Optimizing Mechanical Ventilation: A Clinical and Practical Bedside Method for the Identification and Management of Patient–Ventilator Asynchronies in Critical Care
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
2.6. Ethical Considerations
3. Results
4. How to Identify Patient–Ventilator Asynchronies
5. Classification of Asynchronies
5.1. Asynchronies Definition, Identification, and Management
5.1.1. Triggering
Inspiratory Trigger Delay
Ineffective Trigger
Autotrigger
Double Trigger
Reverse Trigger
5.1.2. Cycling
Early Cycling
Late Cycling
5.1.3. Flow
Flow Overshoot
Flow Starvation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Holets, S.R.; Li, M.; Cortes-Puentes, G.A.; Meyer, T.J.; Hanson, A.C.; Schulte, P.J.; Oeckler, R.A. Etiology, Incidence, and Outcomes of Patient–Ventilator Asynchrony in Critically-Ill Patients Undergoing Invasive Mechanical Ventilation. Sci. Rep. 2021, 11, 12390. [Google Scholar] [CrossRef]
- Kyo, M.; Shimatani, T.; Hosokawa, K.; Taito, S.; Kataoka, Y.; Ohshimo, S.; Shime, N. Patient–Ventilator Asynchrony, Impact on Clinical Outcomes and Effectiveness of Interventions: A Systematic Review and Meta-Analysis. J. Intensive Care 2021, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Pedram, S.; Best, A.M.; Epstein, S.K. Observational Study of Patient-Ventilator Asynchrony and Relationship to Sedation Level. J. Crit. Care 2009, 24, 74–80. [Google Scholar] [CrossRef]
- Bailey, J.M. Management of Patient–Ventilator Asynchrony. Anesthesiology 2021, 134, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Rodriguez, P.; Cabello, B.; Lellouche, F.; Brochard, L. Patient-Ventilator Asynchrony during Assisted Mechanical Ventilation. Intensive Care Med. 2006, 32, 1515–1522. [Google Scholar] [CrossRef]
- Beitler, J.R.; Sands, S.A.; Loring, S.H.; Owens, R.L.; Malhotra, A.; Spragg, R.G.; Matthay, M.A.; Thompson, B.T.; Talmor, D. Quantifying Unintended Exposure to High Tidal Volumes from Breath Stacking Dyssynchrony in ARDS: The BREATHE Criteria. Intensive Care Med. 2016, 42, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.K. How Often Does Patient-Ventilator Asynchrony Occur and What Are the Consequences? Respir. Care 2011, 56, 25–38. [Google Scholar] [CrossRef] [PubMed]
- De Haro, C.; Ochagavia, A.; López-Aguilar, J.; Fernandez-Gonzalo, S.; Navarra-Ventura, G.; Magrans, R.; Montanyà, J.; Blanch, L.; the Asynchronies in the Intensive Care Unit (ASYNICU) Group. Patient-Ventilator Asynchronies during Mechanical Ventilation: Current Knowledge and Research Priorities. Intensive Care Med. Exp. 2019, 7, 43. [Google Scholar] [CrossRef]
- Sousa, M.L.D.A.; Magrans, R.; Hayashi, F.K.; Blanch, L.; Kacmarek, R.M.; Ferreira, J.C. Predictors of Asynchronies during Assisted Ventilation and Its Impact on Clinical Outcomes: The EPISYNC Cohort Study. J. Crit. Care 2020, 57, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Docci, M.; Rodrigues, A.; Dubo, S.; Ko, M.; Brochard, L. Does Patient-Ventilator Asynchrony Really Matter? Curr. Opin. Crit. Care, 2024; online ahead of print. [Google Scholar]
- Gholami, B.; Phan, T.S.; Haddad, W.M.; Cason, A.; Mullis, J.; Price, L.; Bailey, J.M. Replicating Human Expertise of Mechanical Ventilation Waveform Analysis in Detecting Patient-Ventilator Cycling Asynchrony Using Machine Learning. Comput. Biol. Med. 2018, 97, 137–144. [Google Scholar] [CrossRef]
- Notó, M.; Blanch, L.; de Haro, C. Bridging the Gap Between Detection, Understanding, and Future Innovation in Patient-Ventilator Asynchronies. Respir. Care 2024, 69, 902. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lyu, S.; Mireles-Cabodevila, E.; Miller, A.G.; Albuainain, F.A.; Ibarra-Estrada, M.; Li, J. Survey of Ventilator Waveform Interpretation Among ICU Professionals. Respir. Care 2024, 69, 773. [Google Scholar] [CrossRef]
- Georgopoulos, D.; Prinianakis, G.; Kondili, E. Bedside Waveforms Interpretation as a Tool to Identify Patient-Ventilator Asynchronies. Intensive Care Med. 2006, 32, 34–47. [Google Scholar] [CrossRef]
- Ramirez, I.I.; Arellano, D.H.; Adasme, R.S.; Landeros, J.M.; Salinas, F.A.; Vargas, A.G.; Vasquez, F.J.; Lobos, I.A.; Oyarzun, M.L.; Restrepo, R.D. Ability of ICU Health-Care Professionals to Identify Patient-Ventilator Asynchrony Using Waveform Analysis. Respir. Care 2017, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Subirà, C.; De Haro, C.; Magrans, R.; Fernández, R.; Blanch, L. Minimizing Asynchronies in Mechanical Ventilation: Current and Future Trends. Respir. Care 2018, 63, 464–478. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M. Monitoring of Patient-Ventilator Interaction at the Bedside. Respir. Care 2011, 56, 61–72. [Google Scholar] [CrossRef]
- Dres, M.; Rittayamai, N.; Brochard, L. Monitoring Patient–Ventilator Asynchrony. Curr. Opin. Crit. Care 2016, 22, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, L.; Cinnella, G.; Costa, R.; Cortegiani, A.; Tullo, L.; Rauseo, M.; Conti, G.; Gregoretti, C. Patient-Ventilator Asynchronies: Clinical Implications and Practical Solutions. Respir. Care 2020, 65, 1751–1766. [Google Scholar] [CrossRef]
- Pham, T.; Telias, I.; Beitler, J.R. Esophageal Manometry. Respir. Care 2020, 65, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Holanda, M.A.; Vasconcelos, R.D.S.; Ferreira, J.C.; Pinheiro, B.V. Patient-Ventilator Asynchrony. J. Bras. Pneumol. 2018, 44, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Blanch, L.; Villagra, A.; Sales, B.; Montanya, J.; Lucangelo, U.; Luján, M.; García-Esquirol, O.; Chacón, E.; Estruga, A.; Oliva, J.C.; et al. Asynchronies during Mechanical Ventilation Are Associated with Mortality. Intensive Care Med. 2015, 41, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Mireles-Cabodevila, E.; Siuba, M.T.; Chatburn, R.L. A Taxonomy for Patient-Ventilator Interactions and a Method to Read Ventilator Waveforms. Respir. Care 2022, 67, 129–148. [Google Scholar] [CrossRef]
- Hamahata, N.; Sato, R.; Daoud, E. Go with the Flow—Clinical Importance of Flow Curves during Mechanical Ventilation: A Narrative Review. Can. J. Respir. Ther. 2020, 56, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Oto, B.; Annesi, J.; Foley, R.J. Patient–Ventilator Dyssynchrony in the Intensive Care Unit: A Practical Approach to Diagnosis and Management. Anaesth. Intensive Care 2021, 49, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Murias, G.; Lucangelo, U.; Blanch, L. Patient-Ventilator Asynchrony. Curr. Opin. Crit. Care 2016, 22, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Aquino Esperanza, J.; Sarlabous, L.; De Haro, C.; Magrans, R.; Lopez-Aguilar, J.; Blanch, L. Monitoring Asynchrony During Invasive Mechanical Ventilation. Respir. Care 2020, 65, 847–869. [Google Scholar] [CrossRef] [PubMed]
- Branson, R.D.; Blakeman, T.C.; Robinson, B.R. Asynchrony and DyspneaDiscussion. Respir. Care 2013, 58, 973–989. [Google Scholar] [CrossRef]
- Antonogiannaki, E.-M.; Georgopoulos, D.; Akoumianaki, E. Patient-Ventilator Dyssynchrony. Korean J. Crit. Care Med. 2017, 32, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.M.; Guntupalli, K.K. Review of Ventilatory Techniques to Optimize Mechanical Ventilation in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. COPD 2007, 2, 441–452. [Google Scholar]
- Pham, T.; Telias, I.; Piraino, T.; Yoshida, T.; Brochard, L.J. Asynchrony Consequences and Management. Crit. Care Clin. 2018, 34, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Akoumianaki, E.; Lyazidi, A.; Rey, N.; Matamis, D.; Perez-Martinez, N.; Giraud, R.; Mancebo, J.; Brochard, L.; Richard, J.-C.M. Mechanical Ventilation-Induced Reverse-Triggered Breaths. Chest 2013, 143, 927–938. [Google Scholar] [CrossRef]
- Núñez Silveira, J.M.; Gallardo, A.; García-Valdés, P.; Ríos, F.; Rodriguez, P.O.; Felipe Damiani, L. Reverse Triggering during Mechanical Ventilation: Diagnosis and Clinical Implications. Med. Intensiva Engl. Ed. 2023, 47, 648–657. [Google Scholar] [CrossRef]
- Dexter, A.M.; Clark, K. Ventilator Graphics: Scalars, Loops, & Secondary Measures. Respir. Care 2020, 65, 739–759. [Google Scholar] [CrossRef] [PubMed]

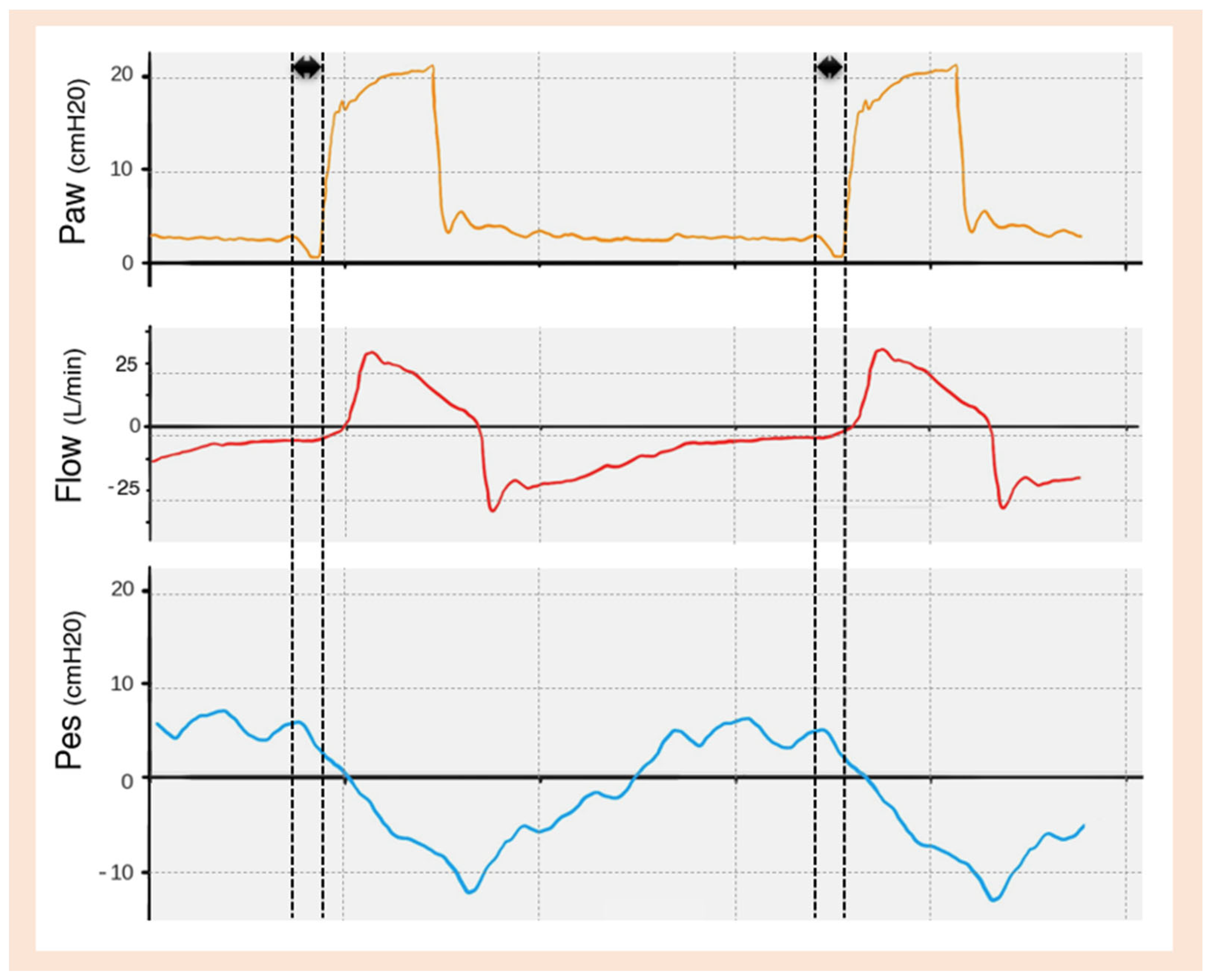
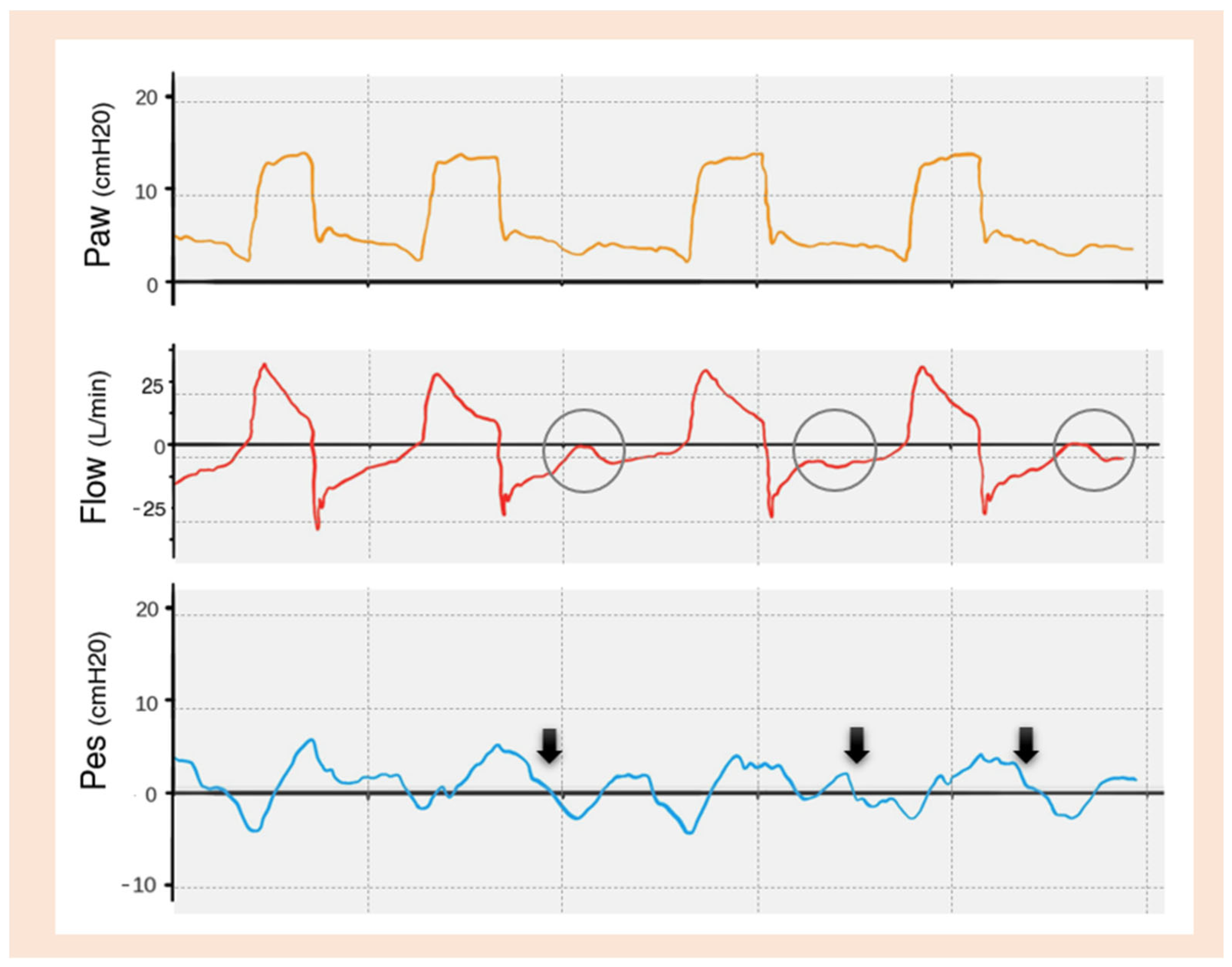


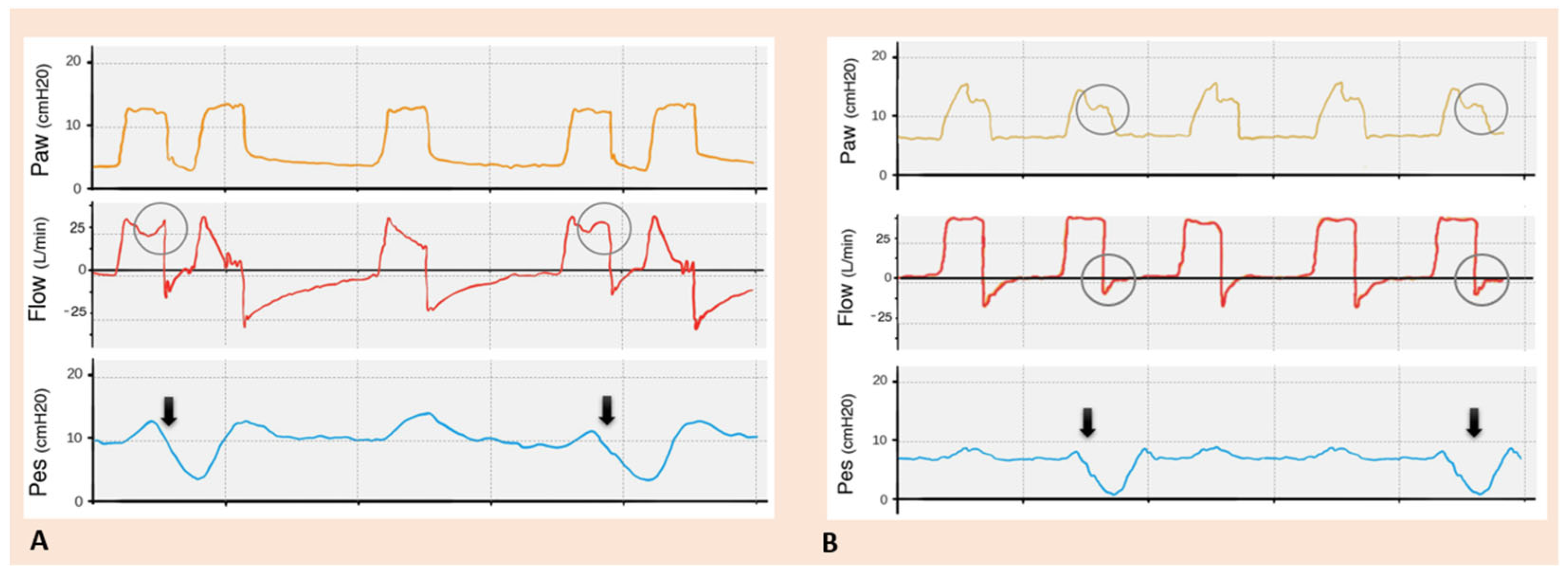
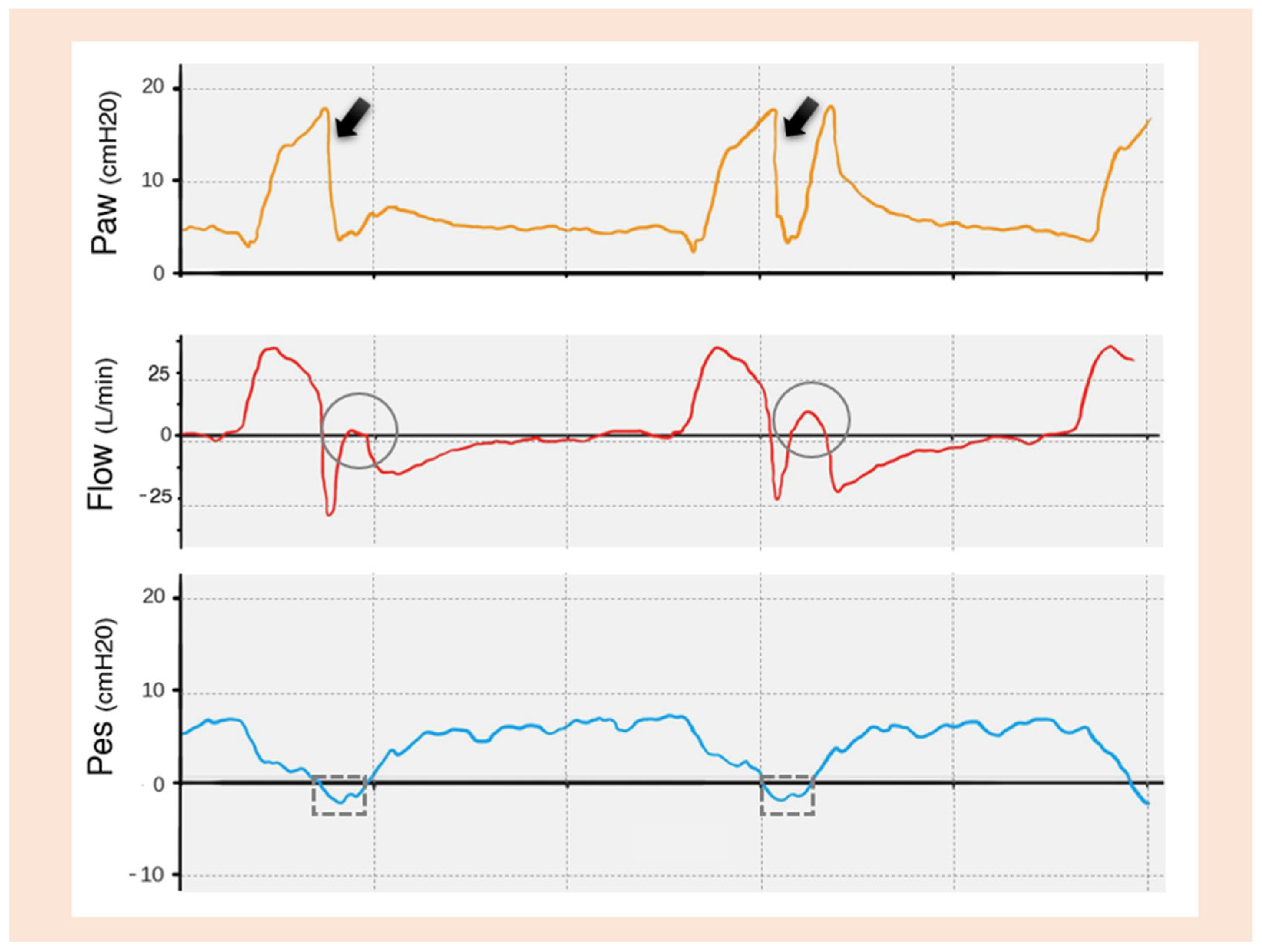
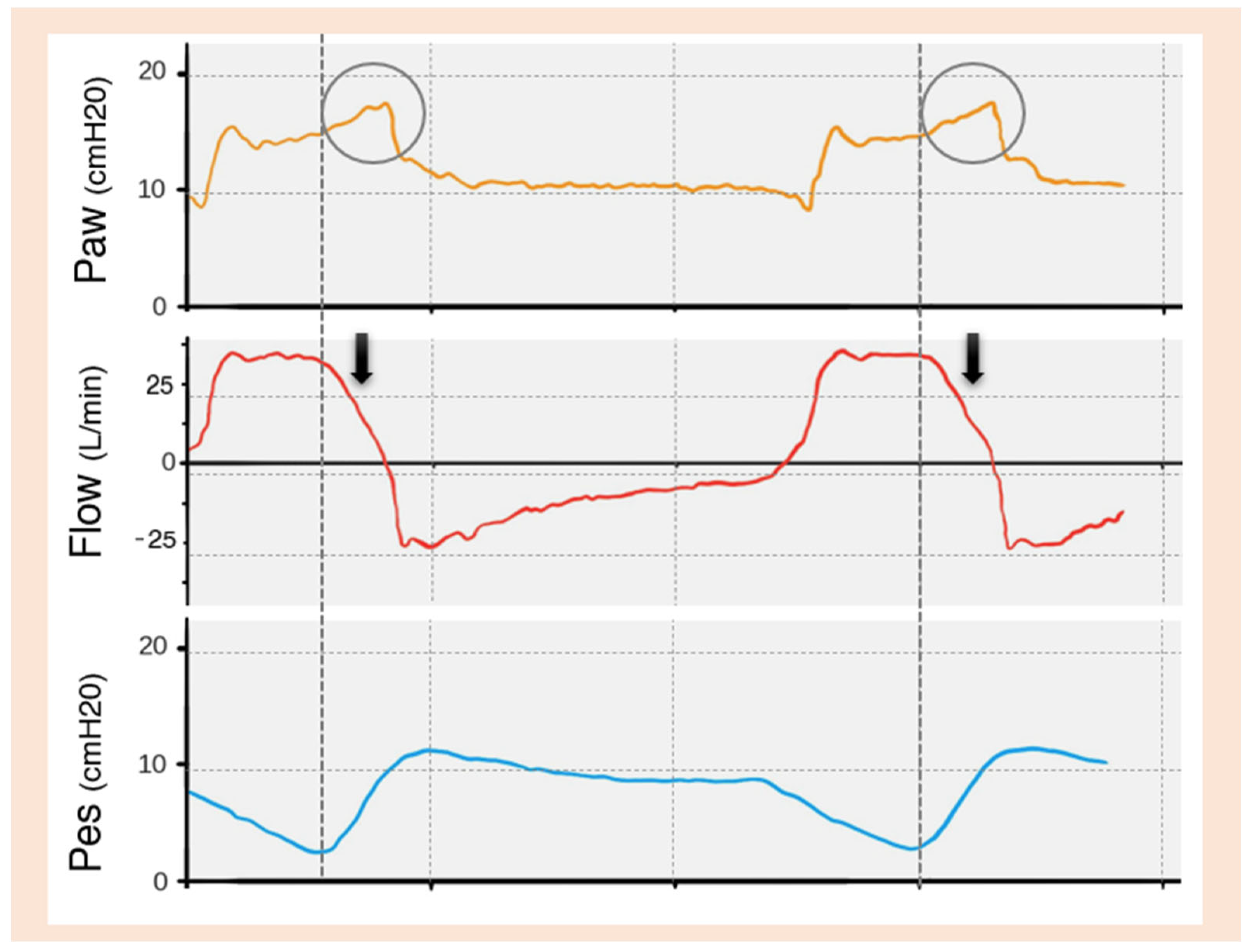
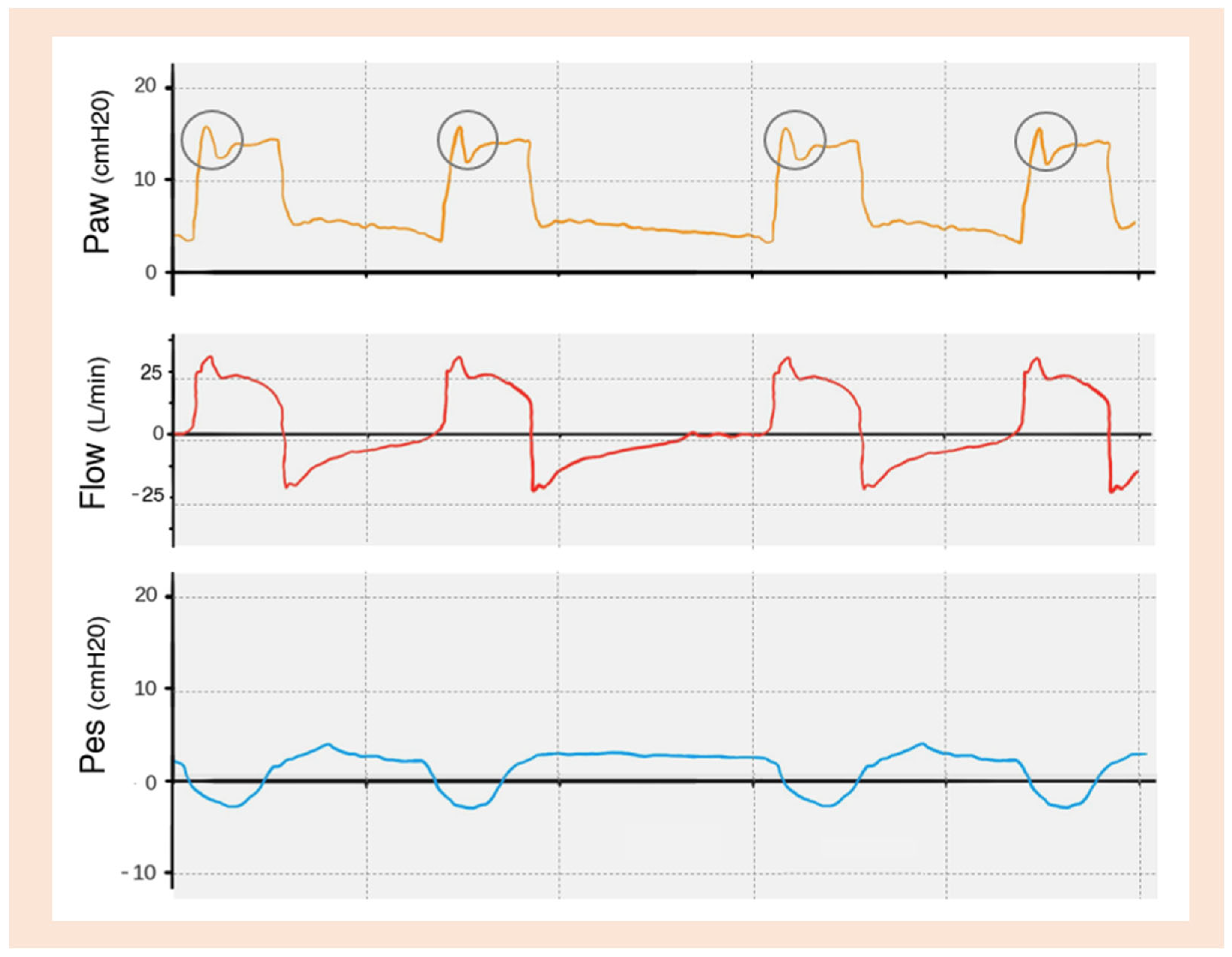

| TRIGGERING | Inspiratory trigger delay |
| Ineffective trigger | |
| Autotrigger | |
| Double trigger | |
| Reverse trigger | |
| CYCLING | Early cycling |
| Late cycling | |
| FLOW | Flow overshoot |
| Flow starvation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, V.; Cidade, J.P.; Medeiros, I.; Póvoa, P. Optimizing Mechanical Ventilation: A Clinical and Practical Bedside Method for the Identification and Management of Patient–Ventilator Asynchronies in Critical Care. J. Clin. Med. 2025, 14, 214. https://doi.org/10.3390/jcm14010214
Costa V, Cidade JP, Medeiros I, Póvoa P. Optimizing Mechanical Ventilation: A Clinical and Practical Bedside Method for the Identification and Management of Patient–Ventilator Asynchronies in Critical Care. Journal of Clinical Medicine. 2025; 14(1):214. https://doi.org/10.3390/jcm14010214
Chicago/Turabian StyleCosta, Vasco, José Pedro Cidade, Inês Medeiros, and Pedro Póvoa. 2025. "Optimizing Mechanical Ventilation: A Clinical and Practical Bedside Method for the Identification and Management of Patient–Ventilator Asynchronies in Critical Care" Journal of Clinical Medicine 14, no. 1: 214. https://doi.org/10.3390/jcm14010214
APA StyleCosta, V., Cidade, J. P., Medeiros, I., & Póvoa, P. (2025). Optimizing Mechanical Ventilation: A Clinical and Practical Bedside Method for the Identification and Management of Patient–Ventilator Asynchronies in Critical Care. Journal of Clinical Medicine, 14(1), 214. https://doi.org/10.3390/jcm14010214





