Comparison of Cerebral Blood Flow During General Anesthesia in Elderly Patients with and Without Dementia: A Prospective Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Declarations
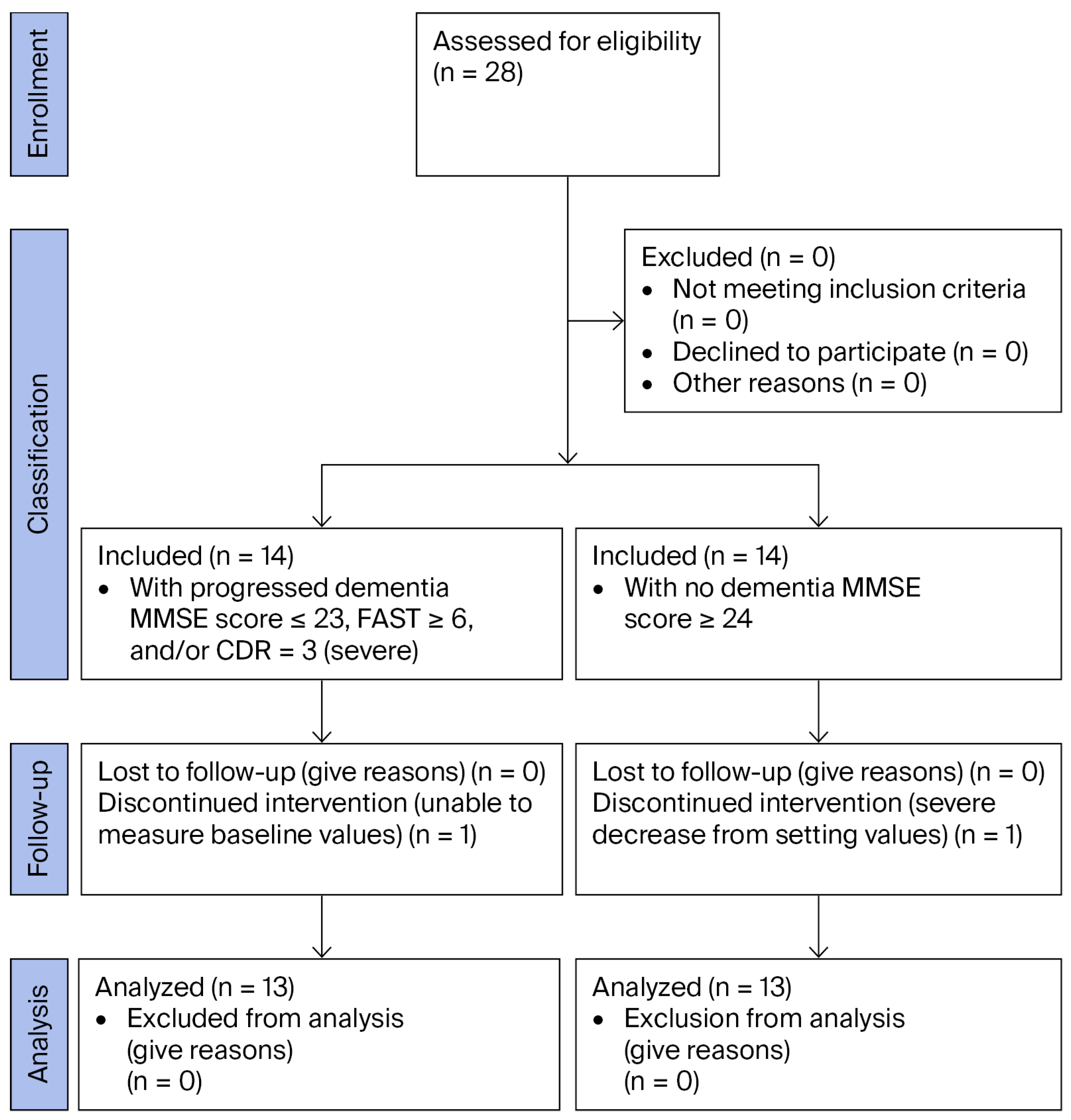
2.2. Study Participants
2.3. Measurements
2.3.1. Pretreatment Fasting
2.3.2. Monitoring and Anesthesia Method
2.3.3. Measurement Items and Timing
2.4. Statistical Analyses
3. Results
3.1. Patients’ Background Characteristics
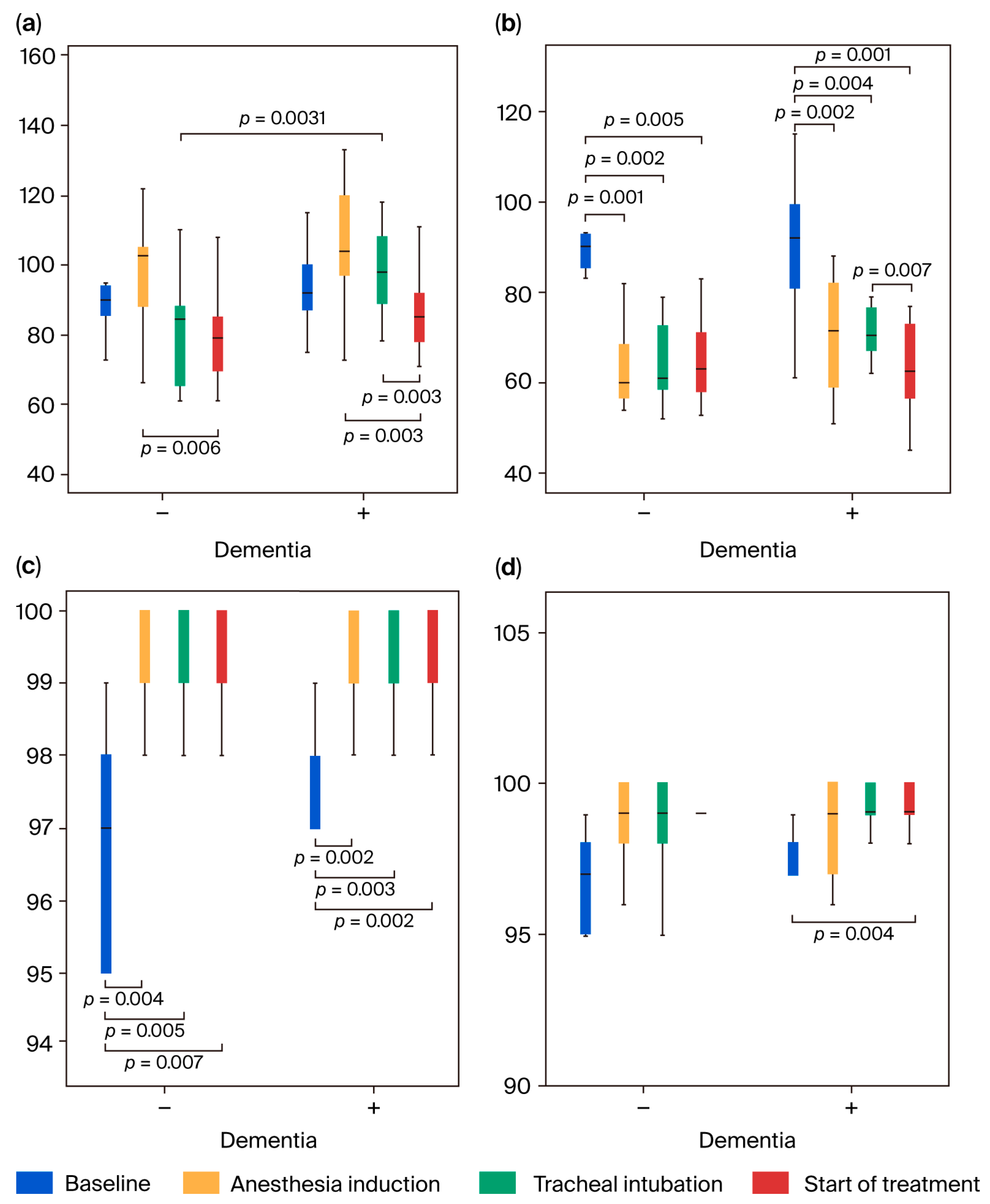
3.2. Comparison of Patients’ Characteristics and Vital Signs
3.3. NIRS Measurement Parameters
3.3.1. Change in nTHI
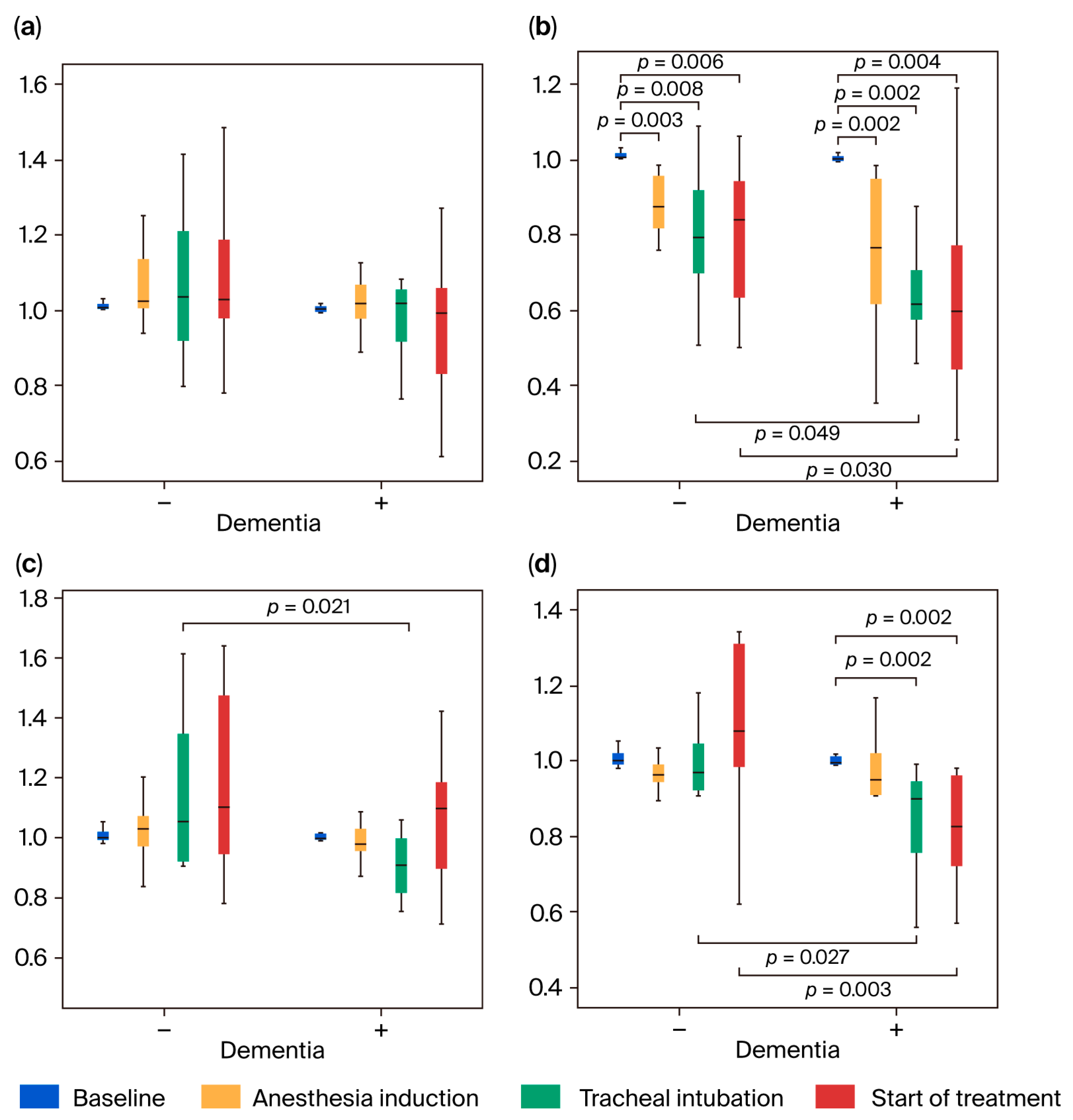
3.3.2. Changes in TOI

3.4. Changes in Cognitive Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- So, E.; Kim, H.J.; Karm, M.H.; Seo, K.S.; Chang, J.; Lee, J.H. A retrospective analysis of outpatient anesthesia management for dental treatment of patients with severe Alzheimer’s disease. J. Dent. Anesth. Pain. Med. 2017, 17, 271–280. [Google Scholar] [CrossRef]
- Kim, T.; Chi, S.I.; Kim, H.; Seo, K.S. Analysis of behavioral management for dental treatment in patients with dementia using the Korean National Health Insurance data. J. Dent. Anesth. Pain. Med. 2021, 21, 461–469. [Google Scholar] [CrossRef]
- Jockusch, J.; Hopfenmler, W.; Ettinger, R.; Nitschke, I. Outpatient, dental care of adult vulnerable patients under general anaesthesia- a retrospective evaluation of need for treatment and dental follow-up care. Clin. Oral. Investig. 2021, 25, 2407–2417. [Google Scholar] [CrossRef]
- White, S.; Griffiths, R.; Baxter, M.; Beanland, T.; Cross, J.; Dhesi, J.; Docherty, A.B.; Foo, I.; Jolly, G.; Jones, J.; et al. Guidelines for the peri-operative care of people with dementia. Anaesthesia 2019, 74, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Sieber, F.; Pauldine, R. Geriatric anesthesia. In Miller’s Anesthesia, 8th ed.; Miller, R.D., Ed.; Elsevier: Philadelphia, PA, USA, 2015; Volume 2, p. 2418. [Google Scholar]
- Needham, M.J.; Webb, C.E.; Bryden, D.C. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br. J. Anaesth. 2017, 119 (Suppl. S1), i115–i125. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S.; Johnson, T.; Kuipers, H.M.; Kristensen, D.; Siersma, V.D.; Vila, P.; Jolles, J.; Papaioannou, A.; Abildstrom, H.; Silverstein, J.H.; et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol. Scand. 2003, 47, 260–266. [Google Scholar] [CrossRef]
- Jin, I.H.; Lew, M.W. The Impact of Total Intravenous Anesthesia and Volatile Anesthetics on Minimizing Cancer Recurrence and Postoperative Cognition. Cureus 2025, 17, e87379. [Google Scholar] [CrossRef] [PubMed]
- Rortgen, D.; Kloos, J.; Fries, M.; Grottke, O.; Rex, S.; Rossaint, R.; Coburn, M. Comparison of early cognitive function and recovery after desflurane or sevoflurane anaesthesia in the elderly: A double-blinded randomized controlled trial. Br. J. Anaesth. 2010, 104, 167–174. [Google Scholar] [CrossRef]
- Seitz, D.P.; Gill, S.S.; Bell, C.M.; Austin, P.C.; Gruneir, A.; Anderson, G.M.; Rochon, P.A. Postoperative medical complications associated with anesthesia in older adults with dementia. J. Am. Geriatr. Soc. 2014, 62, 2102–2109. [Google Scholar] [CrossRef]
- Chen, P.L.; Yang, C.W.; Tseng, Y.K.; Sun, W.Z.; Wang, J.L.; Wang, S.J.; Oyang, Y.J.; Fuh, J.L. Risk of dementia after anaesthesia and surgery. Br. J. Psych. 2014, 204, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Fuh, J.L. Exposure to general anesthesia and the risk of dementia. J. Pain. Res. 2015, 8, 711–718. [Google Scholar] [CrossRef]
- Sprung, J.; Roberts, R.O.; Knopman, D.S.; Olive, D.M.; Gappa, J.L.; Sifuentes, V.L.; Behrend, T.L.; Farmer, J.D.; Weingarten, T.N.; Hanson, A.C.; et al. Association of mild cognitive impairment with exposure to general anesthesia for surgical and nonsurgical procedures: A population-based study. Mayo Clin. Proc. 2016, 91, 208–217. [Google Scholar] [CrossRef]
- Bowles, A.E.J.; Larson, E.B.; Pong, R.P.; Walker, R.D.; Anderson, M.L.; Yu, O.; Gray, S.L.; Crane, P.K.; Dublin, S. Anesthesia exposure and risk of dementia and Alzheimer’s disease: A prospective study. J. Am. Geriatr. Soc. 2016, 64, 602–607. [Google Scholar] [CrossRef]
- Sprung, J.; Roberts, R.O.; Knopman, D.S.; Price, L.L.; Schulz, H.P.; Tatsuyama, C.L.; Weingarten, T.N.; Schroeder, D.R.; Hanson, A.C.; Petersen, R.C.; et al. Mild Cognitive Impairment and Exposure to General Anesthesia for Surgeries and Procedures: A Population-Based Case Control Study. Anesth. Analg. 2017, 124, 1277–1290. [Google Scholar] [CrossRef]
- Sprung, J.; Roberts, R.O.; Weingarten, T.N.; Cavalcante, A.N.; Knopman, D.S.; Petersen, R.C.; Hanson, A.C.; Schroeder, D.R.; Warner, D.O. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br. J. Anaesth. 2017, 119, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dong, Y.; Huang, W.; Bao, M. General anesthesia exposure and risk of dementia: A meta-analysis of epidemiological studies. Oncotarget 2017, 8, 59628–59637. [Google Scholar] [CrossRef]
- Kim, C.T.; Myung, W.; Lewis, M.; Lee, H.; Kim, S.E.; Lee, K.; Lee, C.; Choi, J.; Kim, H.; Carroll, B.J.; et al. Exposure to general anesthesia and risk of dementia: A nationwide population-based cohort study. J. Alzheimer Dis. 2018, 63, 395–405. [Google Scholar] [CrossRef]
- Huang, H.; Tanner, J.; Parvataneni, H.; Rice, M.; Horgas, A.; Ding, M.; Price, C. Impact of total knee arthroplasty with general anesthesia on brain networks: Cognitive efficiency and ventricular volume predict functional connectivity decline in older adults. J. Alzheimer Dis. 2018, 62, 319–333. [Google Scholar] [CrossRef]
- Eriksson, L.I.; Lundholm, C.; Narasimhalu, K.; Sandin, R.; Jin, Y.P.; Gatz, M.; Pedersen, N.L. Hospitalization, surgery, and incident dementia. Alzheimer Dement. 2019, 15, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.M.; Zhang, Y.W.; Sheng, R.W.; Gao, W.; Kang, Q.R.; Gao, Y.C.; Qiu, X.D.; Rui, Y.F. General anesthesia versus regional anesthesia in the elderly patients undergoing hip fracture surgeries: A systematic review and meta-analysis of randomized clinical trials. World J. Surg. 2023, 47, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, G.; Zhang, B.; Moir, R.D.; Xia, W.; Marcantonio, E.R.; Culley, D.J.; Crosby, G.; Tanzi, R.E.; Xie, Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases β-amyloid protein levels. Arch. Neurol. 2009, 66, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Eckenhoff, R.G.; Johansson, J.S.; Wei, H.; Carnini, A.; Kang, B.; Wei, W.; Pidikiti, R.; Keller, J.M.; Eckenhoff, M.F. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology 2004, 101, 703–709. [Google Scholar] [CrossRef]
- Han, F.; Zhao, I.; Zhao, G. Prolonged volatile anesthetic exposure exacerbates cognitive impairment and neuropathology in the 5xFAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2021, 84, 1551–1562. [Google Scholar] [CrossRef]
- Mandal, P.K.; Fodale, V. Isoflurane and desflurane at clinically relevant concentrations induce amyloid beta-peptide oligomerization: An NMR study. Biochem. Biophys. Res. Commun. 2009, 379, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Dong, Y.; Maeda, U.; Moir, R.; Inouye, S.K.; Culley, D.J.; Crosby, G.; Tanzi, R.E. Isoflurane-induced apoptosis: A potential pathogenic link between delirium and dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, Y.; Zhang, G.; Moir, R.D.; Xia, W.; Yue, Y.; Tian, M.; Culley, D.J.; Crosby, G.; Tanzi, R.E.; et al. The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. J. Biol. Chem. 2008, 283, 11866–11875. [Google Scholar] [CrossRef]
- Pan, C.; Xu, Z.; Dong, Y.; Zhang, Y.; Zhang, J.; McAuliffe, S.; Yue, Y.; Li, T.; Xie, Z. The potential dual effects of anesthetic isoflurane on hypoxia-induced caspase-3 activation and increases in β-site amyloid precursor protein-cleaving enzyme levels. Anesth. Analg. 2011, 113, 145–152. [Google Scholar] [CrossRef]
- Planel, E.; Bretteville, A.; Liu, L.; Virag, L.; Du, A.L.; Yu, W.H.; Dickson, D.W.; Whittington, R.A.; Duff, K.E. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J. 2009, 23, 2595–2604. [Google Scholar] [CrossRef]
- Bianch, S.L.; Tran, T.; Liu, C.; Li, Y.; Keller, J.M.; Eckenhoff, R.G.; Eckenhoff, M.F. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol. Aging 2008, 29, 1002–1010. [Google Scholar] [CrossRef]
- Yamamoto, N.; Arima, H.; Sugiura, T.; Hirate, H.; Taniura, H.; Suzuki, K.; Sobue, K. Propofol and thiopental suppress amyloid fibril formation and GM1 ganglioside expression through the γ-aminobutyric acid A receptor. Anesthesiology 2013, 118, 1408–1416. [Google Scholar] [CrossRef]
- Yamamoto, N.; Arima, H.; Sugiura, T.; Hirate, H.; Kusama, N.; Suzuki, K.; Sobue, K. Midazolam inhibits the formation of amyloid fibrils and GM1 ganglioside-rich microdomains in presynaptic membranes through the gamma-aminobutyric acid A receptor. Biochem. Biophy. Res. Commun. 2015, 457, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.A.; Virág, L.; Gratuze, M.; Shpuntoff, H.L.; Cheheltanan, M.; Petry, F.; Poitras, I.; Morin, F.; Planel, E. Administration of the benzodiazepine midazolam increases tau phosphorylation in the mouse brain. Neurobiol. Aging 2019, 75, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, Y.; Morimoto, Y.; Hayashi, M.; Iida, T. Comparison of intravenous sedation using midazolam during dental treatment in elderly patients with/without dementia: A prospective, controlled clinical trial. Sci. Rep. 2021, 11, 3617. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hayashi, M.; Yao, Y.; Nishizaki, H.; Ishii, H.; Mikuzuki, L.; Nishizaki, H.; Ishii, H.; Mikuzuki, L.; Hara, K. Comparison of intravenous sedation using midazolam versus dexmedetomidine in elderly patients with dementia: A randomized cross-over trial. Sci. Rep. 2022, 12, 6293. [Google Scholar] [CrossRef]
- Tsoi, K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, S.Y.S.; Kwok, T.C.Y. Cognitive tests to detect dementia: A systematic review and meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society of Anesthesiologists. Practice Guidelines for Preoperative Fasting. Available online: https://anesth.or.jp/files/pdf/kangae2.pdf (accessed on 26 July 2025).
- Yoshitani, K.; Kawaguchi, M.; Ishida, K.; Maekawa, K.; Miyawaki, H.; Tanaka, S.; Uchino, H.; Kakinohana, M.; Koide, Y.; Yokota, M.; et al. Guidelines for the use of cerebral oximetry by nearinfrared spectroscopy in cardiovascular anesthesia: A report by the cerebrospinal Division of the Academic Committee of the Japanese Society of Cardiovascular Anesthesiologists (JSCVA). J. Anesth. 2019, 33, 167–196. [Google Scholar] [CrossRef]
- Kim, D.H.; Kwak, Y.L.; Nam, S.; Kim, M.S.; Kim, E.M.; Shim, J.K. Assessment of cerebral oxygen supply-demand balance by near-infrared spectroscopy during induction of anesthesia in patients undergoing coronary artery bypass graft surgery: Comparison of midazolam with propofol. Korean J. Anesthesiol. 2009, 57, 428–433. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Cerebral blood flow, cerebrospinal fluid, and brain metabolism. In Guyton and Hall Textbook of Medical Physiology, 14th ed.; Hall, J.E., Hall, M.E., Eds.; Elsevier: Philadelphia, PA, USA, 2021; pp. 777–784. [Google Scholar]
- Patel, P.M.; Drummond, J.C.; Lemkuil, B.P. Cerebral physiology and the effects of anesthetic drugs. In Miller’s Anesthesia, 8th ed.; Miller, R.D., Ed.; Elsevier: Philadelphia, PA, USA, 2015; Volume 1, pp. 387–393, 396–406. [Google Scholar]
- Karwacki, Z.; Niewiadomski, S.; Witkowska, M.; Dzieranowski, J.; Szczyrba, S.; Cichomska, M. The influence of propofol on middle cerebral artery flow velocity (VMCA) in patients with unruptured intracranial aneurysms during induction of general anaesthesia. Anaesth. Intensive Ther. 2018, 50, 349–358. [Google Scholar] [CrossRef]
- Arican, S.; Bakdik, S.; Hacıbeyoğlu, G.; Yilmaz, R.; Koç, O.; Tavlan, A.; Uzun, S.T. The effects of sevoflurane anesthesia on hemodynamics and cerebral artery diameters in endovascular treatment of intracranial aneurysm: A pilot study. Ulus. Travma Acil Cerrahi Derg. 2021, 27, 200–206. [Google Scholar] [CrossRef]
- Thiel, A.; Zickmann, B.; Roth, H.; Hempelmann, G. Effects of Intravenous anesthetic agents on middle cerebral artery blood flow velocity during induction of general anesthesia. J. Clin. Monit. 1995, 11, 92–98. [Google Scholar] [CrossRef]
- Vuyk, J.; Sitsen, E.; Reekers, M. Intravenous anesthetics. In Miller’s Anesthesia, 8th ed.; Miller, R.D., Ed.; Elsevier: Philadelphia, PA, USA, 2015; pp. 838–843. [Google Scholar]
- Soejima, T.; Uchida, Y.; Saito, H.; Morimoto, Y. A prospective observational study of cerebral circulatory changes during induction of general anesthesia using near-infrared time-resolved spectroscopy and transcranial doppler ultrasonography. Masui 2020, 69, 530–537. (In Japanese) [Google Scholar]
- Chaix, I.; Manquat, E.; Liu, N.; Casadio, M.C.; Ludes, P.O.; Tantot, A.; Lopes, J.P.; Touchard, C.; Mateo, J.; Mebazaa, A.; et al. Impact of hypotension on cerebral perfusion during general anesthesia induction: A prospective observational study in adults. Acta Anaesthesiol. Scand. 2020, 64, 592–601. [Google Scholar] [CrossRef]
- Holzer, A.; Greher, M.; Hetz, H.; Standhardt, H.; Donner, A.; Heinzl, H.; Zimpfer, M.; Illievich, U.M. Influence of aortic blood flow velocity on change of middle cerebral artery blood flow velocity during isoflurane and sevoflurane anesthesia. Eur. J. Anaesth. 2001, 18, 238–244. [Google Scholar] [CrossRef]
- Goettel, N.; Patet, C.; Rossi, A.; Burkhart, C.S.; Czosnyka, M.; Strebel, S.P.; Steiner, L.A. Monitoring of cerebral blood flow autoregulation in adults undergoing sevoflurane anesthesia: A prospective cohort study of two age groups. J. Clin. Monit. Comput. 2016, 30, 255–264. [Google Scholar] [CrossRef]
- Juhasz, M.; Molnar, L.; Fulesdi, B.; Vegh, T.; Pall, D.; Molnar, C. Effect of sevoflurane on systemic and cerebral circulation, cerebral autoregulation and CO2 reactivity. BMC Anesthesiol. 2019, 19, 109. [Google Scholar] [CrossRef]
- Khalid, S.G.; Ali, S.M.; Liu, H.; Qurashi, A.G.; Ali, U. Photoplethysmography temporal marker.based machine learning classifier for anesthesia drug detection. Med. Biol. Eng. Comp. 2022, 60, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zhu, J.; Gao, Y.; Luo, Z.; Wang, H.; Zhu, Z.; Yu, Y.; Shi, H.; Bao, H. To clarify features of photoplethysmography in monitoring balanced anesthesia, compared with cerebral state index. Med. Sci. Monit. 2014, 20, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, F.; Chen, X.; Feng, Y.; Miao, J.; Chen, S.; Jiao, C.; Chen, H. Photoplethysmography-derived approximate entropy and sample entropy as measures of analgesia depth during propofol-remifentanil anesthesia. J. Clin. Monit. Comp. 2021, 35, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Abdelmalak, B.; Farag, E. Neurological biomarkers in the perioperative period. Br. J. Anaesth. 2011, 107, 844–858. [Google Scholar] [CrossRef]
- Villalobos, D.; Reese, M.; Wright, M.C.; Wong, M.; Syed, A.; Park, J.; Hall, A.; Browndyke, J.N.; Martucci, K.T.; Devinney, M.J.; et al. Perioperative changes in neurocognitive and Alzheimer’s disease-related cerebrospinal fluid biomarkers in older patients randomized to isoflurane or propofol for anaesthetic maintenance. Br. J. Anaesth. 2023, 131, 328–337. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Seyedmirzaei, H.; Karami, S. Neuroimaging biomarkers and CSF sTREM2 levels in Alzheimer’s disease: A longitudinal study. Sci. Rep. 2024, 14, 15318. [Google Scholar] [CrossRef] [PubMed]

| Patients With Dementia (n = 13) | Patients Without Dementia (n = 13) | p Value | Mann–Whitney U Test | |
|---|---|---|---|---|
| Age (years) | 70 (65–84) | 68 (54.5–77) | 0.113 | 53.5 |
| Sex (male/female) | 6/7 | 10/3 | 0.186 | 58.5 |
| Height (cm) | 157 (147.8–168.0) | 158.4 (156.4–165) | 0.724 | 77.0 |
| Weight (kg) | 49 (43.5–59.7) | 50.8 (50–56.1) | 0.579 | 73.0 |
| Body mass index | 20.4 (19.4–23.1) | 20.4 (18.9–22.6) | 0.880 | 81.5 |
| Treatment time (min) | 177 (159.5–195) | 147 (122–174) | 0.029 | 42.0 |
| Anesthesia time (min) | 230 (215–258) | 196 (161.5–234.5) | 0.016 | 38.0 |
| Time between induction and intubation (min) | 17 (15–21) | 12 (9–18.5) | 0.125 | 54.5 |
| Time between intubation and treatment (min) | 17 (14–20) | 16.0 (14.5–25.5) | 0.960 | 83.0 |
| Induction | ||||
| Fentanyl (μg) | 75 (75–75) | 75 (50–100) | 0.949 | 21.0 |
| Remifentanil (μg/kg/min) | 0.2 (0.1–0.3) | 0.20 (0.2–0.35) | 0.582 | 6.5 |
| Sevoflurane (%) | 5.0 (3.5–5.0) | 5.0 (5.0–5.0) | 0.439 | 13.5 |
| Maintenance | ||||
| Fentanyl (μg) | 25 (25–50) | 50 (25–50) | 0.517 | 7.5 |
| Remifentanil (μg/kg/min) | ||||
| High | 0.10 (0.10–0.15) | 0.15 (0.11–0.19) | 0.261 | 28.0 |
| Low | 0.06 (0.05–0.07) | 0.1 (0.035–0.1) | 0.340 | 30.5 |
| Sevoflurane (%) | ||||
| High | 1.0 (1.0–1.5) | 1.0 (1.0–1.5) | 0.362 | 66.5 |
| Low | 0.5 (0.5–1.0) | 1.0 (1.0–1.0) | 0.064 | 48.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, Y.; Hayashi, M.; Tanaka, Y.; Nishizaki, H.; Shirakawa, M.; Tamura, R.; Mikuzuki, L. Comparison of Cerebral Blood Flow During General Anesthesia in Elderly Patients with and Without Dementia: A Prospective Controlled Clinical Trial. J. Clin. Med. 2025, 14, 6692. https://doi.org/10.3390/jcm14196692
Morimoto Y, Hayashi M, Tanaka Y, Nishizaki H, Shirakawa M, Tamura R, Mikuzuki L. Comparison of Cerebral Blood Flow During General Anesthesia in Elderly Patients with and Without Dementia: A Prospective Controlled Clinical Trial. Journal of Clinical Medicine. 2025; 14(19):6692. https://doi.org/10.3390/jcm14196692
Chicago/Turabian StyleMorimoto, Yoshinari, Megumi Hayashi, Yohei Tanaka, Hitomi Nishizaki, Masayoshi Shirakawa, Ryota Tamura, and Lou Mikuzuki. 2025. "Comparison of Cerebral Blood Flow During General Anesthesia in Elderly Patients with and Without Dementia: A Prospective Controlled Clinical Trial" Journal of Clinical Medicine 14, no. 19: 6692. https://doi.org/10.3390/jcm14196692
APA StyleMorimoto, Y., Hayashi, M., Tanaka, Y., Nishizaki, H., Shirakawa, M., Tamura, R., & Mikuzuki, L. (2025). Comparison of Cerebral Blood Flow During General Anesthesia in Elderly Patients with and Without Dementia: A Prospective Controlled Clinical Trial. Journal of Clinical Medicine, 14(19), 6692. https://doi.org/10.3390/jcm14196692






