Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging
Abstract
1. Introduction
2. Materials and Methods
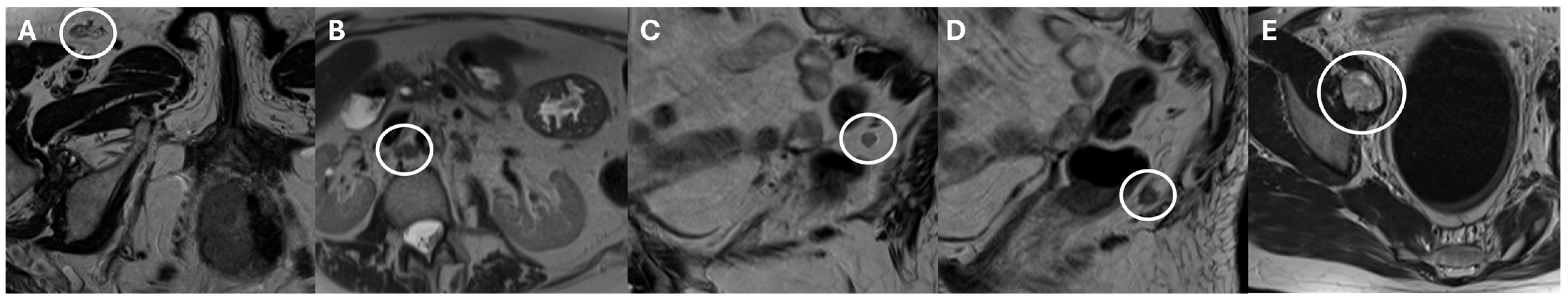
3. Node-RADS Score
4. Node-RADS’s Diagnostic Value
4.1. Neck
4.2. Chest
4.3. Abdomen
4.4. Summary of Evidence on Node-RADS’s Diagnostic Performance
5. Node-RADS’s Reliability
Summary of Evidence on Node-RADS’s Inter-Observer Reliability
6. Other Issues
6.1. Node-RADS’s Prognostic Role
6.2. Diffusion-Weighted Imaging in Node-RADS
6.3. Texture Analysis and Node-RADS
6.4. Radiomics and Node-RADS
6.5. Nomograms and Node-RADS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Eycken, E.V.; Weir, H.K.; Gospodarowicz, M. The TNM Classification of Malignant Tumours—Towards Common Understanding and Reasonable Expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Elsholtz, F.H.J.; Asbach, P.; Haas, M.; Becker, M.; Beets-Tan, R.G.H.; Thoeny, H.C.; Padhani, A.R.; Hamm, B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): A Concept for Standardized Assessment of Lymph Nodes in Cancer. Eur. Radiol. 2021, 31, 6116–6124. [Google Scholar] [CrossRef] [PubMed]
- Prativadi, R.; Dahiya, N.; Kamaya, A.; Bhatt, S. Chapter 5 Ultrasound Characteristics of Benign vs. Malignant Cervical Lymph Nodes. Semin. Ultrasound CT MRI 2017, 38, 506–515. [Google Scholar] [CrossRef]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Rettenbacher, T.; Testa, A.C.; Epstein, E.; Guiggi, I.; et al. Terms, Definitions and Measurements to Describe Sonographic Features of Lymph Nodes: Consensus Opinion from the Vulvar International Tumor Analysis (VITA) Group. Ultrasound Obstet. Gynecol. 2021, 57, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hedgire, S.; Harisinghani, M. Radiologic Assessment of Lymph Nodes in Oncologic Patients. Curr. Radiol. Rep. 2013, 2, 36. [Google Scholar] [CrossRef]
- Brown, G.; Richards, C.J.; Bourne, M.W.; Newcombe, R.G.; Radcliffe, A.G.; Dallimore, N.S.; Williams, G.T. Morphologic Predictors of Lymph Node Status in Rectal Cancer with Use of High-Spatial-Resolution MR Imaging with Histopathologic Comparison. Radiology 2003, 227, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Curtin, H.D.; Ishwaran, H.; Mancuso, A.A.; Dalley, R.W.; Caudry, D.J.; McNeil, B.J. Comparison of CT and MR Imaging in Staging of Neck Metastases. Radiology 1998, 207, 123–130. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic Resonance Imaging for Clinical Management of Rectal Cancer: Updated Recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Consensus Meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Parillo, M.; Quattrocchi, C.C. Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery. Surgeries 2024, 5, 764–773. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Vertulli, D.; Perillo, G.; Montanari, E.; Mallio, C.A.; Quattrocchi, C.C. Assessment of Reason for Exam Imaging Reporting and Data System (RI-RADS) in Inpatient Diagnostic Imaging Referrals. Insights Imaging 2024, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Reporting and Data Systems. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems (accessed on 1 December 2024).
- Parillo, M.; Mallio, C.A.; Van der Molen, A.J.; Rovira, À.; Dekkers, I.A.; Karst, U.; Stroomberg, G.; Clement, O.; Gianolio, E.; Nederveen, A.J.; et al. The Role of Gadolinium-Based Contrast Agents in Magnetic Resonance Imaging Structured Reporting and Data Systems (RADS). Magn. Reson. Mater. Phy. 2024, 37, 15–25. [Google Scholar] [CrossRef]
- Parillo, M.; van der Molen, A.J.; Asbach, P.; Elsholtz, F.H.J.; Laghi, A.; Ronot, M.; Wu, J.S.; Mallio, C.A.; Quattrocchi, C.C. The Role of Iodinated Contrast Media in Computed Tomography Structured Reporting and Data Systems (RADS): A Narrative Review. Quant. Imaging Med. Surg. 2023, 13, 7621–7631. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Li, J.; Leng, J.; Qiu, Y.; Ma, X. Diagnostic Performance of Node Reporting and Data System Magnetic Resonance Imaging Score in Detecting Metastatic Cervical Lymph Nodes of Nasopharyngeal Carcinoma. Clin. Med. Insights Oncol. 2024, 18, 11795549241231564. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, C.; Zhang, H.; Zheng, G.; Jia, C.; Liu, Z.; Wang, Q.; Mu, Y.; Yang, X.; Mao, N.; et al. Deep Learning-Based Automatic Pipeline System for Predicting Lateral Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Carcinoma Using Computed Tomography: A Multi-Center Study. Chin. J. Cancer Res. 2024, 36, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Pediconi, F.; Maroncelli, R.; Pasculli, M.; Galati, F.; Moffa, G.; Marra, A.; Polistena, A.; Rizzo, V. Performance of MRI for Standardized Lymph Nodes Assessment in Breast Cancer: Are We Ready for Node-RADS? Eur. Radiol. 2024, 34, 7734–7745. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.-J.; Schnarkowski, B.; Pappisch, J.; Kerkhoff, T.; Wirtz, H.; Höhn, A.-K.; Krämer, S.; Denecke, T.; Leonhardi, J.; Frille, A. CT Texture Analysis and Node-RADS CT Score of Mediastinal Lymph Nodes—Diagnostic Performance in Lung Cancer Patients. Cancer Imaging 2022, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, M.; Zheng, X.; Yao, Y.; Huang, K.; Chen, S.; Xu, T.; Xu, Z.; Lin, D. Validation of the Node Reporting and Data System (Node-RADS) for Standardized CT Evaluation of Regional Lymph Nodes in Esophageal Squamous Cell Carcinoma Patients. Eur. Radiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Bai, X.; Peng, C.; Liu, B.; Zhou, S.; Liu, H.; Chen, Y.; Guo, H.; Hao, Y.; Liu, X.; Zhao, J.; et al. Diagnostic and Prognostic Value of MRI-Based Node-RADS for the Assessment of Regional Lymph Node Metastasis in Renal Cell Carcinoma. Diagn. Interv. Imaging 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Loch, F.N.; Beyer, K.; Kreis, M.E.; Kamphues, C.; Rayya, W.; Schineis, C.; Jahn, J.; Tronser, M.; Elsholtz, F.H.J.; Hamm, B.; et al. Diagnostic Performance of Node Reporting and Data System (Node-RADS) for Regional Lymph Node Staging of Gastric Cancer by CT. Eur. Radiol. 2024, 34, 3183–3193. [Google Scholar] [CrossRef]
- Jiang, C.; Fang, W.; Wei, N.; Ma, W.; Dai, C.; Liu, R.; Cai, A.; Feng, Q. Node Reporting and Data System Combined With Computed Tomography Radiomics Can Improve the Prediction of Nonenlarged Lymph Node Metastasis in Gastric Cancer. J. Comput. Assist. Tomogr. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Leonhardi, J.; Sabanov, A.; Schnarkowski, B.; Hoehn, A.-K.; Sucher, R.; Seehofer, D.; Denecke, T.; Meyer, H.-J. CT Texture Analysis and Node-RADS CT Score of Lymph Nodes in Patients With Perihilar Cholangiocarcinoma. Anticancer Res. 2023, 43, 5089–5097. [Google Scholar] [CrossRef] [PubMed]
- Leonhardi, J.; Mehdorn, M.; Stelzner, S.; Scheuermann, U.; Höhn, A.-K.; Seehofer, D.; Schnarkowski, B.; Denecke, T.; Meyer, H.-J. Diagnostic Accuracy and Reliability of CT-Based Node-RADS for Colon Cancer. Abdom. Radiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Maggialetti, N.; Greco, C.N.; Lucarelli, N.M.; Morelli, C.; Cianci, V.; Sasso, S.; Rubini, D.; Scardapane, A.; Stabile Ianora, A.A. Applications of New Radiological Scores: The Node-Rads in Colon Cancer Staging. Radiol. Med. 2023, 128, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yu, S.; Chen, P.; Tang, M.; Wen, L.; Sun, Y.; Yang, Y.; Zhang, Y.; Fu, Y.; Lu, Q.; et al. Diagnostic Performance of Node-RADS Score for Mesorectal Lymph Node Metastasis in Rectal Cancer. Abdom. Radiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Niu, Y.; Wen, L.; Yang, Y.; Zhang, Y.; Fu, Y.; Lu, Q.; Wang, Y.; Yu, X.; Yu, X. Diagnostic Performance of Node Reporting and Data System (Node-RADS) for Assessing Mesorectal Lymph Node in Rectal Cancer by CT. BMC Cancer 2024, 24, 716. [Google Scholar] [CrossRef]
- Ninkova, R.V.; Calabrese, A.; Curti, F.; Riccardi, S.; Gennarini, M.; Miceli, V.; Cupertino, A.; Di Donato, V.; Pernazza, A.; Rizzo, S.M.; et al. The Performance of the Node Reporting and Data System 1.0 (Node-RADS) and DWI-MRI in Staging Patients with Cervical Carcinoma According to the New FIGO Classification (2018). Radiol. Med. 2024, 129, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lou, J.; Liu, J.; Dong, L.; Wu, Q.; Wu, Y.; Yu, X.; Wang, M. Performance of Node Reporting and Data System (Node-RADS): A Preliminary Study in Cervical Cancer. BMC Med. Imaging 2024, 24, 28. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, C.; Flammia, R.S.; Lucciola, S.; Proietti, F.; Pecoraro, M.; Bucca, B.; Licari, L.C.; Borrelli, A.; Bologna, E.; Landini, N.; et al. Performance of Node-RADS Scoring System for a Standardized Assessment of Regional Lymph Nodes in Bladder Cancer Patients. Cancers 2023, 15, 580. [Google Scholar] [CrossRef]
- Lucciola, S.; Pisciotti, M.L.; Frisenda, M.; Magliocca, F.; Gentilucci, A.; Del Giudice, F.; Canale, V.; Scarrone, E.; Busetto, G.M.; Carrieri, G.; et al. Predictive Role of Node-Rads Score in Patients with Prostate Cancer Candidates for Radical Prostatectomy with Extended Lymph Node Dissection: Comparative Analysis with Validated Nomograms. Prostate Cancer Prostatic Dis. 2023, 26, 379–387. [Google Scholar] [CrossRef]
- Zhong, J.; Mao, S.; Chen, H.; Wang, Y.; Yin, Q.; Cen, Q.; Lu, J.; Yang, J.; Hu, Y.; Xing, Y.; et al. Node-RADS: A Systematic Review and Meta-Analysis of Diagnostic Performance, Category-Wise Malignancy Rates, and Inter-Observer Reliability. Eur. Radiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Dalah, E.Z.; Nisbet, A.; Reise, S.; Bradley, D. Evaluating Commercial Image Registration Packages for Radiotherapy Treatment Planning. Appl. Radiat. Isot. 2008, 66, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.; Loft, M.K.; Dam, C.; Pedersen, M.R.V.; Timm, S.; Rafaelsen, S.R. Intra- and Interobserver Variability in Magnetic Resonance Imaging Measurements in Rectal Cancer Patients. Cancers 2021, 13, 5120. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; Robertis, R.D.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef]
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical Examination versus Magnetic Resonance Imaging in the Pretreatment Staging of Cervical Carcinoma: Systematic Review and Meta-Analysis. Eur. Radiol. 2013, 23, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, J.M.; Park, J.; Joo, I.; Yoon, J.H.; Lee, D.H.; Ganeshan, B.; Han, J.K. Hepatocellular Carcinoma: Texture Analysis of Preoperative Computed Tomography Images Can Provide Markers of Tumor Grade and Disease-Free Survival. Korean J. Radiol. 2019, 20, 569–579. [Google Scholar] [CrossRef]
- Meyer, H.-J.; Hamerla, G.; Höhn, A.K.; Surov, A. CT Texture Analysis-Correlations with Histopathology Parameters in Head and Neck Squamous Cell Carcinomas. Front. Oncol. 2019, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, H.; Kim, S.; Han, K.; Rhee, H.; Kim, D.-K.; Kwon, H.; Hong, H.; Lim, J.S. Radiomics Analysis of Contrast-Enhanced CT for Classification of Hepatic Focal Lesions in Colorectal Cancer Patients: Its Limitations Compared to Radiologists. Eur. Radiol. 2021, 31, 8786–8796. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Lu, L.; Schwartz, L.H.; Qian, M.; Tejpar, S.; Eggleton, P.; Zhao, B.; Piessevaux, H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J. Natl. Cancer Inst. 2020, 112, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Milonas, D.; Venclovas, Z.; Muilwijk, T.; Jievaltas, M.; Joniau, S. External Validation of Memorial Sloan Kettering Cancer Center Nomogram and Prediction of Optimal Candidate for Lymph Node Dissection in Clinically Localized Prostate Cancer. Cent. Eur. J. Urol. 2020, 73, 19–25. [Google Scholar] [CrossRef]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Fossati, N.; Zaffuto, E.; Bandini, M.; Dell’Oglio, P.; Bravi, C.A.; Fallara, G.; Pellegrino, F.; Nocera, L.; Karakiewicz, P.I.; et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. Eur. Urol. 2017, 72, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Mattei, A.; Fiori, C.; Fossati, N.; Stabile, A.; Beauval, J.-B.; Malavaud, B.; Roumiguié, M.; et al. A Novel Nomogram to Identify Candidates for Extended Pelvic Lymph Node Dissection Among Patients with Clinically Localized Prostate Cancer Diagnosed with Magnetic Resonance Imaging-Targeted and Systematic Biopsies. Eur. Urol. 2019, 75, 506–514. [Google Scholar] [CrossRef]


| Investigators | Study Design | Imaging Technique | Number of Patients/Lymph Nodes | Cancer Type | Main Findings |
|---|---|---|---|---|---|
| Yang et al. [14] | Retrospective | MRI | 119/203 for correlation and diagnostic performance analysis; 300 for interobserver agreement analysis | Nasopharyngeal carcinoma |
|
| Yu et al. [15] | Retrospective and prospective | CT | 519/1266 | Papillary thyroid carcinoma |
|
| Pediconi et al. [16] | Retrospective | MRI | 192/1134 | Breast cancer |
|
| Meyer et al. [17] | Retrospective | CT | 91/91 | Lung cancer |
|
| Fang et al. [18] | Retrospective | CT | 173/3550 | Esophageal carcinoma |
|
| Bai et al. [19] | Retrospective | MRI | 216/216 | Renal cell carcinoma |
|
| Loch et al. [20] | Retrospective | CT | 91/443 | Gastric adenocarcinoma |
|
| Jiang et al. [21] | Retrospective | CT | 376/605 | Gastric cancer |
|
| Leonhardi et al. [22] | Retrospective | CT | 28/50 | Perihilar cholangiocarcinoma |
|
| Leonhardi et al. [23] | Retrospective | CT | 108/108 | Colon cancer |
|
| Maggialetti et al. [24] | Retrospective | CT | 67/67 | Colon cancer |
|
| Niu et al. [25] | Retrospective | MRI | 154/154 | Rectal cancer |
|
| Niu et al. [26] | Retrospective | CT | 146/292 | Rectal cancer |
|
| Ninkova et al. [27] | Retrospective | MRI | 68/68 | Cervical cancer |
|
| Wu et al. [28] | Retrospective | MRI | 81/729 | Cervical cancer |
|
| Leonardo et al. [29] | Retrospective | CT | 49/396 | Bladder cancer |
|
| Lucciola et al. [30] | Retrospective | MRI | 150/150 | Prostate cancer |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parillo, M.; Quattrocchi, C.C. Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging. J. Clin. Med. 2025, 14, 263. https://doi.org/10.3390/jcm14010263
Parillo M, Quattrocchi CC. Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging. Journal of Clinical Medicine. 2025; 14(1):263. https://doi.org/10.3390/jcm14010263
Chicago/Turabian StyleParillo, Marco, and Carlo Cosimo Quattrocchi. 2025. "Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging" Journal of Clinical Medicine 14, no. 1: 263. https://doi.org/10.3390/jcm14010263
APA StyleParillo, M., & Quattrocchi, C. C. (2025). Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging. Journal of Clinical Medicine, 14(1), 263. https://doi.org/10.3390/jcm14010263






