Premature Ventricular Contraction-Induced Cardiomyopathy: Contemporary Evidence from Risk Stratification, Pathophysiology, and Management
Abstract
1. Introduction
2. Risk Factors
3. Pathophysiology
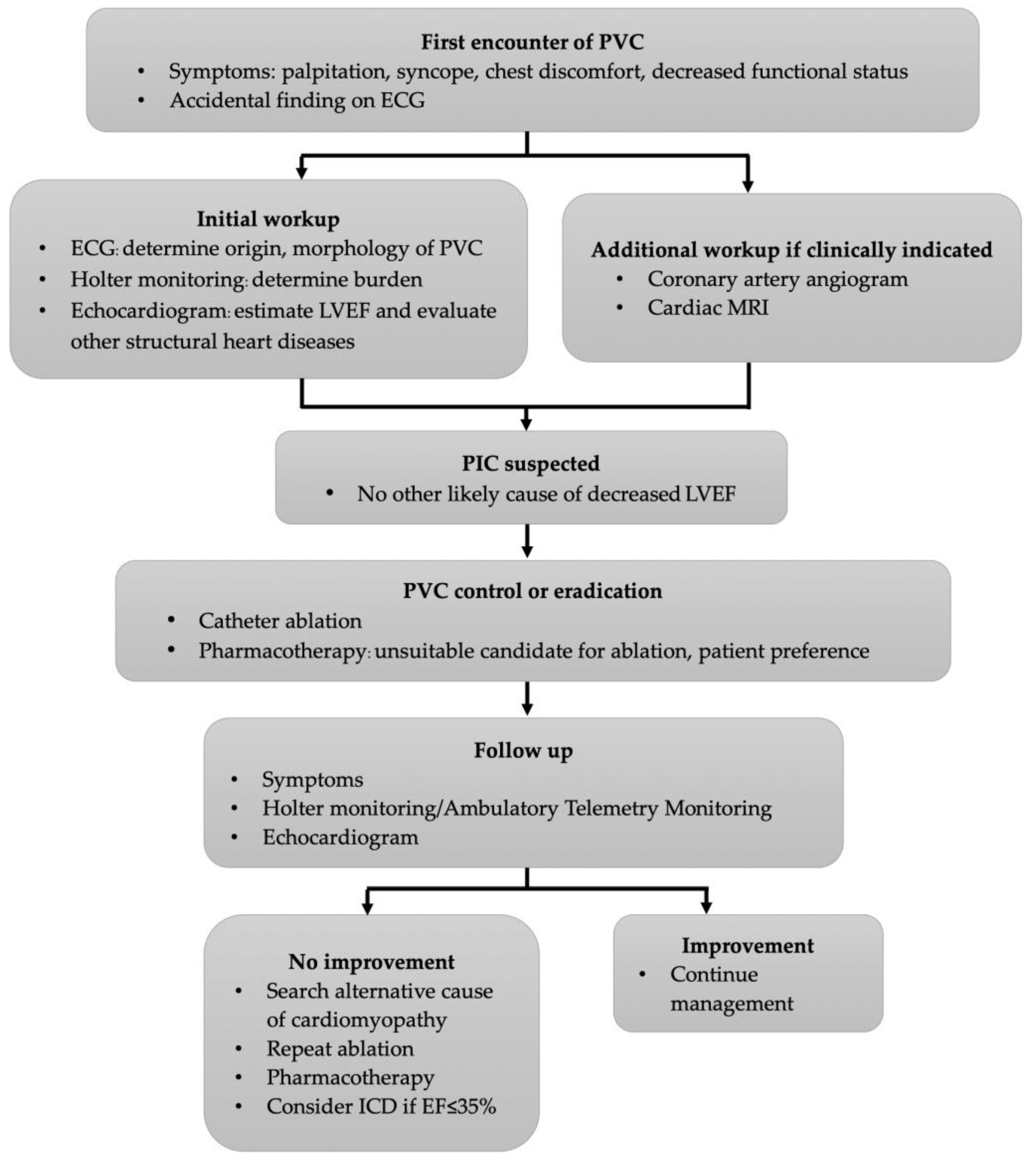
4. Diagnosis
5. Treatment
5.1. Lifestyle Modification
5.2. Catheter Ablation
5.3. Pharmacologic Treatment
5.4. Prognosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, H.L.; Whitlock, J.A.; Sprague, M.K.; Kennedy, L.J.; Buckingham, T.A.; Goldberg, R.J. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N. Engl. J. Med. 1985, 312, 193–197. [Google Scholar] [CrossRef]
- Mountantonakis, S.E.; Frankel, D.S.; Gerstenfeld, E.P.; Dixit, S.; Lin, D.; Hutchinson, M.D.; Riley, M.; Bala, R.; Cooper, J.; Callans, D.; et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: Effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011, 8, 1608–1614. [Google Scholar] [CrossRef]
- Sheldon, S.H.; Gard, J.J.; Asirvatham, S.J. Premature Ventricular Contractions and Non-sustained Ventricular Tachycardia: Association with Sudden Cardiac Death, Risk Stratification, and Management Strategies. Indian Pacing Electrophysiol. J. 2010, 10, 357–371. [Google Scholar] [PubMed]
- Latchamsetty, R.; Bogun, F. Premature Ventricular Complex-Induced Cardiomyopathy. JACC Clin. Electrophysiol. 2019, 5, 537–550. [Google Scholar] [CrossRef]
- El Kadri, M.; Yokokawa, M.; Labounty, T.; Mueller, G.; Crawford, T.; Good, E.; Jongnarangsin, K.; Chugh, A.; Ghanbari, H.; Latchamsetty, R.; et al. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm. 2015, 12, 706–713. [Google Scholar] [CrossRef]
- Sarrazin, J.F.; Labounty, T.; Kuhne, M.; Crawford, T.; Armstrong, W.F.; Desjardins, B.; Good, E.; Jongnarangsin, K.; Chugh, A.; Oral, H.; et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009, 6, 1543–1549. [Google Scholar] [CrossRef]
- Niwano, S.; Wakisaka, Y.; Niwano, H.; Fukaya, H.; Kurokawa, S.; Kiryu, M.; Hatakeyama, Y.; Izumi, T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009, 95, 1230–1237. [Google Scholar] [CrossRef]
- Takemoto, M.; Yoshimura, H.; Ohba, Y.; Matsumoto, Y.; Yamamoto, U.; Mohri, M.; Yamamoto, H.; Origuchi, H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J. Am. Coll. Cardiol. 2005, 45, 1259–1265. [Google Scholar] [CrossRef]
- Del Carpio Munoz, F.; Syed, F.F.; Noheria, A.; Cha, Y.M.; Friedman, P.A.; Hammill, S.C.; Munger, T.M.; Venkatachalam, K.L.; Shen, W.K.; Packer, D.L.; et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J. Cardiovasc. Electrophysiol. 2011, 22, 791–798. [Google Scholar] [CrossRef]
- Baman, T.S.; Lange, D.C.; Ilg, K.J.; Gupta, S.K.; Liu, T.Y.; Alguire, C.; Armstrong, W.; Good, E.; Chugh, A.; Jongnarangsin, K.; et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010, 7, 865–869. [Google Scholar] [CrossRef]
- Bogun, F.; Crawford, T.; Reich, S.; Koelling, T.M.; Armstrong, W.; Good, E.; Jongnarangsin, K.; Marine, J.E.; Chugh, A.; Pelosi, F.; et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm. 2007, 4, 863–867. [Google Scholar] [CrossRef]
- Kanei, Y.; Friedman, M.; Ogawa, N.; Hanon, S.; Lam, P.; Schweitzer, P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann. Noninvasive Electrocardiol. 2008, 13, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Olgun, H.; Yokokawa, M.; Baman, T.; Kim, H.M.; Armstrong, W.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Crawford, T.; Oral, H.; et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011, 8, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Hasdemir, C.; Ulucan, C.; Yavuzgil, O.; Yuksel, A.; Kartal, Y.; Simsek, E.; Musayev, O.; Kayikcioglu, M.; Payzin, S.; Kultursay, H.; et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: The incidence, clinical and electrophysiologic characteristics, and the predictors. J. Cardiovasc. Electrophysiol. 2011, 22, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Kim, H.M.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Alguire, C.; Armstrong, W.; Crawford, T.; Jongnarangsin, K.; Oral, H.; et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm. 2012, 9, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Kim, H.M.; Good, E.; Crawford, T.; Chugh, A.; Pelosi, F., Jr.; Jongnarangsin, K.; Latchamsetty, R.; Armstrong, W.; Alguire, C.; et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012, 9, 1460–1464. [Google Scholar] [CrossRef]
- Yarlagadda, R.K.; Iwai, S.; Stein, K.M.; Markowitz, S.M.; Shah, B.K.; Cheung, J.W.; Tan, V.; Lerman, B.B.; Mittal, S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005, 112, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Wijnmaalen, A.P.; Delgado, V.; Schalij, M.J.; van Huls van Taxis, C.F.; Holman, E.R.; Bax, J.J.; Zeppenfeld, K. Beneficial effects of catheter ablation on left ventricular and right ventricular function in patients with frequent premature ventricular contractions and preserved ejection fraction. Heart 2010, 96, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.; Roukoz, H.; Adabag, S.; Benditt, D.G.; Anand, I.; Li, J.M.; Zakharova, M.; Tholakanahalli, V. Improvement of left ventricular diastolic function and left atrial reverse remodeling after catheter ablation of premature ventricular complexes. J. Interv. Card Electrophysiol. 2013, 38, 179–185. [Google Scholar] [CrossRef]
- Shanmugam, N.; Chua, T.P.; Ward, D. F‘requent’ ventricular bigeminy—A reversible cause of dilated cardiomyopathy. How frequent is f‘requent’? Eur. J. Heart Fail. 2006, 8, 869–873. [Google Scholar] [CrossRef]
- Duffee, D.F.; Shen, W.K.; Smith, H.C. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clin. Proc. 1998, 73, 430–433. [Google Scholar] [CrossRef]
- Carballeira Pol, L.; Deyell, M.W.; Frankel, D.S.; Benhayon, D.; Squara, F.; Chik, W.; Kohari, M.; Deo, R.; Marchlinski, F.E. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014, 11, 299–306. [Google Scholar] [CrossRef]
- Potfay, J.; Kaszala, K.; Tan, A.Y.; Sima, A.P.; Gorcsan, J., 3rd; Ellenbogen, K.A.; Huizar, J.F. Abnormal Left Ventricular Mechanics of Ventricular Ectopic Beats: Insights Into Origin and Coupling Interval in Premature Ventricular Contraction-Induced Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2015, 8, 1194–1200. [Google Scholar] [CrossRef]
- Sun, Y.; Blom, N.A.; Yu, Y.; Ma, P.; Wang, Y.; Han, X.; Swenne, C.A.; van der Wall, E.E. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: An echocardiographic evaluation. Int. J. Cardiovasc. Imaging 2003, 19, 295–299. [Google Scholar] [CrossRef]
- Costa, S.; Saguner, A.M.; Gasperetti, A.; Akdis, D.; Brunckhorst, C.; Duru, F. The Link Between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 644279. [Google Scholar] [CrossRef]
- Cannata, A.; Manca, P.; Nuzzi, V.; Gregorio, C.; Artico, J.; Gentile, P.; Pio Loco, C.; Ramani, F.; Barbati, G.; Merlo, M.; et al. Sex-Specific Prognostic Implications in Dilated Cardiomyopathy After Left Ventricular Reverse Remodeling. J. Clin. Med. 2020, 9, 2426. [Google Scholar] [CrossRef]
- Marcus, G.M. Evaluation and Management of Premature Ventricular Complexes. Circulation 2020, 141, 1404–1418. [Google Scholar] [CrossRef]
- Simpson, R.J., Jr.; Cascio, W.E.; Schreiner, P.J.; Crow, R.S.; Rautaharju, P.M.; Heiss, G. Prevalence of premature ventricular contractions in a population of African American and white men and women: The Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2002, 143, 535–540. [Google Scholar] [CrossRef]
- Ghannam, M.; Yokokawa, M.; Liang, J.J.; Cochet, H.; Jais, P.; Dabagh, G.S.; Latchamsetty, R.; Jongnarangsin, K.; Morady, F.; Bogun, F. Clinical significance of myocardial scar in patients with frequent premature ventricular complexes undergoing catheter ablation. Heart Rhythm. 2021, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Latchamsetty, R.; Yokokawa, M.; Morady, F.; Kim, H.M.; Mathew, S.; Tilz, R.; Kuck, K.H.; Nagashima, K.; Tedrow, U.; Stevenson, W.G.; et al. Multicenter Outcomes for Catheter Ablation of Idiopathic Premature Ventricular Complexes. JACC Clin. Electrophysiol. 2015, 1, 116–123. [Google Scholar] [CrossRef]
- Sadron Blaye-Felice, M.; Hamon, D.; Sacher, F.; Pascale, P.; Rollin, A.; Duparc, A.; Mondoly, P.; Derval, N.; Denis, A.; Cardin, C.; et al. Premature ventricular contraction-induced cardiomyopathy: Related clinical and electrophysiologic parameters. Heart Rhythm. 2016, 13, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.E.; Rahmutula, D.; Szilagyi, J.; Alhede, C.; Sievers, R.; Fang, Q.; Olgin, J.; Gerstenfeld, E.P. Left Ventricular Dyssynchrony Predicts the Cardiomyopathy Associated With Premature Ventricular Contractions. J. Am. Coll. Cardiol. 2018, 72, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Kowlgi, G.N.; Ramirez, R.J.; Balderas-Villalobos, J.; Jovin, D.; Parker, C.; Om, E.; Airapetov, S.; Kaszala, K.; Tan, A.Y.; et al. Eccentric hypertrophy in an animal model of mid- and long-term premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. O2 2021, 2, 80–88. [Google Scholar] [CrossRef]
- Huizar, J.F.; Kaszala, K.; Potfay, J.; Minisi, A.J.; Lesnefsky, E.J.; Abbate, A.; Mezzaroma, E.; Chen, Q.; Kukreja, R.C.; Hoke, N.N.; et al. Left ventricular systolic dysfunction induced by ventricular ectopy: A novel model for premature ventricular contraction-induced cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2011, 4, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Villalobos, J.; Medina-Contreras, J.M.L.; Lynch, C.; Kabadi, R.; Hayles, J.; Ramirez, R.J.; Tan, A.Y.; Kaszala, K.; Samso, M.; Huizar, J.F.; et al. Mechanisms of adaptive hypertrophic cardiac remodeling in a large animal model of premature ventricular contraction-induced cardiomyopathy. IUBMB Life 2023, 75, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Rahmutula, D.; Duggirala, S.; Nazer, B.; Fang, Q.; Olgin, J.; Sievers, R.; Gerstenfeld, E.P. Diffuse fibrosis leads to a decrease in unipolar voltage: Validation in a swine model of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2016, 13, 547–554. [Google Scholar] [CrossRef]
- Hasdemir, C.; Yuksel, A.; Camli, D.; Kartal, Y.; Simsek, E.; Musayev, O.; Isayev, E.; Aydin, M.; Can, L.H. Late gadolinium enhancement CMR in patients with tachycardia-induced cardiomyopathy caused by idiopathic ventricular arrhythmias. Pacing Clin. Electrophysiol. 2012, 35, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Eltit, J.M.; Kaszala, K.; Tan, A.; Jiang, M.; Zhang, M.; Tseng, G.N.; Huizar, J.F. Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2014, 11, 2064–2072. [Google Scholar] [CrossRef]
- Balderas-Villalobos, J.; Medina-Contreras, J.M.L.; Lynch, C.; Kabadi, R.; Ramirez, R.J.; Tan, A.Y.; Kaszala, K.; Samso, M.; Huizar, J.F.; Eltit, J.M. Alterations of sarcoplasmic reticulum-mediated Ca(2+) uptake in a model of premature ventricular contraction (PVC)-induced cardiomyopathy. Mol. Cell Biochem. 2023, 478, 1447–1456. [Google Scholar] [CrossRef]
- Deyell, M.W.; Park, K.M.; Han, Y.; Frankel, D.S.; Dixit, S.; Cooper, J.M.; Hutchinson, M.D.; Lin, D.; Garcia, F.; Bala, R.; et al. Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm. 2012, 9, 1465–1472. [Google Scholar] [CrossRef]
- Callans, D.J. Premature Ventricular Contraction-induced Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2017, 6, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.K.; Klarich, K.W.; Grogan, M.; Cha, Y.M. Premature ventricular contraction-induced cardiomyopathy: A treatable condition. Circ. Arrhythm. Electrophysiol. 2012, 5, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Panizo, J.G.; Barra, S.; Mellor, G.; Heck, P.; Agarwal, S. Premature Ventricular Complex-induced Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2018, 7, 128–134. [Google Scholar] [CrossRef]
- Huizar, J.F.; Ellenbogen, K.A.; Tan, A.Y.; Kaszala, K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2328–2344. [Google Scholar] [CrossRef] [PubMed]
- Altintas, B.; Ozkalayci, F.; Cinier, G.; Kaya, I.; Aktan, A.; Kup, A.; Onuk, R.; Ozcan, S.; Uslu, A.; Akyuz, A.; et al. The effect of idiopathic premature ventricular complexes on left ventricular ejection fraction. Ann. Noninvasive Electrocardiol. 2020, 25, e12702. [Google Scholar] [CrossRef]
- Hamon, D.; Blaye-Felice, M.S.; Bradfield, J.S.; Chaachoui, N.; Tung, R.; Elayi, C.S.; Vaseghi, M.; Dhanjal, T.S.; Boyle, N.G.; Maury, P.; et al. A New Combined Parameter to Predict Premature Ventricular Complexes Induced Cardiomyopathy: Impact and Recognition of Epicardial Origin. J. Cardiovasc. Electrophysiol. 2016, 27, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Xie, E.; Chamera, E.; Lima, J.A.C.; Chrispin, J. Cardiac MRI structural and functional predictors of left ventricular ejection fraction recovery following PVC catheter ablation. Sci. Rep. 2021, 11, 8265. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, A.; Mont, L.; Nava, S.; Chueca, E.; Bartholomay, E.; Brugada, J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation 2004, 109, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.V.; Lu, Y.Y.; Morton, J.B.; Santucci, P.A.; Akar, J.G.; Green, A.; Wilber, D.J. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: Electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation 2006, 113, 1659–1666. [Google Scholar] [CrossRef]
- Jame, S.; Liu, Z.; Kolias, T.; Liang, J.; Labounty, T.; Ghannam, M.; Latchamsetty, R.; Jongnarangsin, K.; Morady, F.; Bogun, F. Strain Analysis in Patients with Frequent Premature Ventricular Complexes and Preserved Left Ventricular Function Undergoing Ablation. J. Clin. Med. 2023, 12, 3017. [Google Scholar] [CrossRef]
- Lukas Laws, J.; Lancaster, M.C.; Ben Shoemaker, M.; Stevenson, W.G.; Hung, R.R.; Wells, Q.; Marshall Brinkley, D.; Hughes, S.; Anderson, K.; Roden, D.; et al. Arrhythmias as Presentation of Genetic Cardiomyopathy. Circ Res 2022, 130, 1698–1722. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e533–e557. [Google Scholar] [CrossRef]
- Marcus, G.M.; Rosenthal, D.G.; Nah, G.; Vittinghoff, E.; Fang, C.; Ogomori, K.; Joyce, S.; Yilmaz, D.; Yang, V.; Kessedjian, T.; et al. Acute Effects of Coffee Consumption on Health among Ambulatory Adults. N. Engl. J. Med. 2023, 388, 1092–1100. [Google Scholar] [CrossRef]
- Kerola, T.; Dewland, T.A.; Vittinghoff, E.; Heckbert, S.R.; Stein, P.K.; Marcus, G.M. Modifiable Predictors of Ventricular Ectopy in the Community. J. Am. Heart Assoc. 2018, 7, e010078. [Google Scholar] [CrossRef]
- Ahmed, S.; Hisamatsu, T.; Kadota, A.; Fujiyoshi, A.; Segawa, H.; Torii, S.; Takashima, N.; Kondo, K.; Nakagawa, Y.; Ueshima, H.; et al. Ventricular Premature Complexes and Their Associated Factors in a General Population of Japanese Men. Am. J. Cardiol. 2022, 169, 51–56. [Google Scholar] [CrossRef]
- DeBacker, G.; Jacobs, D.; Prineas, R.; Crow, R.; Vilandre, J.; Kennedy, H.; Blackburn, H. Ventricular premature contractions: A randomized non-drug intervention trial in normal men. Circulation 1979, 59, 762–769. [Google Scholar] [CrossRef]
- Lee, A.; Denman, R.; Haqqani, H.M. Ventricular Ectopy in the Context of Left Ventricular Systolic Dysfunction: Risk Factors and Outcomes Following Catheter Ablation. Heart Lung Circ. 2019, 28, 379–388. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Samir, R. Reversal of premature ventricular complexes induced cardiomyopathy. Influence of concomitant structural heart disease. Indian Heart J. 2018, 70, 410–415. [Google Scholar] [CrossRef]
- Zhong, L.; Lee, Y.H.; Huang, X.M.; Asirvatham, S.J.; Shen, W.K.; Friedman, P.A.; Hodge, D.O.; Slusser, J.P.; Song, Z.Y.; Packer, D.L.; et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: A single-center retrospective study. Heart Rhythm. 2014, 11, 187–193. [Google Scholar] [CrossRef]
- Yokokawa, M.; Good, E.; Crawford, T.; Chugh, A.; Pelosi, F., Jr.; Latchamsetty, R.; Jongnarangsin, K.; Armstrong, W.; Ghanbari, H.; Oral, H.; et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm. 2013, 10, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Mansour, M.C.; Ruskin, J.N.; Heist, E.K. Atrial Fibrillation-Mediated Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2019, 12, e007809. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, R.; Tang, H.; Li, G.; Guan, X.; Yang, Y.; Sun, Y.; Xiao, X.; Yu, X.; Yin, X.; et al. E/E’ Is a New Independent Predictor of Recovered Ejection Fraction in Patients With Systolic Heart Failure Undergoing Ablation for Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 707996. [Google Scholar] [CrossRef] [PubMed]
- Medi, C.; Kalman, J.M.; Haqqani, H.; Vohra, J.K.; Morton, J.B.; Sparks, P.B.; Kistler, P.M. Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: Long-term outcome after catheter ablation. J. Am. Coll. Cardiol. 2009, 53, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Krittayaphong, R.; Bhuripanyo, K.; Punlee, K.; Kangkagate, C.; Chaithiraphan, S. Effect of atenolol on symptomatic ventricular arrhythmia without structural heart disease: A randomized placebo-controlled study. Am. Heart J. 2002, 144, e10. [Google Scholar] [CrossRef] [PubMed]
- Stec, S.; Sikorska, A.; Zaborska, B.; Krynski, T.; Szymot, J.; Kulakowski, P. Benign symptomatic premature ventricular complexes: Short- and long-term efficacy of antiarrhythmic drugs and radiofrequency ablation. Kardiol. Pol. 2012, 70, 351–358. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E.; Boccuzzi, S.J.; Cruess, D.; Nattel, S. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation 1991, 83, 52–60. [Google Scholar] [CrossRef]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L.; et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef]
- Hyman, M.C.; Mustin, D.; Supple, G.; Schaller, R.D.; Santangeli, P.; Arkles, J.; Lin, D.; Muser, D.; Dixit, S.; Nazarian, S.; et al. Class IC antiarrhythmic drugs for suspected premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2018, 15, 159–163. [Google Scholar] [CrossRef]
- Singh, S.N.; Fletcher, R.D.; Fisher, S.G.; Singh, B.N.; Lewis, H.D.; Deedwania, P.C.; Massie, B.M.; Colling, C.; Lazzeri, D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 77–82. [Google Scholar] [CrossRef]
- Penela, D.; Martinez, M.; Fernandez-Armenta, J.; Aguinaga, L.; Tercedor, L.; Ordonez, A.; Acosta, J.; Marti-Almor, J.; Bisbal, F.; Rossi, L.; et al. Influence of myocardial scar on the response to frequent premature ventricular complex ablation. Heart 2019, 105, 378–383. [Google Scholar] [CrossRef]
- Mohanty, S.; Burkhardt, J.D.; Di Biase, L.; Mohanty, P.; Shetty, S.S.; Gianni, C.; Della Rocca, D.G.; Baho, K.K.; Morris, T.; Mayedo, A.; et al. Best ablation strategy in patients with premature ventricular contractions with multiple morphology: A single-centre experience. Europace 2023, 25, euad038. [Google Scholar] [CrossRef] [PubMed]
- Penela, D.; Teres, C.; Fernandez-Armenta, J.; Aguinaga, L.; Tercedor, L.; Soto-Iglesias, D.; Jauregui, B.; Ordonez, A.; Acosta, J.; Bisbal, F.; et al. Premature ventricular complex site of origin and ablation outcomes in patients with prior myocardial infarction. Heart Rhythm. 2021, 18, 27–33. [Google Scholar] [CrossRef]
- Koca, H.; Koca, F.; Icen, Y.K.; Demirtas, A.O.; Aslan, M.Z.; Sumbul, H.E.; Coskun, M.; Erdogdu, T.; Koc, M. Impaired left ventricular global longitudinal strain improves with radiofrequency catheter ablation in patients with PVC-induced cardiomyopathy. Pacing Clin. Electrophysiol. 2020, 43, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Penela, D.; Jauregui, B.; Fernandez-Armenta, J.; Aguinaga, L.; Tercedor, L.; Ordonez, A.; Acosta, J.; Bisbal, F.; Vassanelli, F.; Teres, C.; et al. Influence of baseline QRS on the left ventricular ejection fraction recovery after frequent premature ventricular complex ablation. Europace 2020, 22, 274–280. [Google Scholar] [CrossRef]
- Krishnan, B.; Sankar, A.; Anand, I.; Adabag, S.; Li, J.M.; McFalls, E.O.; Benditt, D.G.; Shivkumar, K.; Tholakanahalli, V.N. Post-Extrasystolic Potentiation as a Predictor of Recovery of Left Ventricular Dysfunction After Radiofrequency Catheter Ablation. JACC Clin. Electrophysiol. 2017, 3, 1283–1291. [Google Scholar] [CrossRef]
- Maeda, S.; Chik, W.W.; Liang, J.J.; Kelesidis, I.; Arroyo, R.C.; D‘Souza, B.; Sadek, M.M.; Muser, D.; Squara, F.; Venugopal, D.; et al. Recovery of renal dysfunction after catheter ablation of outflow tract ventricular arrhythmias in patients with ventricular premature depolarization-mediated cardiomyopathy. J. Interv. Card Electrophysiol. 2017, 48, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sadron Blaye-Felice, M.; Hamon, D.; Sacher, F.; Pascale, P.; Rollin, A.; Bongard, V.; Duparc, A.; Mondoly, P.; Derval, N.; Denis, A.; et al. Reversal of left ventricular dysfunction after ablation of premature ventricular contractions related parameters, paradoxes and exceptions to the rule. Int. J. Cardiol. 2016, 222, 31–36. [Google Scholar] [CrossRef]
- Penela, D.; Acosta, J.; Aguinaga, L.; Tercedor, L.; Ordonez, A.; Fernandez-Armenta, J.; Andreu, D.; Sanchez-Millan, P.J.; Cabanelas, N.; Tolosana, J.M.; et al. Ablation of frequent PVC in patients meeting criteria for primary prevention ICD implant: Safety of withholding the implant. Heart Rhythm. 2015, 12, 2434–2442. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, J.; Na, H.; Chun, K.J.; Im, S.I.; Park, S.J.; Kim, J.S.; On, Y.K. Cardiomyopathy With Frequent Ventricular Premature Depolarization—Predicting Irreversible Ventricular Dysfunction. Circ. J. 2015, 79, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Di Biase, L.; Ryschon, K.; Biria, M.; Swarup, V.; Reddy, Y.M.; Verma, A.; Bommana, S.; Burkhardt, D.; Dendi, R.; et al. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J. Am. Coll. Cardiol. 2012, 60, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Benditt, D.G.; Yu, J.; Graf, B. Effects of catheter ablation of “asymptomatic” frequent ventricular premature complexes in patients with reduced (<48%) left ventricular ejection fraction. Am. J. Cardiol. 2012, 110, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Rayan, M.; Tawfik, M.; Alabd, A.; Gamal, A. Ivabradine, a novel heart rate slower: Is it a sword of double blades in patients with idiopathic dilated cardiomyopathy? Anadolu. Kardiyol. Derg. 2011, 11, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.M.; Maury, P.; Shah, D.; Duparc, A.; Galinier, M.; Delay, M.; Morice, R.; Alfares, A.; Barnay, C. Reversal of dilated cardiomyopathy by the elimination of frequent left or right premature ventricular contractions. J. Interv. Card Electrophysiol. 2007, 20, 9–13. [Google Scholar] [CrossRef]
| Population | Pattern | PVC-Associated Symptom | Method of PVC Burden Assessment | N/Age/Female/Mean F/U | F/U Duration from PVC to Cardiomyopathy | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Frequent PVC (>1000/day) | RVOT, LVOT | No | 2–3 times Holter monitoring | 239/43 ± 13 years/50.6%/5.6 ± 1.7 years | 5.6 ± 1.7 years | PVC burden > 20,000/24 h was associated with LVEF decline and increase LVEDd. | [7] |
| Patients underwent RFA with PVC-associated symptoms | RVOT | Yes | 24 h Holter monitoring | 40/50 ± 2 years/77.8%/8 ± 1 months | Cross-sectional | PVC burden > 20% was associated with a decrease in LVEF, enlarged LVEDd, LVESd, degree of MR, and a higher NYHA functional class. | [8] |
| Patients underwent RFA with PVC-associated symptoms | Any | Yes | 24 or 48 h Holter monitoring | 70/42 ± 17 years/57%/- | Cross-sectional | PVC burden > 10,000 or >20,000/24 h was associated with a decline in LVEF. PVC burden > 10% or >20% was associated with LVEF decline and increase LVEDd. Increased PVC duration > 140 ms was associated with a lower LVEF. The threshold for PVC burden associated with LVEF decline for RV and LV origin PVC was 10% and 20%, respectively. | [9] |
| Patients underwent RFA | Any | Any | 24 h Holter monitoring | 174/48 ± 13 years/50%/- | Cross-sectional | PVC burden > 24% was a predictor of impaired LVEF with a sensitivity of 79% and specificity of 78% (AUC 0.89). There was no difference in RV and LV origin PVC for PVC-induced cardiomyopathy. | [11] |
| Frequent PVC > 10/h underwent RFA with symptomatic PVC-associated | Any | Yes | 24 h Holter monitoring | 60/45 ± 11 years/63.3%/6 months | Cross-sectional | The LVEF, LVESd, and LVEDd were correlated with PVC burden. | [10] |
| Frequent PVC > 10/h | RVOT | - | 24 h Holter monitoring | 108/50 ± 16 years/69%/- | Cross-sectional | Higher PVC burden was associated with LV dysfunction. PVC > 10,000/24 h was an independent predictor of LV dysfunction. | [12] |
| Frequent PVC referred for RFA | RVOT | Any | 24 h Holter monitoring | 27/47 ± 15 years/59.3%/- | Cross-sectional | There was no difference in PVC/24 h between impaired and normal LVEF. | [17] |
| Frequent PVC referred for RFA | Any | Any | 24 h Holter monitoring | 51/49 ± 15 years/27.5%/- | Cross-sectional | PVC interpolation was associated with a higher PVC burden and impaired LVEF. | [13] |
| Frequent PVC | Any | Any | 24 h Holter monitoring | 183/49 ± 15 years/59.4%/- | Cross-sectional | PVC burden was associated with cardiomyopathy, but not the total heart rate. PVC burden > 16% was a predictor of cardiomyopathy with a sensitivity of 100% and specificity of 87% (AUC 0.96). | [14] |
| Frequent PVC referred for RFA | Any | Any | 24 h Holter monitoring | 241/48 ± 14 years/52%/- | Cross-sectional | Asymptomatic, longer palpitation duration (>30 months), and PVC burden were independent predictors of PVC-induced cardiomyopathy. | [15] |
| Frequent PVC referred for RFA | Any | Any | 24 h Holter monitoring | 294/48 ± 14 years/46.6%/- | Cross-sectional | PVC QRS duration, PVC epicardial origin, PVC burden, and symptom duration were independent predictors of cardiomyopathy. Broad PVC QRS was associated with a lower PVC burden threshold for cardiomyopathy. PVC QRS duration > 150 ms was a predictor of cardiomyopathy with a sensitivity of 80% and specificity of 52% (AUC 0.66). | [16] |
| PVC ≥ 10%/24 h underwent ablation with normal baseline LVEF | Any | Any | 24 h Holter monitoring | 45/53 ± 16.5 years/50% | Median 14 (8–32) months | PVC QRS duration ≥ 153 ms and non-outflow tract origin were predictors of LV dysfunction. There was no association with PVC burden. | [22] |
| PVC-Induced Cardiomyopathy (PIC) | Cardiomyopathy-Induced PVC | |
|---|---|---|
| PVC morphology | Monomorphic, smooth contours with sharp QRS complex | Multifocal, broad notching, and slurred QRS |
| PVC burden | Higher | Lower |
| Origin | RVOT, LVOT | Non-outflow tracts |
| Echocardiogram | Global hypokinesia | Regional hypokinesia |
| CMR | Absent or minimal myocardial fibrosis | Significant fibrosis |
| Intervention | Patients with LV Dysfunction | Presence of Concomitant SHD | PVC Burden (Initial) | PVC Burden (Final) | LVEF (Initial) | LVEF (Final) | Ref. |
|---|---|---|---|---|---|---|---|
| RFCA | 40 | None | 20% | N/A | 38% | 49% | [72] |
| RFCA | 171 | 48% ICM | 24% | N/A | 39% | 42% (If successful ablation) | [73] |
| RFCA | 67 | 100% ICM | 29% | 4.6% | 34% | 42% | [74] |
| RFCA | 120 | 11% ICM 13% NICM | 25% | 4.6% | 41% | 59% | [29] |
| RFCA | 39 | 28% ICM | 21% | N/A | 44% | 52% | [75] |
| RFCA | 215 | 37% SHD | 23% | 1% | 35% | 44% | [76] |
| RFCA | 54 | 19% ICM 19% NICM | 28% | 0.8% | 40% | 52% | [58] |
| RFCA | 77 | 23% ICM 18% DCM 9% VHD | 28% | 3.6% | 37% | 49% | [59] |
| 65% Flecainide 35% Propafenone | 20 | N/A | 36% | 10% (Overall) 11% (Flecainide) 7% (Propafenone) | 37% | 49% (Overall) 49% (Flecainide) 48% (Propafenone) | [70] |
| RFCA | 31 | None | 23% | N/A | 35% | 42% | [77] * |
| RFCA | 55 | None | 25% | 0.4% (If successful ablation) | 35% | 50% | [78] |
| RFCA | 96 | 23% ICM 8% DCM 3% VHD | 26% | 4% (Overall) 1% (If successful ablation) | 38% | 50% (Overall) 54% (If successful ablation) | [79] |
| RFCA | 66 | 17% ICM 5% NCCM 2% VHD | 21% | N/A | 28% | 42% | [80] |
| RFCA | 30 | 100% NICM | 23% | 4.7% (Overall) 1% (If successful ablation) | 38% | 45% | [5] |
| RFCA | 245 | None | 27% | 5% | 38% | 50% | [30] |
| 68% RFCA 32% AAD (4% Flecainide, 5% Propafenone, 23% Amiodarone) | 57 | None | 30% | <6% | 35% | 67% LVEF normalization 33% persistent LV dysfunction | [81] * |
| 44% RFCA 56% AAD | 121 | 83% DCM 17% ICM | 23% | 9.2% (Overall) Reduction of 25,366 and 12,278 PVC/day in RFCA and AAD group, respectively | 37% | 42% (Overall) LVEF normalized in 47% and 21% of patients in RFCA and AAD group, respectively | [60] |
| RFCA | 75 | None | 26% | 2% | 39% | 59% | [61] * |
| RFCA | 65 | 65% DCM 35% ICM | 10–20% (41%) 20–30% (31%) >30% (28%) | N/A | 26% | 33% | [82] |
| RFCA | 24 | 38% DCM 38% ICM | 15% | 1% | 32% | 43% | [83] |
| RFCA | 37 | None | 29% | N/A | 37% | 18% increase (70%) 0% increase (30%) | [40] * |
| RFCA | 69 | 29% NICM | 29% | 3.5% | 35% | 46% | [2] |
| Ivabradine | 35 | 100% DCM | 18% | 10.5% | 33% | 38% | [84] |
| RFCA | 57 | N/A | 33% | 1.9% | 35% | 54% | [10] |
| RFCA | 15 | 100% ICM | 22% | 2.6% | 38% | 51% | [6] |
| RFCA | 6 | None | 17,717 PVC/day | 268 PVC/day | 42% | 57% | [85] |
| RFCA | 22 | None | 37% | 0.7% (If successful ablation) | 34% | 53% | [11] |
| RFCA | 8 | None | 17,541 PVC/day | 2054 PVC/day (Overall) 507 PVC/day (If successful ablation) | 39% | 59% (Overall) 62% (If successful ablation) | [17] |
| Amiodarone | 336 | 72% ICM 28% NICM | 254 PVC/hour | 44 PVC/hour | 25% | 35% | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attachaipanich, T.; Thiravetyan, B.; Tribuddharat, N.; Jaroonpipatkul, S.; Navaravong, L. Premature Ventricular Contraction-Induced Cardiomyopathy: Contemporary Evidence from Risk Stratification, Pathophysiology, and Management. J. Clin. Med. 2024, 13, 2635. https://doi.org/10.3390/jcm13092635
Attachaipanich T, Thiravetyan B, Tribuddharat N, Jaroonpipatkul S, Navaravong L. Premature Ventricular Contraction-Induced Cardiomyopathy: Contemporary Evidence from Risk Stratification, Pathophysiology, and Management. Journal of Clinical Medicine. 2024; 13(9):2635. https://doi.org/10.3390/jcm13092635
Chicago/Turabian StyleAttachaipanich, Tanawat, Ben Thiravetyan, Narisara Tribuddharat, Surachat Jaroonpipatkul, and Leenhapong Navaravong. 2024. "Premature Ventricular Contraction-Induced Cardiomyopathy: Contemporary Evidence from Risk Stratification, Pathophysiology, and Management" Journal of Clinical Medicine 13, no. 9: 2635. https://doi.org/10.3390/jcm13092635
APA StyleAttachaipanich, T., Thiravetyan, B., Tribuddharat, N., Jaroonpipatkul, S., & Navaravong, L. (2024). Premature Ventricular Contraction-Induced Cardiomyopathy: Contemporary Evidence from Risk Stratification, Pathophysiology, and Management. Journal of Clinical Medicine, 13(9), 2635. https://doi.org/10.3390/jcm13092635





