Risk Factors and Impact of Intra-Articular Scarring After Open Reduction and Internal Fixation in Mandibular Condylar Head Fractures—A Prospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Classification of Mandibular Condylar Head Fractures (CHFs)
2.3. Clinical Variables
2.4. Imaging and Volume Measurements
2.5. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozakiewicz, M.; Walczyk, A. Current Frequency of Mandibular Condylar Process Fractures. J. Clin. Med. 2023, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Hirjak, D.; Machon, V.; Beno, M.; Galis, B.; Kupcova, I. Surgical Treatment of Condylar Head Fractures, the Way to Minimize the Postraumatic TMJ Ankylosis. Bratisl. Med. J. 2017, 118, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ramanujam, S.; Kumaravelu, R.; Cheeman, R.S.; Periera, R.J.; Titus, S. Open vs. Closed Management of Condylar Fracture Our Experience of 100 Cases in a Suburban Tertiary Care Hospital. Craniomaxillofacial Trauma Reconstr. 2024, 17, 4–12. [Google Scholar] [CrossRef]
- Shakya, S.; Zhang, X.; Liu, L. Key Points in Surgical Management of Mandibular Condylar Fractures. Chin. J. Traumatol. 2020, 23, 63–70. [Google Scholar] [CrossRef]
- Bertin, E.; Meyer, C.; Louvrier, A.; Weber, E.; Barrabé, A.; Pons, M. Intraoperative Cone-Beam Computed Tomography for Open Reduction and Internal Fixation of Condylar Head Fractures. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 593–597. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Pruszyńska, P. Lateral Pterygoid Muscle Alteration in Patients Treated Surgically Due to Mandibular Head Fractures. J. Clin. Med. 2023, 12, 4789. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Ellis, E. Surgical Treatment of Adult Mandibular Condylar Fractures Provides Better Outcomes Than Closed Treatment: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2015, 73, 482–493. [Google Scholar] [CrossRef]
- Chieng, C.Y.; Patel, A.; Nazir, H.; Ali, S.; Bhatti, N.; Mcleod, N. Condyle Head Fracture Management: A Systematic Review of Outcomes. J. Cranio-Maxillofac. Surg. 2024, 52, 1476–1484. [Google Scholar] [CrossRef]
- Bhatti, N.; Mohamedbhai, H.; Poon, X.; Khan, P.; Van Der Cruyssen, F.; Simon, H. Open Management of Condylar Head Fractures: The First 50 Cases: What Have We Learnt and Where Are We Going? Br. J. Oral Maxillofac. Surg. 2024; in press. [Google Scholar] [CrossRef]
- Schneider, M.; Erasmus, F.; Gerlach, K.L.; Kuhlisch, E.; Loukota, R.A.; Rasse, M.; Schubert, J.; Terheyden, H.; Eckelt, U. Open Reduction and Internal Fixation versus Closed Treatment and Mandibulomaxillary Fixation of Fractures of the Mandibular Condylar Process: A Randomized, Prospective, Multicenter Study with Special Evaluation of Fracture Level. J. Oral Maxillofac. Surg. 2008, 66, 2537–2544. [Google Scholar] [CrossRef]
- Neff, A.; Cornelius, C.-P.; Rasse, M.; Torre, D.D.; Audigé, L. The Comprehensive AOCMF Classification System: Condylar Process Fractures—Level 3 Tutorial. Craniomaxillofacial Trauma Reconstr. 2014, 7, 44–58. [Google Scholar] [CrossRef]
- Skroch, L.; Fischer, I.; Meisgeier, A.; Kozolka, F.; Apitzsch, J.; Neff, A. Condylar Remodeling after Osteosynthesis of Fractures of the Condylar Head or Close to the Temporomandibular Joint. J. Cranio-Maxillofac. Surg. 2020, 48, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Scheunemann, L.-M.; Grill, F.; Stimmer, H.; Wolff, K.-D.; Neff, A. Prognostic Factors for Long-Term Results after Condylar Head Fractures: A Comparative Study of Non-Surgical Treatment versus Open Reduction and Osteosynthesis. J. Cranio-Maxillofac. Surg. 2020, 48, 1138–1145. [Google Scholar] [CrossRef]
- Pujol, N.; Boisrenoult, P.; Beaufils, P. Post-Traumatic Knee Stiffness: Surgical Techniques. Orthop. Traumatol. Surg. Res. 2015, 101, S179–S186. [Google Scholar] [CrossRef] [PubMed]
- Johner, J.-P.; Essig, H.; Neff, A.; Wagner, M.E.H.; Blumer, M.; Gander, T. Volumetric Evaluated Bone Resorption After Open Reduction and Internal Fixation of Condylar Head Fractures of the Mandible. J. Oral Maxillofac. Surg. 2021, 79, 1902–1913. [Google Scholar] [CrossRef]
- Pienkohs, S.P.; Meisgeier, A.; Herrmann, J.; Graf, L.; Reichert, C.S.; Trento, G.; Neff, A. Factors Affecting the Duration of Surgery in the Management of Condylar Head Fractures. J. Clin. Med. 2023, 12, 7172. [Google Scholar] [CrossRef] [PubMed]
- Helkimo, M. Studies on Function and Dysfunction of the Masticatory System. II. Index for Anamnestic and Clinical Dysfunction and Occlusal State. Sven. Tandlakare Tidskr. Swed. Dent. J. 1974, 67, 101–121. [Google Scholar]
- John, M.T.; Hirsch, C.; Reiber, T.; Dworkin, S.F. Translating the Research Diagnostic Criteria for Temporomandibular Disorders into German: Evaluation of Content and Process. J. Orofac. Pain 2006, 20, 43–52. [Google Scholar]
- Kapos, F.P.; Exposto, F.G.; Oyarzo, J.F.; Durham, J. Temporomandibular Disorders: A Review of Current Concepts in Aetiology, Diagnosis and Management. Oral Surg. 2020, 13, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Warzocha, J.; Gadomska-Krasny, J.; Mrowiec, J. Etiologic Factors of Temporomandibular Disorders: A Systematic Review of Literature Containing Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) and Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) from 2018 to 2022. Healthcare 2024, 12, 575. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Salinas Fredricson, A.; Krüger Weiner, C.; Ulmner, M.; Naimi-Akbar, A. Craniomaxillofacial Trauma Increases the Risk of Temporomandibular Joint Disorders and Days of Work Disability—A SWEREG-TMD Registry-Based Study. Int. J. Oral Maxillofac. Surg. 2024; in press. [Google Scholar] [CrossRef]
- Maini, K.; Dua, A. Temporomandibular Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ohrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Gawda, P. Analysis of the Use of Sample Size and Effect Size Calculations in a Temporomandibular Disorders Randomised Controlled Trial—Short Narrative Review. J. Pers. Med. 2024, 14, 655. [Google Scholar] [CrossRef]
- Lyles, R.H.; Lin, H.; Williamson, J.M. A Practical Approach to Computing Power for Generalized Linear Models with Nominal, Count, or Ordinal Responses. Stat. Med. 2007, 26, 1632–1648. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1969; ISBN 978-0-12-179050-9. [Google Scholar]
- Sapre, V.; Dhanwani, Y.; Saluja, N.; Jaiswal, A.M.; Chandanwale, R. Clinical Outcomes of Arthroscopic Adhesiolysis: A Case Series of 40 Patients with Postoperative Knee Stiffness. Cureus 2024, 16, e63378. [Google Scholar] [CrossRef]
- Asim, M.A.; Ibrahim, M.W.; Javed, M.U.; Zahra, R.; Qayyum, M.U. Functional Outcomes Of Open Versus Closed Treatment of Unilateral Mandibular Condylar Fractures. J. Ayub Med. Coll. Abbottabad JAMC 2019, 31, 67–71. [Google Scholar]
- Smolka, W.; Cornelius, C.-P.; Lechler, C. Resorption Behaviour of the Articular Surface Dome and Functional Outcome After Open Reduction and Internal Fixation of Mandibular Condylar Head Fractures Using Small-Fragment Positional Screws. J. Cranio-Maxillofac. Surg. 2018, 46, 1953–1959. [Google Scholar] [CrossRef]
- Neuhaus, M.-T.; Gellrich, N.-C.; Sander, A.K.; Lethaus, B.; Halama, D.; Zimmerer, R.M. No Significant Bone Resorption After Open Treatment of Mandibular Condylar Head Fractures in the Medium-Term. J. Clin. Med. 2022, 11, 2868. [Google Scholar] [CrossRef] [PubMed]
- McLeod, N.M.; Saeed, N.R.; Gerber, B. Remodelling of Mandibular Condylar Head after Fixation of Fractures with Ultrasound Activated Resorbable Pins: A Retrospective Case Series. J. Cranio-Maxillofac. Surg. 2023, 51, 460–466. [Google Scholar] [CrossRef]
- Park, H.; Ahn, S.; Lee, B. Quantitative Assessment of Condylar Remodeling After Open Reduction and Internal Fixation in Mandibular Condylar Head Fractures. J. Craniofac. Surg. 2024, 35, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Jussila, P.; Krooks, L.; Näpänkangas, R.; Päkkilä, J.; Lähdesmäki, R.; Pirttiniemi, P.; Raustia, A. The Role of Occlusion in Temporomandibular Disorders (TMD) in the Northern Finland Birth Cohort (NFBC) 1966. CRANIO® 2019, 37, 231–237. [Google Scholar] [CrossRef]
- Lekaviciute, R.; Kriauciunas, A. Relationship Between Occlusal Factors and Temporomandibular Disorders: A Systematic Literature Review. Cureus 2024, 16, e54130. [Google Scholar] [CrossRef] [PubMed]
- Kermer, C.; Undt, G.; Rasse, M. Surgical Reduction and Fixation of Intracapsular Condylar Fractures. A Follow up Study. Int. J. Oral Maxillofac. Surg. 1998, 27, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.; Druelle, C.; Schlund, M.; Bricout, N.; Nicot, R. Complications in Orthognathic Surgery: A Retrospective Study of 5025 Cases. Int. Orthod. 2019, 17, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Fama, F.; Cicciu, M.; Sindoni, A.; Nastro-Siniscalchi, E.; Falzea, R.; Cervino, G.; Polito, F.; De Ponte, F.; Gioffre-Florio, M. Maxillofacial and Concomitant Serious Injuries: An Eight-Year Single Center Experience. Chin. J. Traumatol. 2017, 20, 4–8. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, M.; Zhou, Q.; Liu, Q. Delayed Open Reduction and Single Screw Internal Fixation as a Treatment Option in Cases of Failed Non-Surgical Treatment of Bilateral Condylar Head Fractures with Fragmentation. J. Cranio-Maxillofac. Surg. 2016, 44, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bi, R.; Jiang, N.; Zhu, S.; Li, Y. Clinical Outcomes of Open Treatment of Old Condylar Head Fractures in Adults. J. Cranio-Maxillofac. Surg. 2021, 49, 480–487. [Google Scholar] [CrossRef]
- Hayashi, D.; Gould, E.S.; Ho, C.; Caruana, D.L.; Komatsu, D.E.; Yang, J.; Zhu, C.; Mufti, M.; Nicholson, J. Severity of Heterotopic Ossification in Patients Following Surgery for Hip Fracture: A Retrospective Observational Study. BMC Musculoskelet. Disord. 2019, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.; Handa, S.; August, M.; Keith, D.A. Are There Identifiable Risk Factors Associated with Heterotopic Ossification of the Temporomandibular Joint? J. Oral Maxillofac. Surg. 2022, 80, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.; Abdelhaq, A.; Piggott, R.P.; Osman, M.; McElwain, J.P.; Leonard, M. Heterotopic Ossification Following Acetabular Fixation: Incidence and Risk Factors: 10-Year Experience of a Tertiary Centre. Injury 2016, 47, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.M.; Elsoufy, M.A. Arthroscopic Arthrolysis for Arthrofibrosis of the Knee after Total Knee Replacement. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2011, 7, 130–133. [Google Scholar] [CrossRef]
- Gittings, D.; Hesketh, P.; Dattilo, J.; Zgonis, M.; Kelly, J.; Mehta, S. Arthroscopic Lysis of Adhesions Improves Knee Range of Motion after Fixation of Intra-Articular Fractures about the Knee. Arch. Orthop. Trauma Surg. 2016, 136, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Sirvent, E.; Ibarzábal-Gil, A.; Rodríguez-Merchán, E.C. Complications of the Surgical Treatment of Fractures of the Tibial Plateau: Prevalence, Causes, and Management. EFORT Open Rev. 2022, 7, 554–568. [Google Scholar] [CrossRef]
- Fabricant, P.D.; Tepolt, F.A.; Kocher, M.S. Range of Motion Improvement Following Surgical Management of Knee Arthrofibrosis in Children and Adolescents. J. Pediatr. Orthop. 2018, 38, e495–e500. [Google Scholar] [CrossRef]
- Lindahl, L.; Hollender, L. Condylar Fractures of the Mandible. Int. J. Oral Surg. 1977, 6, 153–165. [Google Scholar] [CrossRef] [PubMed]




| Fractures n = 96 | Patients n = 80 | Mean Age in Years (SD) | Median | Gender (% of Fractures) | |
|---|---|---|---|---|---|
| Unilateral Fractures | 64 | 64 | 44.9 | 48.5 | Male 45.3% |
| (19) | Female 54.7% | ||||
| Bilateral Fractures | 32 | 16 | 48.8 | 48 | Male 43.75% |
| (19) | Female 56.25% | ||||
| Major Fragmentation | 28 | 26 | 51.6 | 51 | Male 32.1% |
| (20) | Female 67.9% | ||||
| Minor Fragmentation | 23 | 23 | 47.1 | 49 | Male 39.1% |
| (19) | Female 60.9% | ||||
| Non-fragmented | 45 | 39 | 42.1 | 48 | Male 55.6% |
| (19) | Female 44.4% |
| Predictor | p | OR with 95% CI |
|---|---|---|
| Patient age | 0.869 | 0.998 [0.977, 1.020] |
| Gender (female) | 0.474 | 0.73 [0.312, 1.717] |
| Concomitant fractures | 0.073 | 1.8 [0.192, 1.075] |
| Dental support zone missing | 0.022 * | 3.36 [1.191, 9.459] |
| Screw protrusion (T2) | 0.536 | 1.31 [0.554, 3.108] |
| Osteosynthesis loosening (T2) | 0.079 | 2.24 [0.910, 5.506] |
| Number of microplates (T2) | 0.572 | 1.108 [0.777, 1.581] |
| Major fragmentation | 0.029 * | 3.182 [1.123, 9.013] |
| Minor fragmentation | 0.658 | 0.805 [0.308, 2.103] |
| Fracture age | 0.390 | 0.965 [0.891, 1.046] |
| Osseous alteration (T2) | 0.458 | 0.592 [0.148, 2.361] |
| Months between initial surgery and RMI | 0.374 | 0.90 [0.717, 1.133] |
| Predictor | p | OR with 95% CI |
|---|---|---|
| Patient age | 0.419 | 1.01 [0.984, 1.039] |
| Gender (female) | 0.603 | 1.347 [0.438, 4.138] |
| Concomitant fractures | 0.201 | 2 [0.691, 5.787] |
| Dental support zone missing | 0.071 | 0.344 [0.108, 1.097] |
| Screw protrusion (T2) | 0.387 | 0.602 [0.191, 1.897] |
| Osteosynthesis loosening (T2) | - | 1 [n.a.] |
| Number of microplates | 0.103 | 1.460 [0.927, 2.302] |
| Major fragmentation | 0.472 | 0.643 [0.193, 2.144] |
| Minor fragmentation | 0.435 | 0.58 [0.148, 2.275] |
| Fracture age | 0.018 * | 1.113 [1.018, 1.216] |
| Osseous alteration (T2) | 0.041 * | 4.171 [1.058, 16.452] |
| Months between initial surgery and RMI | 0.254 | 1.128 [0.917, 1.389] |
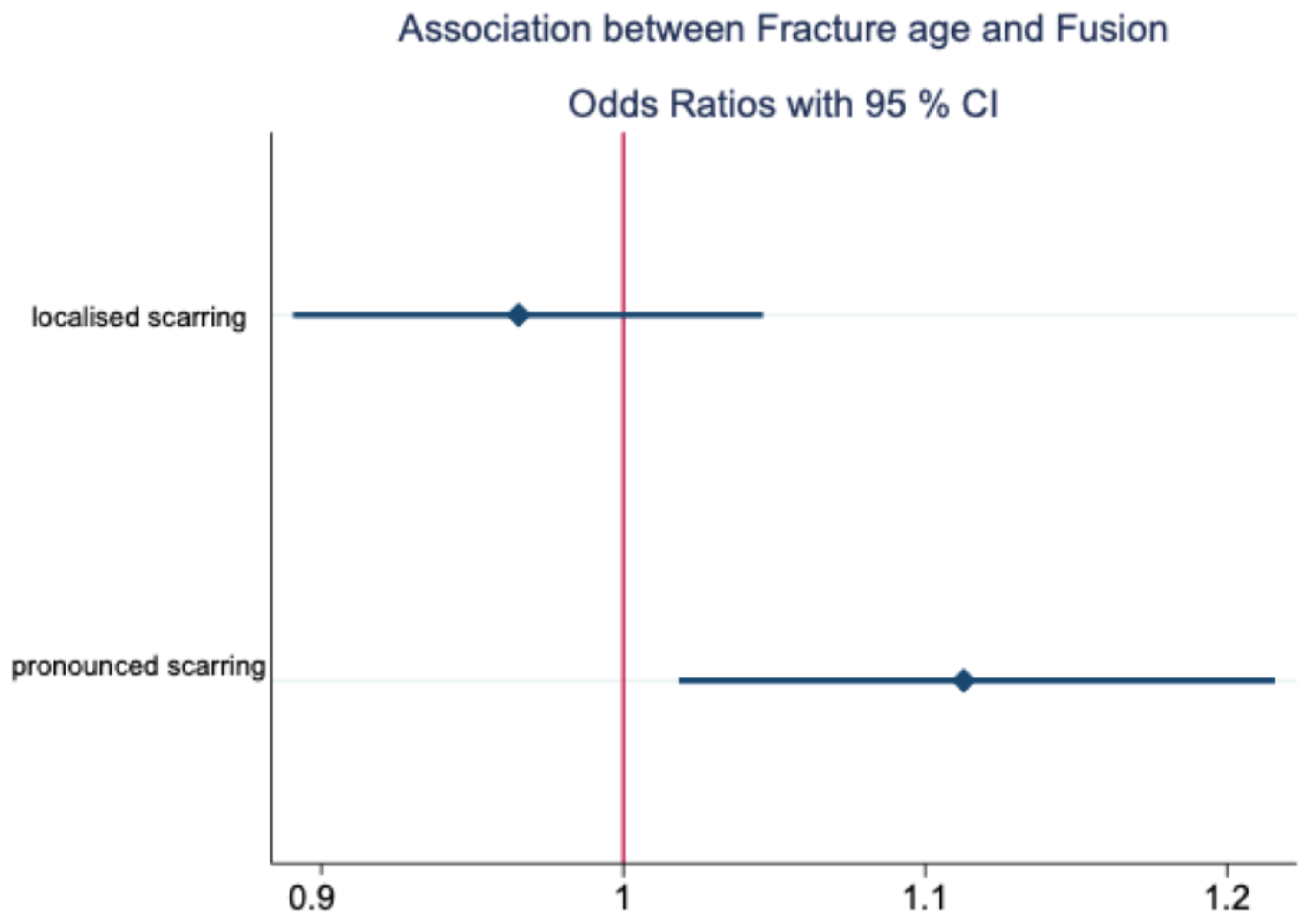
| Dependent Variable | Predictor | p | OR with 95% CI |
|---|---|---|---|
| Di | 0.422 | 0.778 [0.421, 1.436] | |
| Localised scarring | RDC/TMD | 0.594 | 0.847 [0.460, 1.559] |
| Di | 0.410 | 1.445 [0.603, 3.464] | |
| Pronounced scarring | RDC/TMD | 0.350 | 1.488 [0.647, 3.424] |
| Preoperative Di | Postoperative Di | Total | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 0 | 21 | 2 | 0 | 0 | 23 |
| 1 | 11 | 5 | 0 | 0 | 16 |
| 2 | 10 | 2 | 0 | 0 | 12 |
| 3 | 2 | 1 | 0 | 0 | 3 |
| Total | 44 | 10 | 0 | 0 | 54 |
| Preoperative RDC/TMD | Postoperative RDC/TMD | Total | |||
| 0 | 1 | 2 | 3 | ||
| 0 | 3 | 2 | 21 | 0 | 27 |
| 1 | 2 | 3 | 19 | 0 | 23 |
| 2 | 1 | 2 | 11 | 0 | 14 |
| 3 | 0 | 0 | 2 | 0 | 2 |
| Total | 6 | 7 | 53 | 0 | 66 |
| Predictor | Regression Coefficient B with 95% CI | p |
|---|---|---|
| Patient age | −0.141 [−0.280, −0.002] | 0.047 * |
| Major fragmentation | n.a. [−9411, 1757] | 0.176 |
| Female patient | −3.576 [−8.483, 1.331] | 0.151 |
| Osseous alteration | 2.338 [−18.921, −13.665] | 0.570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichert, C.S.; Pienkohs, S.P.; Skroch, L.; Meisgeier, A.; Neff, A. Risk Factors and Impact of Intra-Articular Scarring After Open Reduction and Internal Fixation in Mandibular Condylar Head Fractures—A Prospective Analysis. J. Clin. Med. 2025, 14, 266. https://doi.org/10.3390/jcm14010266
Reichert CS, Pienkohs SP, Skroch L, Meisgeier A, Neff A. Risk Factors and Impact of Intra-Articular Scarring After Open Reduction and Internal Fixation in Mandibular Condylar Head Fractures—A Prospective Analysis. Journal of Clinical Medicine. 2025; 14(1):266. https://doi.org/10.3390/jcm14010266
Chicago/Turabian StyleReichert, Clarissa Sophie, Simon Patrik Pienkohs, Linda Skroch, Axel Meisgeier, and Andreas Neff. 2025. "Risk Factors and Impact of Intra-Articular Scarring After Open Reduction and Internal Fixation in Mandibular Condylar Head Fractures—A Prospective Analysis" Journal of Clinical Medicine 14, no. 1: 266. https://doi.org/10.3390/jcm14010266
APA StyleReichert, C. S., Pienkohs, S. P., Skroch, L., Meisgeier, A., & Neff, A. (2025). Risk Factors and Impact of Intra-Articular Scarring After Open Reduction and Internal Fixation in Mandibular Condylar Head Fractures—A Prospective Analysis. Journal of Clinical Medicine, 14(1), 266. https://doi.org/10.3390/jcm14010266







