The Safety of Negative-Pressure Wound Therapy in Melanoma and Sarcoma Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Strategy
2.2. The Assessment of the Quality of the Included Studies
2.3. Statistical Analysis
3. Results
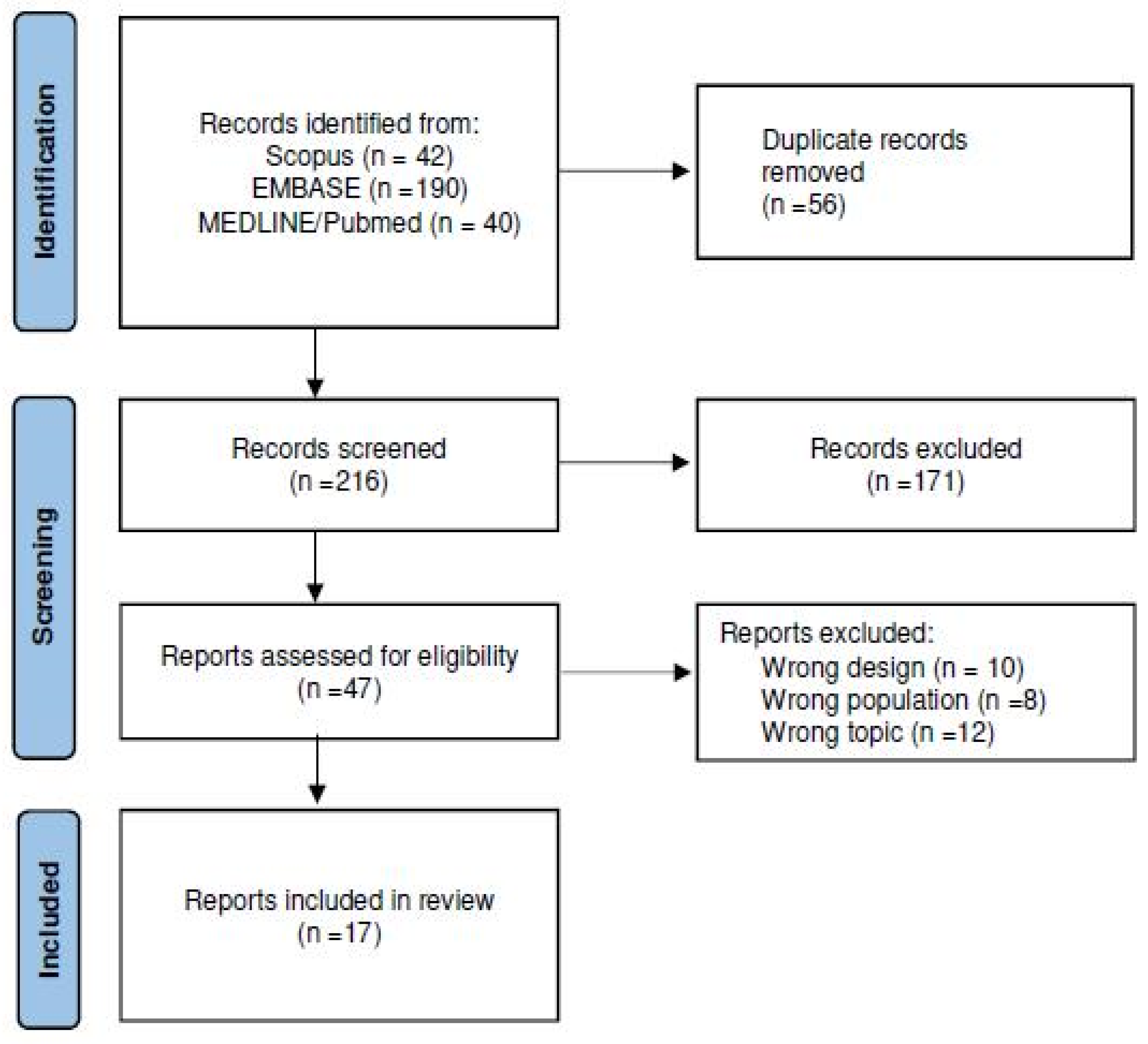
3.1. Search Results
3.2. Study Characteristics
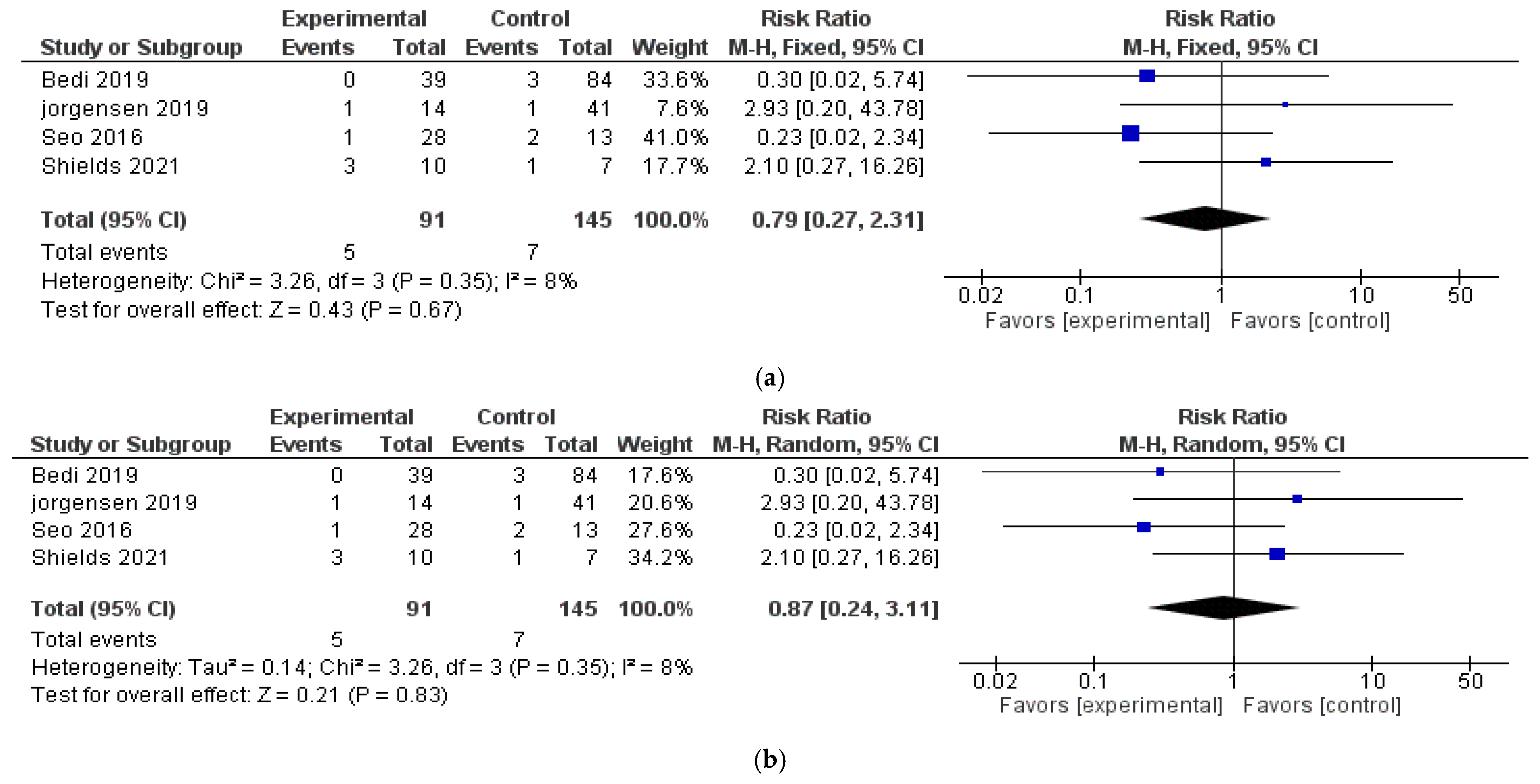
3.3. Local Recurrence Rate (LRR)
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPWT | Negative-Pressure Wound Therapy |
| sNPWT | Single-Use Negative-Pressure Wound Therapy |
| VAC | Vacuum-Assisted Closure |
| CM | Cutaneous Melanoma |
| STS | Soft Tissue Sarcoma |
References
- Apelqvist, J.; Willy, C.; Fagerdahl, A.M.; Fraccalvieri, M.; Malmsjö, M.; Piaggesi, A.; Probst, A.; Vowden, P. EWMA Document: Negative Pressure Wound Therapy. J. Wound Care 2017, 26 (Suppl. S3), S1–S154, Erratum in J. Wound Care 2018, 27, 253. [Google Scholar] [CrossRef] [PubMed]
- Poteet, S.J.; Schulz, S.A.; Povoski, S.P.; Chao, A.H. Negative pressure wound therapy: Device design, indications, and the evidence supporting its use. Expert Rev. Med. Devices 2021, 18, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ker, H.; Al-Murrani, A.; Rolfe, G.; Martin, R. WOUND Study: A Cost-Utility Analysis of Negative Pressure Wound Therapy After Split-Skin Grafting for Lower Limb Skin Cancer. J. Surg. Res. 2019, 235, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Mermerkaya, U.; Bekmez, S.; Alkan, E.; Ayvaz, M.; Tokgozoglu, M. Evaluation of vacuum-assisted closure in patients with wound complications following tumour surgery. Int. Wound J. 2016, 13, 394–397. [Google Scholar] [CrossRef]
- Hays, T.R.; Singh, G.; Saragossi, J.; Park, J.; Shekar, S.; Marquez, J.E.; Dagum, A.B.; Khan, S.U.; Khan, F.A.; Bui, D.T. Negative-Pressure Wound Therapy versus Standard Surgical Dressings after Malignant Tumor Resection: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2022, 150, 655e–670e. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Yao, X.-F.; Lin, Y.-S.; Wang, J.-Y.; Chang, C.-C. Oncologic feasibility for negative pressure wound therapy application in surgical wounds: A meta-analysis. Int. Wound J. 2022, 19, 573–582. [Google Scholar] [CrossRef]
- Lik, P.; Nejc, D. Management of hard-to-heal wounds arising as a result of surgical oncology treatment—Usage of the modern wound dressings. Pol. Przegl. Chir. 2019, 91, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Loos, B.; Kopp, J.; Hohenberger, W.; Horch, R.E. Post-malignancy irradiation ulcers with exposed alloplastic materials can be salvaged with topical negative pressure therapy (TNP). Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2007, 33, 920–925. [Google Scholar] [CrossRef]
- Senchenkov, A.; Petty, P.M.; Knoetgen, J., 3rd; Moran, S.L.; Johnson, C.H.; Clay, R.P. Outcomes of skin graft reconstructions with the use of Vacuum Assisted Closure (VAC®) dressing for irradiated extremity sarcoma defects. World J. Surg. Oncol. 2007, 5, 138. [Google Scholar] [CrossRef]
- Heller, L.; Ballo, M.T.; Cormier, J.N.; Oates, S.D.; Butler, C.E. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann. Plast. Surg. 2008, 60, 58–63. [Google Scholar] [CrossRef]
- Agostini, T.; Dini, M.; Quattrini Li, A.; Grassetti, L.; Mori, A.; Spinelli, G.; Russo, G.L.; Lazzeri, D. A novel combined surgical approach to head and neck dermatofibrosarcoma protuberans. J. Cranio-Maxillo-Facial Surg. 2013, 41, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sun, M.; Dai, H.; Xu, J.; Wang, X.; Guo, R.; Wang, Y.; Xue, C. The application of keystone flap combined with vacuum-assisted closure in the repair of sacrococcygeal skin defect after tumor resection. J. Surg. Oncol. 2019, 119, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Baysal, Ö.; Sağlam, F.; Akgülle, A.H.; Sofulu, Ö.; Yiğit, O.; Şirin, E.; Erol, B. Factors affecting postmusculoskeletal tumour surgery wound problem treatment with negative pressure wound therapy. Int. Wound J. 2020, 17, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Lembo, F.; Cecchino, L.R.; Parisi, D.; Portincasa, A. Role of a new acellular dermal matrix in a multistep combined treatment of dermatofibrosarcoma protuberans of the lumbar region: A case report. J. Med. Case Rep. 2021, 15, 180. [Google Scholar] [CrossRef]
- Miura, T.; Yamamoto, Y.; Murao, N.; Maeda, T.; Osawa, M.; Hayashi, T.; Funayama, E. Combined internal and external negative pressure wound therapy: Breakthrough treatment for lymphocutaneous intractable fistula. Surg. Today 2021, 51, 1630–1637. [Google Scholar] [CrossRef]
- Korovin, S.; Ostafiichuk, V.; Diedkov, S.; Kukushkina, M. Experience of Vacuum-Assisted Closure in the Surgical Treatment of Malignant Skin Tumors after Skin Grafting. Open Access Maced. J. Med. Sci. 2022, 10, 2520–2522. [Google Scholar] [CrossRef]
- Gjorup, C.A.; Andersen, P.S. Excellent outcome of healing by secondary intention after wide local excision of the weight-bearing heel. JPRAS Open 2022, 32, 178–181. [Google Scholar] [CrossRef]
- Fourman, M.S.; Ramsey, D.C.; Newman, E.T.; Schwab, J.H.; Chen, Y.L.; Hung, Y.P.; Chebib, I.; Deshpande, V.; Nielsen, G.P.; DeLaney, T.F.; et al. Assessing the Safety and Utility of Wound VAC Temporization of the Sarcoma or Benign Aggressive Tumor Bed Until Final Margins Are Achieved. Ann. Surg. Oncol. 2022, 29, 2290–2298. [Google Scholar] [CrossRef]
- Chen, Y.U.; Xu, S.F.; Xu, M.; Yu, X.C. Use of negative pressure wound therapy as an adjunct to the treatment of extremity soft-tissue sarcoma with ulceration or impending ulceration. Oncol. Lett. 2016, 12, 757–763. [Google Scholar] [CrossRef]
- Oh, B.H.; Lee, S.H.; Nam, K.A.; Lee, H.B.; Chung, K.Y. Comparison of negative pressure wound therapy and secondary intention healing after excision of acral lentiginous melanoma on the foot. Br. J. Dermatol. 2013, 168, 333–338. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Thomsen, J.B.; Sørensen, J.A. Prophylactic incisional negative pressure wound therapy shows promising results in prevention of wound complications following inguinal lymph node dissection for Melanoma: A retrospective case-control series. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2019, 72, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kim, J.; Nam, K.A.; Zheng, Z.; Oh, B.H.; Chung, K.Y. Reconstruction of large wounds using a combination of negative pressure wound therapy and punch grafting after excision of acral lentiginous melanoma on the foot. J. Dermatol. 2016, 43, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.; King, D.M.; DeVries, J.; Hackbarth, D.A.; Neilson, J.C. Does Vacuum-assisted Closure Reduce the Risk of Wound Complications in Patients With Lower Extremity Sarcomas Treated With Preoperative Radiation? Clin. Orthop. Relat. Res. 2019, 477, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.W.; Razii, N.; Doonan, J.; Mahendra, A.; Gupta, S. Closed incision negative pressure wound therapy versus conventional dressings following soft-tissue sarcoma excision: A prospective, randomized controlled trial. Bone Jt. Open 2021, 2, 1049–1056. [Google Scholar] [CrossRef]
- Review Manager (RevMan) [Computer Program]; Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Kask, G.; Repo, J.P.; Tukiainen, E.J.; Blomqvist, C.; Barner-Rasmussen, I. Soft Tissue Sarcoma of Lower Extremity: Functional Outcome and Quality of Life. Ann. Surg. Oncol. 2021, 28, 6892–6905. [Google Scholar] [CrossRef]
- Hassani, M.; Mate, K.K.V.; Turcotte, R.; Denis-Larocque, G.; Ghodsi, E.; Tsimicalis, A.; Goulding, K. Uncovering the gaps: A systematic mixed studies review of quality of life measures in extremity soft tissue sarcoma. J. Surg. Oncol. 2023, 128, 430–443. [Google Scholar] [CrossRef]
- Dal Pos, S.; Mazza, M.; Gianesini, C.M.; Cavallin, F.; Del Fiore, P.; Tropea, S.; Parisi, A.; Ghirelli, A.; Buzzaccarini, M.S.; Scarzello, G.; et al. Wound Healing Complication in Radio-Treated Limb Soft Tissue Sarcoma Patients: A Single Referral Centre Experience. Int. Wound J. 2025, 22, e70175. [Google Scholar] [CrossRef]
- Coindre, J.M. Grading of soft tissue sarcomas: Review and update. Arch. Pathol. Lab. Med. 2006, 130, 1448–1453. [Google Scholar] [CrossRef]
- Mocellin, S. Soft Tissue Tumors: A Practical and Comprehensive Guide to Sarcomas and Benign Neoplasms; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Sugiura, H.; Tsukushi, S.; Yoshida, M.; Nishida, Y. What Is the Success of Repeat Surgical Treatment of a Local Recurrence After Initial Wide Resection of Soft Tissue Sarcomas? Clin. Orthop. Relat. Res. 2018, 476, 1791–1800. [Google Scholar] [CrossRef]
- Ramanathan, R.C.; A’Hern, R.; Fisher, C.; Meirion, J.T.T. Prognostic Index for Extremity Soft Tissue Sarcomas with Isolated Local Recurrence. Ann. Surg. Oncol. 2001, 8, 278–289. [Google Scholar] [CrossRef]
- García-Ortega, D.Y.; Clara-Altamirano, M.A.; Martín-Tellez, K.S.; Caro-Sanchez, C.H.S.; Alvarez-Cano, A.; Lino-Silva, L.S.; Salcedo-Hernandez, R.A.; Ruvalcaba-Gonzalez, C.C.; Martinez-Said, H.; Luna-Ortiz, L. Epidemiological profile of soft tissue sarcomas of the extremities: Incidence, histological subtypes, and primary sites. J. Orthop. 2021, 25, 70–74. [Google Scholar] [CrossRef]
- Dal Pos, S.; Tafaj, S.; Furlan, D.; Volpi, S.; Mazza, M. The application of the negative pressure wound therapy in patients with cutaneous melanoma and soft tissue sarcoma: A case series. Int. J. Clin. Invest. Case Rep. 2024, 3, 20–24. [Google Scholar] [CrossRef]
| Author, Year | Histology | Number of Patients | Median Age (Range) | Margin Status (RO %) | Local Recurrence | LRR (%) | Timing Recurrence (Months) | Mean FU (Months) |
|---|---|---|---|---|---|---|---|---|
| Loos B, 2007 [10] | STS | 1 | 56 | 100 | 0 | 0 | N/A | 18 |
| Senchenkov, A. 2007 [11] | STS | 17 | 65 (42–82) | N/A | 1 | 6 | 24 | N/A |
| Heller L, 2008 [12] | STS | 3 | 70 (62–76) | N/A | 1 | 33 | 5 | 12.3 (9–18) |
| Agostini T, 2013 [13] | STS | 5 | 47 (20–61) | N/A | 0 | 0 | N/A | 15.2 (9–24) |
| Chen Y, 2015 [21] | STS | 5 | 44 (24–68) | 100 | 1 | 20 | 12 | 26 (12–36) |
| Wu M, 2019 [14] | STS | 12 | 54 (34–69) | 100 | 0 | 0 | N/A | 12 (1–24) |
| Baysal Ö, 2020 [15] | STS | 42 | 39 (8–79) | 100 | 0 | 0 | N/A | 30 (5–55) |
| Lembo F, 2021 [16] | STS | 1 | 34 | 100 | 0 | 0 | N/A | 6 |
| Miura T, 2021 [17] | CM | 5 | 66 (58–77) | N/A | 0 | 0 | N/A | N/A |
| Korovin, S, 2022 [18] | CM | 31 | 58 (23–86) | N/A | 2 | 6 | 3 | 20 (6–35) |
| Gjorup G.A. 2022 [19] | CM | 1 | 54 | 100 | 0 | 0 | N/A | 10 |
| Fourman MS. 2022 [20] | STS | 62 | 66 (61–72) | 93 | 5 | 8.1 | 33 (15–51) | 52 (19–85) |
| Author | Histology | Margin Status (RO %) | Control Group | NPWT Group | Control Group | NPWT Group | Timing Recurrence (Months) | Mean FU (Months) |
|---|---|---|---|---|---|---|---|---|
| Patients (Median Age) | LRR (%) | |||||||
| Oh B. H, 2013 [22] | CM | N/A | 13 (64.7) | 9 (58.3) | 0 | 0 | N/A | NA |
| Jørgensen M. G, 2019 [23] | CM | N/A | 41 (57.8) | 14 (60) | 1 | 1 | N/A | 24 (21.9–32.9) |
| Seo J, 2016 [24] | CM | N/A | 13 (64.9) | 28 (58.8) | 2 | 1 | N/A | 77 (62–92) |
| Bedi M, 2019 [25] | STS | 93% | 84 (56.5) | 39 (54) | 3 | 0 | N/A | 39.6 (15–98) |
| Shields D, 2021 [26] | STS | N/A | 7 (50) | 10 (56) | 1 | 3 | N/A | 25 (8–42) |
| ITEMS | Loos [10] | Senchenkov, A. [11] | Heller L [12] | Agostini T [13] | Wu M [14] | Baysal Ö [15] | Lembo F [16] | Miura T [17] | Korovin S [18] | Gjorup G.A. [19] | Fourman MS [20] | Chen Y [21] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clear criteria of inclusion | + | + | + | + | + | + | + | + | + | + | + | + |
| Adequacy of the population | +\− | +\− | +\− | +\− | +\− | + | +\− | +\− | +\− | +\− | +\− | +\− |
| Clear description of characteristics of the participants | + | + | + | + | + | + | + | + | + | + | + | + |
| Adequacy of data collection | + | + | + | + | + | + | + | + | +\− | + | + | +\− |
| Relevance of the measurement tools | +\− | +\− | +\− | +\− | +\− | + | +\− | +\− | +\− | +\− | +\− | +\− |
| Appropriateness of the analysis | +\− | +\− | +\− | +\− | +\− | + | +\− | +\− | +\− | +\− | +\− | − |
| Biases consideration | +\− | +\− | +\− | +\− | +\− | +\− | +\− | +\− | +\− | +\− | +\− | − |
| Clarity of the results | + | + | + | + | + | + | + | + | + | + | + | +\− |
| Conclusion supported by the results | + | + | + | + | + | + | + | + | + | + | + | +\− |
| ITEMS | Oh B. H [22] | Jørgensen M.G [23] | Seo J [24] | Bedi M [25] | Shields D [26] |
|---|---|---|---|---|---|
| Clear criteria of inclusion | + | + | + | + | + |
| Adequacy of the population | +/− | +/− | + | + | + |
| Adequacy of comparison of groups (similar treatment/care other than the exposure or intervention or interest) | +/− | +/− | + | + | + |
| Control group | + | + | + | + | + |
| Completeness of the measurement tools | + | + | + | + | + |
| Adequacy of follow up | + | + | + | + | + |
| Relevance of the outcome measurement tools | + | + | + | + | + |
| Clarity and reliability of the results and | + | + | + | + | + |
| Appropriateness of the analysis | +/− | +/− | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Pos, S.; Tafaj, S.; Hoxhaj, I.; Cassalia, F.; Russano, F.; Tropea, S.; Del Fiore, P.; Mazza, M. The Safety of Negative-Pressure Wound Therapy in Melanoma and Sarcoma Patients: A Systematic Review. J. Clin. Med. 2025, 14, 7044. https://doi.org/10.3390/jcm14197044
Dal Pos S, Tafaj S, Hoxhaj I, Cassalia F, Russano F, Tropea S, Del Fiore P, Mazza M. The Safety of Negative-Pressure Wound Therapy in Melanoma and Sarcoma Patients: A Systematic Review. Journal of Clinical Medicine. 2025; 14(19):7044. https://doi.org/10.3390/jcm14197044
Chicago/Turabian StyleDal Pos, Silvia, Stela Tafaj, Ilda Hoxhaj, Fortunato Cassalia, Francesco Russano, Saveria Tropea, Paolo Del Fiore, and Marcodomenico Mazza. 2025. "The Safety of Negative-Pressure Wound Therapy in Melanoma and Sarcoma Patients: A Systematic Review" Journal of Clinical Medicine 14, no. 19: 7044. https://doi.org/10.3390/jcm14197044
APA StyleDal Pos, S., Tafaj, S., Hoxhaj, I., Cassalia, F., Russano, F., Tropea, S., Del Fiore, P., & Mazza, M. (2025). The Safety of Negative-Pressure Wound Therapy in Melanoma and Sarcoma Patients: A Systematic Review. Journal of Clinical Medicine, 14(19), 7044. https://doi.org/10.3390/jcm14197044







