108284. [Google Scholar] [CrossRef] [PubMed]
- Khamar, P.; Nair, A.P.; Shetty, R.; Vaidya, T.; Subramani, M.; Ponnalagu, M.; Dhamodaran, K.; D’Souza, S.; Ghosh, A.; Pahuja, N.; et al. Dysregulated Tear Fluid Nociception-Associated Factors, Corneal Dendritic Cell Density, and Vitamin D Levels in Evaporative Dry Eye. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2532–2542. [Google Scholar] [CrossRef]
- Hwang, J.; Dermer, H.; Galor, A. Can in vivo confocal microscopy differentiate between sub-types of dry eye disease? A review. Clin. Exp. Ophthalmol. 2021, 49, 373–387. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Spierer, O.; Felix, E.R.; McClellan, A.L.; Parel, J.M.; Gonzalez, A.; Feuer, W.J.; Sarantopoulos, C.D.; Levitt, R.C.; Ehrmann, K.; Galor, A. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Investig. Ophthalmol. Vis. Sci. 2016, 57, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Acosta, M.C.; Borderie, V.; Borras, F.; Gallar, J.; Bury, T.; Laroche, L.; Belmonte, C. Decreased corneal sensitivity in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Pflugfelder, S.C. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am. J. Ophthalmol. 2004, 137, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.C.; Lang, A.J.; Norman, S.B. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety 2011, 28, 596–606. [Google Scholar] [CrossRef]
- Amtmann, D.; Bamer, A.M.; Noonan, V.; Lang, N.; Kim, J.; Cook, K.F. Comparison of the psychometric properties of two fatigue scales in multiple sclerosis. Rehabil. Psychol. 2012, 57, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Chalmers, R.L.; Begley, C.G.; Caffery, B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont. Lens Anterior Eye 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miro, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain. 2018, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.; Feuer, W.; Galor, A.; Bouhassira, D.; Levitt, R.C.; Sarantopoulos, C.D.; Felix, E.R. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019, 160, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Lanza, N.L.; Valenzuela, F.; Perez, V.L.; Galor, A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul. Surf. 2016, 14, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar] [PubMed]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Dabbah, M.A.; Chen, X.; Graham, J.; Ponirakis, G.; Boulton, A.J.; et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Moscardelli, F.; Versura, P.; Campos, E.C. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Taroni, L.; Bernabei, F.; Bolognesi, F.; Biglioli, F.; Sebastiani, S.; Moscardelli, F.; Cazzola, F.E.; Campos, E.C. Longitudinal Morphometric Analysis of Sub-Basal Nerve Plexus in Contralateral Eyes of Patients with Unilateral Neurotrophic Keratitis. Curr. Eye Res. 2019, 44, 1047–1053. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Al-Mohammedi, A.; Chen, X.; Ferdousi, M.; Ponirakis, G.; Kemp, H.; Chopra, R.; Hau, S.; Schargus, M.; Vollert, J.; et al. The Utility of Corneal Nerve Fractal Dimension Analysis in Peripheral Neuropathies of Different Etiology. Transl. Vis. Sci. Technol. 2020, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Abingdon, UK, 1988. [Google Scholar]
- Patel, D.V.; Tavakoli, M.; Craig, J.P.; Efron, N.; McGhee, C.N. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea 2009, 28, 735–740. [Google Scholar] [CrossRef]
- Rosenberg, M.E.; Tervo, T.M.; Immonen, I.J.; Muller, L.J.; Gronhagen-Riska, C.; Vesaluoma, M.H. Corneal structure and sensitivity in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2915–2921. [Google Scholar]
- Labbe, A.; Liang, Q.; Wang, Z.; Zhang, Y.; Xu, L.; Baudouin, C.; Sun, X. Corneal nerve structure and function in patients with non-sjogren dry eye: Clinical correlations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5144–5150. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Labbe, A.; Borderie, V.; Hamiche, T.; Dupas, B.; Laroche, L.; Baudouin, C.; Bouheraoua, N. Increased corneal sub-basal nerve density in patients with Sjogren syndrome treated with topical cyclosporine A. Clin. Exp. Ophthalmol. 2017, 45, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Luzu, J.; Labbe, A.; Reaux-Le Goazigo, A.; Rabut, G.; Liang, H.; Dupas, B.; Blautain, B.; Sene, D.; Baudouin, C. In vivo confocal microscopic study of corneal innervation in Sjogren’s Syndrome with or without small fiber neuropathy. Ocul. Surf. 2022, 25, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Chen, J.; Li, L.; Qi, Y.; Zhang, Q.; Wu, Y.; You, Y.; Yang, L.; Moore, J.; Xu, L.; et al. Relationship between ocular surface pain and corneal nerve loss in dry eye diabetics: A cross-sectional study in Shenyang, China. BMJ Open 2023, 13, e076932. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Tran, V.; Wolffsohn, J.S. The impact of dry eye disease on corneal nerve parameters: A systematic review and meta-analysis. Ophthalmic Physiol. Opt. 2023, 43, 1079–1091. [Google Scholar] [CrossRef]
- Karlsson, P.; Hincker, A.M.; Jensen, T.S.; Freeman, R.; Haroutounian, S. Structural, functional, and symptom relations in painful distal symmetric polyneuropathies: A systematic review. Pain 2019, 160, 286–297. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef]
| Demographics | Population (n = 83) |
|---|---|
| Age, years, mean ± SD, range | 55 ± 5, 48–67 |
| Sex, male, % (n) | 89.2% (74) |
| Race, % (n) | |
| White | 57.8% (48) |
| Black | 38.6% (32) |
| Other | 3.60% (3) |
| Ethnicity, % (n) | |
| Hispanic | 39.8% (33) |
| Non-Hispanic | 60.2% (50) |
| Non-ocular comorbidities, % (n) | |
| Hypertension | 38.6% (32) |
| Hyperlipidemia | 50.6% (42) |
| Diabetes mellitus | 16.9% (14) |
| Sleep apnea | 59.8% (49) |
| Benign prostatic hyperplasia | 13.6% (11) |
| Smoking status, % (n) | |
| Never | 63.4% (52) |
| Former | 19.5% (16) |
| Current | 17.1% (14) |
| Mental health, mean ± SD | |
| Depression, PHQ-9 (range 0–27) | 10.16 ± 7.45 |
| Fatigue, MFIS (range 0–84) | 37.46 ± 23.12 |
| PTSD, PCL-M (range 17–85) | 44.74 ± 17.78 |
| Sleep, PSQI (range 0–21) | 11.94 ± 4.57 |
| Oral medications, % (n) | |
| Antianxiety | 14.8% (12) |
| Antidepressant | 27.2% (22) |
| Antihistamine | 28.4% (23) |
| Betablocker | 13.6% (11) |
| Fish oil | 24.7% (20) |
| Multivitamin | 32.9% (27) |
| NSAID | 37.0% (30) |
| Statins | 43.2% (35) |
| Ocular symptoms, mean ± SD | |
| DEQ5 | 8.82 ± 4.80 |
| OSDI | 32.7 ± 25.19 |
| NPSI-Eye | 15.2 ± 18.25 |
| NRS (right now) | 1.57 ± 2.46 |
| NRS (average of 1 week) | 1.90 ± 2.35 |
| NRS (worst in 1 week) | 2.13 ± 2.66 |
| Ocular surface exam * | |
| Inflammation, MMP9, mean ± SD | 0.84 ± 0.91 |
| Corneal sensation, % (n) | |
| Decreased | 6% (5) |
| Normal | 85.5% (71) |
| Increased | 8.40% (7) |
| Tear break up time, seconds, mean ± SD | 9.57 ± 4.26 |
| Corneal staining, mean ± SD | 0.73 ± 1.42 |
| Schirmer score, mean ± SD | 16.6 ± 9.48 |
| Intraocular pressure, mmHg, mean ± SD | 17.24 ± 3.22 |
| Corneal Structure Metrics * | Mean ± SD |
|---|---|
| Corneal nerve fiber density, number/mm2 | 13.45 ± 8.29 |
| Corneal nerve fiber length, mm/mm2 | 9.79 ± 3.96 |
| Corneal nerve branch density, number/mm2 | 13.03 ± 11.90 |
| Corneal nerve fiber area, mm/mm2 | 0.01 ± 0.01 |
| Corneal nerve fiber width, mm/mm2 | 0.02 ± 0.00 |
| Corneal nerve fiber total branch density, number/mm2 | 25.68 ± 19.07 |
| Corneal fractal dimension | 1.43 ± 0.05 |
| CNFD | CNBD | CNFL | CTBD | CNFA | CNFW | CFD | |
|---|---|---|---|---|---|---|---|
| Ocular Symptoms | |||||||
| Dry Eye Questionnaire 5 | −0.11 | −0.14 | −0.04 | −0.03 | 0.15 | 0.04 | −0.06 |
| Ocular Surface Disease Index | 0.05 | 0.03 | 0.09 | 0.02 | 0.01 | −0.14 | 0.05 |
| NRS Now | 0.06 | 0.08 | 0.12 | 0.13 | 0.08 | 0.03 | 0.05 |
| NRS Avg. Week | 0.07 | 0.02 | 0.12 | 0.10 | 0.08 | 0.00 | 0.07 |
| NRS Worst Week | 0.03 | 0.05 | 0.12 | 0.10 | 0.08 | −0.02 | 0.09 |
| NPSI-Eye | −0.02 | 0.05 | 0.08 | 0.14 | 0.15 | −0.06 | 0.02 |
| Ocular Surface Exam * | |||||||
| Inflammation | −0.08 | −0.15 | −0.11 | −0.20 | 0.01 | −0.09 | −0.15 |
| Corneal Sensation | −0.05 | 0.00 | −0.01 | 0.13 | 0.30 ** | −0.09 | −0.04 |
| Tear Break Up Time | −0.03 | 0.15 | 0.04 | 0.03 | −0.17 | −0.10 | −0.05 |
| Corneal Staining | −0.09 | −0.11 | −0.05 | −0.12 | −0.07 | −0.03 | −0.07 |
| Tear Production | −0.25 ** | −0.16 | −0.26 ** | −0.12 | 0.01 | 0.23 ** | −0.26 ** |
| Intraocular Pressure | −0.14 | −0.11 | −0.17 | −0.10 | 0.22 | 0.06 | −0.20 |
| Beta | p Value | ||
|---|---|---|---|
| Tear Production | Corneal Fractal Dimension | −0.26 | 0.02 |
| Corneal Sensation | Corneal Nerve Fiber Area | 0.30 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoubi, M.; Cabrera, K.; Locatelli, E.V.; Felix, E.R.; Galor, A. Associations between Corneal Nerve Structure and Function in a Veteran Population. J. Clin. Med. 2024, 13, 2513. https://doi.org/10.3390/jcm13092513
Ayoubi M, Cabrera K, Locatelli EV, Felix ER, Galor A. Associations between Corneal Nerve Structure and Function in a Veteran Population. Journal of Clinical Medicine. 2024; 13(9):2513. https://doi.org/10.3390/jcm13092513
Chicago/Turabian StyleAyoubi, Mohammad, Kimberly Cabrera, Elyana VT Locatelli, Elizabeth R. Felix, and Anat Galor. 2024. "Associations between Corneal Nerve Structure and Function in a Veteran Population" Journal of Clinical Medicine 13, no. 9: 2513. https://doi.org/10.3390/jcm13092513
APA StyleAyoubi, M., Cabrera, K., Locatelli, E. V., Felix, E. R., & Galor, A. (2024). Associations between Corneal Nerve Structure and Function in a Veteran Population. Journal of Clinical Medicine, 13(9), 2513. https://doi.org/10.3390/jcm13092513






