Sleep Disorders in Neurodegenerative Diseases with Dementia: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
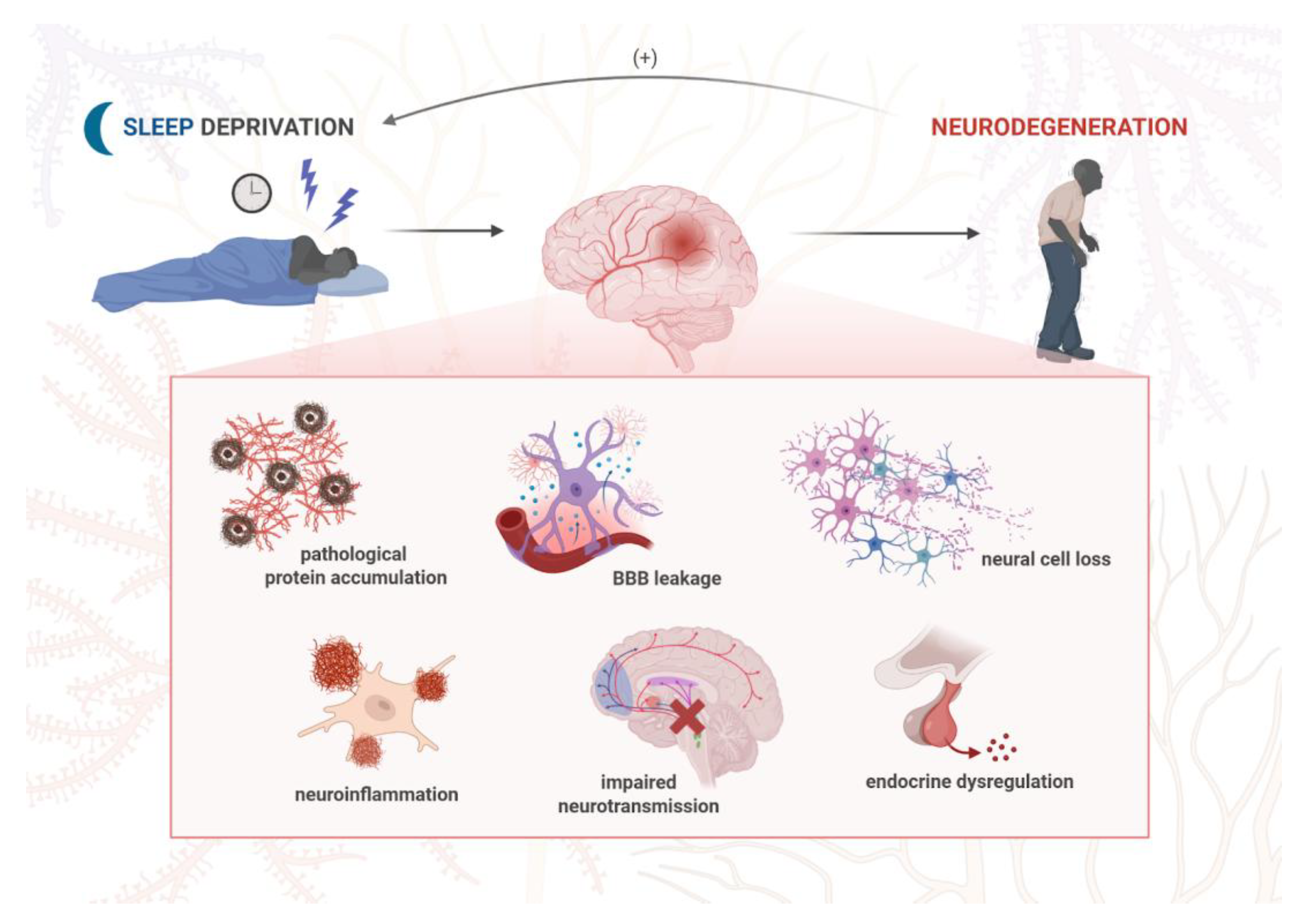
3. Pathophysiology of Neurodegenerative Diseases
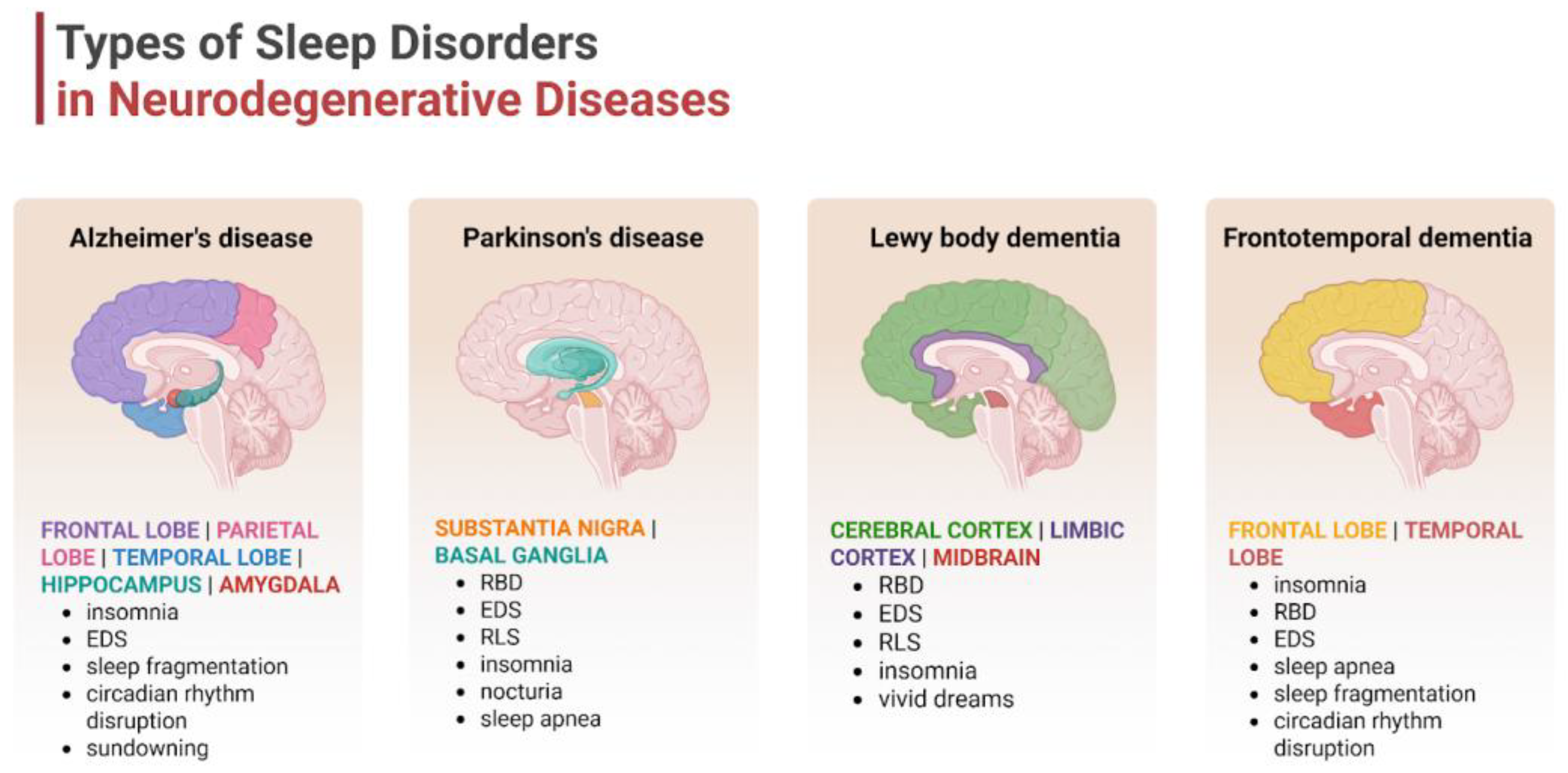
4. Sleep Disorders in Neurodegenerative Diseases with Dementia
4.1. Types of Common Sleep Disorders
4.2. Disease-Specific Sleep Profiles
5. Mental Health Symptoms in Dementia
6. Mechanistic Links Between Sleep Disturbance and Neuropsychiatric Symptoms
6.1. Neuroanatomical Overlap
6.2. Neurochemical Dysregulation
6.3. Neuroinflammation and Stress
6.4. Circadian Misalignment
7. Bidirectional Relationship: Sleep and Mental Health in Dementia
8. Clinical Assessment and Diagnosis
9. Therapeutic Approaches
9.1. Non-Pharmacological Interventions
9.2. Pharmacological Strategies
9.3. Multidisciplinary Management
10. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDB | Sleep-disordered breathing |
| EDS | Excessive daytime sleepiness |
| RLS | Restless legs syndrome |
| REM | Rapid eye movement |
| NREM | Non-rapid eye movement |
| RBD | REM sleep behavior disorder |
| SWS | Slow-wave sleep |
| OSA | Obstructive sleep apnea |
| BMI | Body mass index |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| DLB | Dementia with Lewy bodies |
| FTD | Frontotemporal dementia |
| MSA | Multiple system atrophy |
| PSP | Progressive supranuclear palsy |
| CBD | Corticobasal degeneration |
| MDD | Major depressive disorder |
| DIS | Difficulty initiating sleep |
| MCI | Mild cognitive impairment |
| CSDD | Cornell Scale for Depression in Dementia |
| GDS | Geriatric Depression Scale |
| GAD | Generalized anxiety disorder |
| NPI | Neuropsychiatric inventory |
| NBRS | Neurobehavioral rating scale |
| PAS | Pittsburgh agitation scale |
| PSQI | Pittsburgh sleep quality index |
| SDI | Sleep disorders inventory |
| ESS | Epworth sleepiness scale |
| SCN | Suprachiasmatic nucleus |
| TMN | Tuberomammillary nucleus |
| MCH | Melanin-concentrating hormone |
| LH | Lateral hypothalamus |
| ARAS | Ascending reticular activating system |
| LC | Locus coeruleus |
| RN | Raphe nuclei |
| PAG | Periaqueductal gray matter |
| IntN | Intermediate nucleus |
| PPN | Pedunculopontine nucleus |
| VMH | Ventromedial nucleus |
| SN | Substantia nigra |
| SubC | Subcoeruleus nucleus |
| NM | Nucleus magnocellularis |
| CRHBP | Corticotropin-releasing hormone-binding protein |
| nBM | Nucleus basalis of Meynert |
| GABA | Gamma-aminobutyric acid |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| BBB | Blood–brain barrier |
| EEG | Electroencephalogram |
| ROS | Reactive oxygen species |
| RN | Reactive nitrogen species |
| CRP | C-reactive protein |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor α |
| NLRP3 | NLR family pyrin domain containing 3 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| BDNF | Brain-derived neurotrophic factor |
| TrkB | Tropomyosin receptor kinase B |
| CLOCK | Circadian locomotor output cycles protein kaput |
| PER | Period |
| CRY | Cryptochrome |
| BMAL1 | Brain and muscle ARNT-like protein 1 |
| VIP | Vasoactive intestinal peptide |
| MAO-A | Monoamine oxidase A |
| MTNR1 | Melatonin receptor 1A gene |
| MT1 | Melatonin receptor 1A |
| MT2 | Melatonin receptor 1B |
| CBT-I | Cognitive behavioral therapy for insomnia |
| SRT | Sleep restriction therapy |
| SCT | Stimulus control therapy |
| SH | Sleep hygiene |
| CT | Cognitive therapy |
| BLT | Bright light therapy |
| SAD | Seasonal affective disorder |
| Aβ | Amyloid-β |
| SSRIs | Serotonin reuptake inhibitors |
| NIBS | Non-invasive brain stimulation |
| rTMS | Repetitive transcranial magnetic stimulation |
References
- International, A.D.; Guerchet, M.; Prince, M.; Prina, M. The Global Impact of Dementia 2013–2050: Policy Brief for Heads of Government; Alzheimer’s Disease International: London, UK, 2013. [Google Scholar]
- Li, Z.; Yang, Y.; Liu, Y.; Wang, X.; Ping, F.; Xu, L.; Zhang, H.; Li, W.; Li, Y. Global Burden of Dementia in Younger People: An Analysis of Data from the 2021 Global Burden of Disease Study. eClinicalMedicine 2024, 77, 102868. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Liao, Y.-C.; Tam, K.-W.; Chan, L.; Hsu, T.-H. Effects of Music Therapy on Cognition, Quality of Life, and Neuropsychiatric Symptoms of Patients with Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychiatry Res. 2023, 329, 115498. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, D.; Calo, S.; Dichter, M.N.; Meyer, G.; Möhler, R.; Köpke, S. Non-Pharmacological Interventions for Sleep Disturbances in People with Dementia. Cochrane Database Syst. Rev. 2023, 1, CD011881. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, B.; Adorni, F.; Musicco, M.; Appollonio, I.; Bonanni, E.; Caffarra, P.; Caltagirone, C.; Cerroni, G.; Concari, L.; Cosentino, F.I.I.; et al. Prevalence of Sleep Disturbances in Mild Cognitive Impairment and Dementing Disorders: A Multicenter Italian Clinical Cross-Sectional Study on 431 Patients. Dement. Geriatr. Cogn. Disord. 2012, 33, 50–58. [Google Scholar] [CrossRef]
- Savva, G.M.; Zaccai, J.; Matthews, F.E.; Davidson, J.E.; McKeith, I.; Brayne, C.; Medical Research Council. Cognitive Function and Ageing Study Prevalence, Correlates and Course of Behavioural and Psychological Symptoms of Dementia in the Population. Br. J. Psychiatry 2009, 194, 212–219. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of Neuropsychiatric Symptoms in Dementia and Mild Cognitive Impairment: Results from the Cardiovascular Health Study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Marshall, G.A. Neuropsychiatric Symptoms in Dementia. Continuum 2024, 30, 1744–1760. [Google Scholar] [CrossRef]
- Radue, R.; Walaszek, A.; Asthana, S. Neuropsychiatric Symptoms in Dementia. Handb. Clin. Neurol. 2019, 167, 437–454. [Google Scholar] [CrossRef]
- Bubu, O.M.; Brannick, M.; Mortimer, J.; Umasabor-Bubu, O.; Sebastião, Y.V.; Wen, Y.; Schwartz, S.; Borenstein, A.R.; Wu, Y.; Morgan, D.; et al. Sleep, Cognitive Impairment, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsw032. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Lehoczki, A.; Munkácsy, G.; Fekete, J.T.; Zábó, V.; Purebl, G.; Varga, P.; Ungvari, A.; Győrffy, B. Sleep Disorders Increase the Risk of Dementia, Alzheimer’s Disease, and Cognitive Decline: A Meta-Analysis. GeroScience 2025, 47, 4899–4920. [Google Scholar] [CrossRef]
- Yang, Q.; Li, S.; Yang, Y.; Lin, X.; Yang, M.; Tian, C.; Mao, J. Prolonged Sleep Duration as a Predictor of Cognitive Decline: A Meta-Analysis Encompassing 49 Cohort Studies. Neurosci. Biobehav. Rev. 2024, 164, 105817. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association Between Sleep Duration and Cognitive Decline. JAMA Netw. Open 2020, 3, e2013573. [Google Scholar] [CrossRef]
- Pandya, V.A.; Patani, R. Region-Specific Vulnerability in Neurodegeneration: Lessons from Normal Ageing. Ageing Res. Rev. 2021, 67, 101311. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-C.; Ulane, C.M.; Burke, R.E. Clinical Progression in Parkinson Disease and the Neurobiology of Axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Gülöksüz, S. Shedding Light on the Etiology of Neurodegenerative Diseases and Dementia: The Exposome Paradigm. Npj Ment. Health Res. 2022, 1, 20. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving Neurodegeneration: Common Mechanisms and Strategies for New Treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Overcoming Gaps in the Treatment of Neurodegenerative Disease. eBioMedicine 2020, 60, 103088. [CrossRef]
- Bertram, L.; Tanzi, R.E. The Genetic Epidemiology of Neurodegenerative Disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and Its Involvement in Alzheimer’s Disease: A Review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef]
- Duda, J.E. Pathology and Neurotransmitter Abnormalities of Dementia with Lewy Bodies. Dement. Geriatr. Cogn. Disord. 2004, 17 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef]
- Pasanen, P.; Myllykangas, L.; Siitonen, M.; Raunio, A.; Kaakkola, S.; Lyytinen, J.; Tienari, P.J.; Pöyhönen, M.; Paetau, A. Novel α-Synuclein Mutation A53E Associated with Atypical Multiple System Atrophy and Parkinson’s Disease-Type Pathology. Neurobiol. Aging 2014, 35, 2180.e1–2180.e5. [Google Scholar] [CrossRef]
- Smith, L.; Schapira, A.H.V. GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells 2022, 11, 1261. [Google Scholar] [CrossRef]
- Orme, T.; Guerreiro, R.; Bras, J. The Genetics of Dementia with Lewy Bodies: Current Understanding and Future Directions. Curr. Neurol. Neurosci. Rep. 2018, 18, 67. [Google Scholar] [CrossRef]
- Chan, P.-C.; Lee, H.-H.; Hong, C.-T.; Hu, C.-J.; Wu, D. REM Sleep Behavior Disorder (RBD) in Dementia with Lewy Bodies (DLB). Behav. Neurol. 2018, 2018, 9421098. [Google Scholar] [CrossRef] [PubMed]
- Sobue, G.; Ishigaki, S.; Watanabe, H. Pathogenesis of Frontotemporal Lobar Degeneration: Insights From Loss of Function Theory and Early Involvement of the Caudate Nucleus. Front. Neurosci. 2018, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Greaves, C.V.; Rohrer, J.D. An Update on Genetic Frontotemporal Dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Murley, A.G.; Rowe, J.B. Neurotransmitter Deficits from Frontotemporal Lobar Degeneration. Brain 2018, 141, 1263–1285. [Google Scholar] [CrossRef]
- Ubhi, K.; Low, P.; Masliah, E. Multiple System Atrophy: A Clinical and Neuropathological Perspective. Trends Neurosci. 2011, 34, 581–590. [Google Scholar] [CrossRef]
- Yan, S.; Lu, J.; Duan, B.; Zhu, H.; Liu, D.; Li, L.; Qin, Y.; Li, Y.; Zhu, W. Quantitative Susceptibility Mapping of Multiple System Atrophy and Parkinson’s Disease Correlates with Neurotransmitter Reference Maps. Neurobiol. Dis. 2024, 198, 106549. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, Y.; Jiao, B.; Shen, L. Genetics of Progressive Supranuclear Palsy: A Review. J. Parkinsons Dis. 2021, 11, 93–105. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Paraskevas, P.G.; Stefanis, L.; Kapaki, E. Corticobasal Degeneration and Corticobasal Syndrome: A Review. Clin. Park. Relat. Disord. 2019, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Arienti, F.; Lazzeri, G.; Vizziello, M.; Monfrini, E.; Bresolin, N.; Saetti, M.C.; Picillo, M.; Franco, G.; Di Fonzo, A. Unravelling Genetic Factors Underlying Corticobasal Syndrome: A Systematic Review. Cells 2021, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; Schmidt, R. Genetics of Vascular Cognitive Impairment. Stroke 2019, 50, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Pathology and Pathogenesis of Vascular Cognitive Impairment-a Critical Update. Front. Aging Neurosci. 2013, 5, 17. [Google Scholar] [CrossRef]
- Court, J.A.; Perry, E.K. Neurotransmitter Abnormalities in Vascular Dementia. Int. Psychogeriatr. 2003, 15 (Suppl. S1), S81–S87. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep. Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Liu, X.-C.; Zhou, J.; Cheng, G.-R.; Yang, M.-L.; Hu, F.-F.; Liu, D.; Xie, X.-Y.; Ji, Y.; Lv, Y.; Niu, J.-P.; et al. Association between Insomnia and Its Symptoms and Cognitive Impairment in Community-Dwelling Older Adults in China: A Multicenter Study. J. Alzheimer’s Dis. 2025, 106, 1425–1435. [Google Scholar] [CrossRef]
- Lam, A.; Kong, D.; D’Rozario, A.L.; Ireland, C.; Ahmed, R.M.; Schrire, Z.M.; Mowszowski, L.; Michaelian, J.; Grunstein, R.R.; Naismith, S.L. Sleep Disturbances and Disorders in the Memory Clinic: Self-Report, Actigraphy, and Polysomnography. J. Alzheimer’s Dis. 2025, 106, 78–93. [Google Scholar] [CrossRef]
- Stefani, A.; Antelmi, E.; Arnaldi, D.; Arnulf, I.; During, E.; Högl, B.; Hu, M.M.T.; Iranzo, A.; Luke, R.; Peever, J.; et al. From Mechanisms to Future Therapy: A Synopsis of Isolated REM Sleep Behavior Disorder as Early Synuclein-Related Disease. Mol. Neurodegener. 2025, 20, 19. [Google Scholar] [CrossRef]
- Brenlla, C.; Lazaro-Hernandez, C.; Fernandez, M.; Perez-Montesino, J.; Mena, d.L.; Bargallo, N.; Sanchez, V.; Perissinotti, A.; Niñerola-Baizan, A.; Perez-Soriano, A.; et al. Biomarkers of Neurodegenerative Parkinsonisms: From Current Clinical to Future Biological Definitions—Literature Review and Our Experience. Neurol. Neurochir. Pol. 2025, 59, 97–110. [Google Scholar] [CrossRef]
- Kim, R.; Jin, B.; Kim, H.; Woo, K.A.; Yoon, E.J.; Kim, S.; Shin, J.H.; Nam, H.; Kim, Y.K.; Jeon, B.; et al. Cholinergic Basal Forebrain Degeneration in Isolated REM Sleep Behaviour Disorder. Brain 2025, awaf196. [Google Scholar] [CrossRef]
- Leitner, C.; D’Este, G.; Verga, L.; Rahayel, S.; Mombelli, S.; Sforza, M.; Casoni, F.; Zucconi, M.; Ferini-Strambi, L.; Galbiati, A. Neuropsychological Changes in Isolated REM Sleep Behavior Disorder: A Systematic Review and Meta-Analysis of Cross-Sectional and Longitudinal Studies. Neuropsychol. Rev. 2024, 34, 41–66. [Google Scholar] [CrossRef]
- Ju, E.; Guo, Y.; Burton, C.; Kim, J.; Lee, J.-A. Severe Sleep Disturbances in Persons with Dementia with REM Sleep Behavior Disorder and Family Caregivers: A Mixed Methods Study. Res. Gerontol. Nurs. 2024, 17, 247–255. [Google Scholar] [CrossRef]
- Xu, Q.-H.; Wang, C. Reassessing the Role of Amyloid in Isolated REM Sleep Behavior Disorder. Park. Relat. Disord. 2025, 135, 107809. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Long, J.J.; Chen, Y.; Kim, B.; Bae, S.; Li, Y.; Orandi, B.J.; Chu, N.M.; Mathur, A.; Segev, D.L.; McAdams-DeMarco, M.A. Sleep Disorders and Dementia Risk in Older Patients with Kidney Failure: A Retrospective Cohort Study. Clin. J. Am. Soc. Nephrol. 2024, 19, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Lyu, X.; Dunietz, G.L.; Schulz, P.C.; Bove, R.; Chervin, R.D.; Paulson, H.L.; Shedden, K. Sex-Specific Dementia Risk in Known or Suspected Obstructive Sleep Apnea: A 10-Year Longitudinal Population-Based Study. Sleep Adv. 2024, 5, zpae077. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Sasai-Sakuma, T.; Morita, Y.; Okawa, M.; Inoue, S.; Inoue, Y. Prevalence and Associated Factors of Circadian Rhythm Sleep-Wake Disorders and Insomnia among Visually Impaired Japanese Individuals. BMC Public Health 2021, 21, 31. [Google Scholar] [CrossRef]
- Ghorbani Shirkouhi, S.; Karimi, A.; Khatami, S.S.; Asgari Gashtrodkhani, A.; Kamari, F.; Blaabjerg, M.; Andalib, S. The Clock and the Brain: Circadian Rhythm and Alzheimer’s Disease. Curr. Issues Mol. Biol. 2025, 47, 547. [Google Scholar] [CrossRef] [PubMed]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, Q.; Xie, W.; Gong, S.; Zhuang, S.; Liu, J.; Mao, C.; Liu, C. Circadian Disruption and Sleep Disorders in Neurodegeneration. Transl. Neurodegener. 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Ylikoski, A.; Martikainen, K.; Partinen, M. Parasomnias and Isolated Sleep Symptoms in Parkinson’s Disease: A Questionnaire Study on 661 Patients. J. Neurol. Sci. 2014, 346, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Chahine, L.M.; Amara, A.W.; Videnovic, A. A Systematic Review of the Literature on Disorders of Sleep and Wakefulness in Parkinson’s Disease from 2005 to 2015. Sleep Med. Rev. 2017, 35, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Maggi, G.; Vitale, C.; Cerciello, F.; Santangelo, G. Sleep and Wakefulness Disturbances in Parkinson’s Disease: A Meta-Analysis on Prevalence and Clinical Aspects of REM Sleep Behavior Disorder, Excessive Daytime Sleepiness and Insomnia. Sleep Med. Rev. 2023, 68, 101759. [Google Scholar] [CrossRef]
- Stefanova, N.; Bücke, P.; Duerr, S.; Wenning, G.K. Multiple System Atrophy: An Update. Lancet Neurol. 2009, 8, 1172–1178. [Google Scholar] [CrossRef]
- Moreno-López, C.; Santamaría, J.; Salamero, M.; Del Sorbo, F.; Albanese, A.; Pellecchia, M.T.; Barone, P.; Overeem, S.; Bloem, B.; Aarden, W.; et al. Excessive Daytime Sleepiness in Multiple System Atrophy (SLEEMSA Study). Arch. Neurol. 2011, 68, 223–230. [Google Scholar] [CrossRef]
- Palma, J.-A.; Fernandez-Cordon, C.; Coon, E.A.; Low, P.A.; Miglis, M.G.; Jaradeh, S.; Bhaumik, A.K.; Dayalu, P.; Urrestarazu, E.; Iriarte, J.; et al. Prevalence of REM Sleep Behavior Disorder in Multiple System Atrophy: A Multicenter Study and Meta-Analysis. Clin. Auton. Res. 2015, 25, 69–75. [Google Scholar] [CrossRef]
- Elder, G.J.; Lazar, A.S.; Alfonso-Miller, P.; Taylor, J.-P. Sleep Disturbances in Lewy Body Dementia: A Systematic Review. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- McCarter, S.J.; St Louis, E.K.; Boeve, B.F. Sleep Disturbances in Frontotemporal Dementia. Curr. Neurol. Neurosci. Rep. 2016, 16, 85. [Google Scholar] [CrossRef]
- Abbott, S.M.; Videnovic, A. Sleep Disorders in Atypical Parkinsonism. Mov. Disord. Clin. Pract. 2014, 1, 89–96. [Google Scholar] [CrossRef]
- Arena, J.E.; Weigand, S.D.; Whitwell, J.L.; Hassan, A.; Eggers, S.D.; Höglinger, G.U.; Litvan, I.; Josephs, K.A. Progressive Supranuclear Palsy: Progression and Survival. J. Neurol. 2016, 263, 380–389. [Google Scholar] [CrossRef]
- 2025 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2025, 21, e70235. [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Yang, L.; Zhang, H.; Shi, Y.; Okhravi, H.R.; Vitiello, M.V.; Sanford, L.D.; Tang, X. Sleep in Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Polysomnographic Findings. Transl. Psychiatry 2022, 12, 136. [Google Scholar] [CrossRef]
- Volicer, L.; Harper, D.G.; Manning, B.C.; Goldstein, R.; Satlin, A. Sundowning and Circadian Rhythms in Alzheimer’s Disease. Am. J. Psychiatry 2001, 158, 704–711. [Google Scholar] [CrossRef]
- Lim, A.S.P.; Kowgier, M.; Yu, L.; Buchman, A.S.; Bennett, D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 2013, 36, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Suzuki, K. Current Update on Clinically Relevant Sleep Issues in Parkinson’s Disease: A Narrative Review. J. Park. Dis. 2021, 11, 971–992. [Google Scholar] [CrossRef]
- Feng, F.; Cai, Y.; Hou, Y.; Ou, R.; Jiang, Z.; Shang, H. Excessive Daytime Sleepiness in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Park. Relat. Disord. 2021, 85, 133–140. [Google Scholar] [CrossRef]
- Diaconu, Ș.; Falup-Pecurariu, O.; Țînț, D.; Falup-Pecurariu, C. REM Sleep Behaviour Disorder in Parkinson’s Disease (Review). Exp. Ther. Med. 2021, 22, 812. [Google Scholar] [CrossRef]
- Iranzo, A.; Cochen De Cock, V.; Fantini, M.L.; Pérez-Carbonell, L.; Trotti, L.M. Sleep and Sleep Disorders in People with Parkinson’s Disease. Lancet Neurol. 2024, 23, 925–937. [Google Scholar] [CrossRef]
- Elfil, M.; Bahbah, E.I.; Attia, M.M.; Eldokmak, M.; Koo, B.B. Impact of Obstructive Sleep Apnea on Cognitive and Motor Functions in Parkinson’s Disease. Mov. Disord. 2021, 36, 570–580. [Google Scholar] [CrossRef]
- Yang, X.; Liu, B.; Shen, H.; Li, S.; Zhao, Q.; An, R.; Hu, F.; Ren, H.; Xu, Y.; Xu, Z. Prevalence of Restless Legs Syndrome in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Observational Studies. Sleep Med. 2018, 43, 40–46. [Google Scholar] [CrossRef]
- Marchesi, E.; Negrotti, A.; Angelini, M.; Goldoni, M.; Abrignani, G.; Calzetti, S. A Prospective Study of the Cumulative Incidence and Course of Restless Legs Syndrome in de Novo Patients with Parkinson’s Disease during Chronic Dopaminergic Therapy. J. Neurol. 2016, 263, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko-Alster, N.; Migda, A.; Migda, B.; Kutyłowski, M.; Królicki, L.; Friedman, A. Sleep Disturbances in Progressive Supranuclear Palsy Syndrome (PSPS) and Corticobasal Syndrome (CBS). Neurol. Neurochir. Pol. 2023, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Chokesuwattanaskul, A.; Lertjitbanjong, P.; Thongprayoon, C.; Bathini, T.; Sharma, K.; Mao, M.A.; Cheungpasitporn, W.; Chokesuwattanaskul, R. Impact of Obstructive Sleep Apnea on Silent Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Sleep Med. 2020, 68, 80–88. [Google Scholar] [CrossRef]
- Bessey, L.J.; Walaszek, A. Management of Behavioral and Psychological Symptoms of Dementia. Curr. Psychiatry Rep. 2019, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-W.; Sung, J.-Y.; Wang, W.-H. The Impact of Behavioral and Psychological Symptoms of Dementia on Mental Health, Sleep Quality, and Caregiver’s Burden. Int. J. Geriatr. Psychiatry 2025, 40, e70080. [Google Scholar] [CrossRef]
- Stott, J.; Saunders, R.; Desai, R.; Bell, G.; Fearn, C.; Buckman, J.E.J.; Brown, B.; Nurock, S.; Michael, S.; Ware, P.; et al. Associations between Psychological Intervention for Anxiety Disorders and Risk of Dementia: A Prospective Cohort Study Using National Health-Care Records Data in England. Lancet Healthy Longev. 2023, 4, e12–e22. [Google Scholar] [CrossRef]
- Wolf, M.U.; Goldberg, Y.; Freedman, M. Aggression and Agitation in Dementia. Continuum 2018, 24, 783–803. [Google Scholar] [CrossRef]
- Wong, B.; Wu, P.; Ismail, Z.; Watt, J.; Goodarzi, Z. Detecting Agitation and Aggression in Persons Living with Dementia: A Systematic Review of Diagnostic Accuracy. BMC Geriatr. 2024, 24, 559. [Google Scholar] [CrossRef]
- Pessoa, R.M.d.P.; Maximiano-Barreto, M.A.; Lambert, L.; Leite, É.D.M.; Chagas, M.H.N. The Frequency of Psychotic Symptoms in Types of Dementia: A Systematic Review. Dement. Neuropsychol. 2023, 17, e20220044. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, L.; Van Aubel, E.; Van de Ven, L.; Bouckaert, F.; Luyten, P.; Vandenbulcke, M. The Neuropsychological Profile and Phenomenology of Late Onset Psychosis: A Cross-Sectional Study on the Differential Diagnosis of Very-Late-Onset Schizophrenia-Like Psychosis, Dementia with Lewy Bodies and Alzheimer’s Type Dementia with Psychosis. Arch. Clin. Neuropsychol. 2019, 34, 183–199. [Google Scholar] [CrossRef]
- Connors, M.H.; Teixeira-Pinto, A.; Ames, D.; Woodward, M.; Brodaty, H. Distinguishing Apathy and Depression in Dementia: A Longitudinal Study. Aust. N. Z. J. Psychiatry 2023, 57, 884–894. [Google Scholar] [CrossRef]
- Liguori, C.; Chiaravalloti, A.; Nuccetelli, M.; Izzi, F.; Sancesario, G.; Cimini, A.; Bernardini, S.; Schillaci, O.; Mercuri, N.B.; Fabio, P. Hypothalamic Dysfunction Is Related to Sleep Impairment and CSF Biomarkers in Alzheimer’s Disease. J. Neurol. 2017, 264, 2215–2223. [Google Scholar] [CrossRef]
- Lew, C.H.; Petersen, C.; Neylan, T.C.; Grinberg, L.T. Tau-Driven Degeneration of Sleep- and Wake-Regulating Neurons in Alzheimer’s Disease. Sleep Med. Rev. 2021, 60, 101541. [Google Scholar] [CrossRef] [PubMed]
- Dunmyre, J.R.; Mashour, G.A.; Booth, V. Coupled Flip-Flop Model for REM Sleep Regulation in the Rat. PLoS ONE 2014, 9, e94481. [Google Scholar] [CrossRef] [PubMed]
- Kokošová, V.; Vojtíšek, L.; Baláž, M.; Mangia, S.; Michaeli, S.; Filip, P. Sleep Quality and the Integrity of Ascending Reticular Activating System—A Multimodal MRI Study. Heliyon 2024, 10, e40192. [Google Scholar] [CrossRef]
- Guardia, T.; Geerligs, L.; Tsvetanov, K.A.; Ye, R.; Campbell, K.L. The Role of the Arousal System in Age-Related Differences in Cortical Functional Network Architecture. Hum. Brain Mapp. 2022, 43, 985–997. [Google Scholar] [CrossRef]
- Oishi, Y.; Saito, Y.C.; Sakurai, T. GABAergic Modulation of Sleep-Wake States. Pharmacol. Ther. 2023, 249, 108505. [Google Scholar] [CrossRef]
- Tsunematsu, T.; Ueno, T.; Tabuchi, S.; Inutsuka, A.; Tanaka, K.F.; Hasuwa, H.; Kilduff, T.S.; Terao, A.; Yamanaka, A. Optogenetic Manipulation of Activity and Temporally Controlled Cell-Specific Ablation Reveal a Role for MCH Neurons in Sleep/Wake Regulation. J. Neurosci. 2014, 34, 6896–6909. [Google Scholar] [CrossRef]
- Lim, A.S.P.; Ellison, B.A.; Wang, J.L.; Yu, L.; Schneider, J.A.; Buchman, A.S.; Bennett, D.A.; Saper, C.B. Sleep Is Related to Neuron Numbers in the Ventrolateral Preoptic/Intermediate Nucleus in Older Adults with and without Alzheimer’s Disease. Brain 2014, 137, 2847–2861. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, Q.; Nie, M.; Gu, J.-L.; Hao, W.; Wang, L.; Cao, J.-M. Degeneration and Energy Shortage in the Suprachiasmatic Nucleus Underlies the Circadian Rhythm Disturbance in ApoE−/− Mice: Implications for Alzheimer’s Disease. Sci. Rep. 2016, 6, 36335. [Google Scholar] [CrossRef] [PubMed]
- Londos, E.; Hansson, O.; Rosén, I.; Englund, E. Extreme Sleep Pattern in Lewy Body Dementia: A Hypothalamic Matter? BMJ Case Rep. 2019, 12, e228177. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Mariappan, S.; Rashmi, S.; Choeisoongnern, T.; Sittiprapaporn, P.; Chaiyasut, C. Neurological Insights into Sleep Disorders in Parkinson’s Disease. Brain Sci. 2023, 13, 1202. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Fischer, D.F.; Kalsbeek, A.; Garidou-Boof, M.-L.; van der Vliet, J.; van Heijningen, C.; Liu, R.-Y.; Zhou, J.-N.; Swaab, D.F. Pineal Clock Gene Oscillation Is Disturbed in Alzheimer’s Disease, Due to Functional Disconnection from the “Master Clock”. FASEB J. 2006, 20, 1874–1876. [Google Scholar] [CrossRef] [PubMed]
- Todd, W.D.; Fenselau, H.; Wang, J.L.; Zhang, R.; Machado, N.L.; Venner, A.; Broadhurst, R.Y.; Kaur, S.; Lynagh, T.; Olson, D.P.; et al. A Hypothalamic Circuit for the Circadian Control of Aggression. Nat. Neurosci. 2018, 21, 717–724. [Google Scholar] [CrossRef]
- Yagita, K.; Honda, H.; Ohara, T.; Koyama, S.; Noguchi, H.; Oda, Y.; Yamasaki, R.; Isobe, N.; Ninomiya, T. Association between Hypothalamic Alzheimer’s Disease Pathology and Body Mass Index: The Hisayama Study. Neuropathology 2024, 44, 388–400. [Google Scholar] [CrossRef]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of Preoptic Sleep Neurons Using Retrograde Labelling and Gene Profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef]
- Laurell, A.A.S.; Mak, E.; Dounavi, M.-E.; Underwood, B.R.; Dauvilliers, Y.; Dudas, R.B.; Marguet, O.; Ritchie, C.W.; Koychev, I.; Lawlor, B.A.; et al. Hypothalamic Volume, Sleep, and APOE Genotype in Cognitively Healthy Adults. Alzheimer’s Dement. 2025, 21, e70244. [Google Scholar] [CrossRef]
- Laurell, A.A.; Mak, E.; Underwood, B.R.; Dauvilliers, Y.; Ritchie, C.W.; Koychev, I.; Lawlor, B.A.; Naci, L.; Malhotra, P.; Grau-Rivera, O.; et al. APOE-Ε4 Is Associated with Poor Sleep and Structural Differences of the Hypothalamus in the PREVENT-Dementia and ALFA Cohort Studies. Alzheimer’s Dement. 2024, 20, e088305. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, W.S.; Choi, W.H.; Lim, H.K. Aberrant Brain Stem Morphometry Associated with Sleep Disturbance in Drug-Naïve Subjects with Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.S.; Galgani, A.; Puglisi-Allegra, S.; Busceti, C.L.; Fornai, F. The Connections of Locus Coeruleus with Hypothalamus: Potential Involvement in Alzheimer’s Disease. J. Neural Transm. 2021, 128, 589–613. [Google Scholar] [CrossRef] [PubMed]
- Van Egroo, M.; van Someren, E.J.W.; Grinberg, L.T.; Bennett, D.A.; Jacobs, H.I.L. Associations of 24-Hour Rest-Activity Rhythm Fragmentation, Cognitive Decline, and Postmortem Locus Coeruleus Hypopigmentation in Alzheimer’s Disease. Ann. Neurol. 2024, 95, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryan, J.; Andreescu, C.; Aizenstein, H.; Lim, H.K. Brainstem Morphological Changes in Alzheimer’s Disease. Neuroreport 2015, 26, 411–415. [Google Scholar] [CrossRef]
- Cho, G.; Mecca, A.P.; Buxton, O.M.; Liu, X.; Miner, B. Lower Slow Wave Sleep and Rapid Eye Movement Sleep Are Associated with Brain Atrophy of Alzheimer’s Disease-Vulnerable Regions. J. Clin. Sleep Med. 2025, 21, 1165–1173. [Google Scholar] [CrossRef]
- Holth, J.; Patel, T.; Holtzman, D.M. Sleep in Alzheimer’s Disease—Beyond Amyloid. Neurobiol. Sleep Circadian Rhythm. 2017, 2, 4–14. [Google Scholar] [CrossRef]
- Oh, J.Y.; Walsh, C.M.; Ranasinghe, K.; Mladinov, M.; Pereira, F.L.; Petersen, C.; Falgàs, N.; Yack, L.; Lamore, T.; Nasar, R.; et al. Subcortical Neuronal Correlates of Sleep in Neurodegenerative Diseases. JAMA Neurol. 2022, 79, 498–508. [Google Scholar] [CrossRef]
- Rastegar-Pouyani, S.; Lew, C.; Satpati, A.; Leite, R.E.P.; Suemoto, C.K.; Spina, S.; Seeley, W.W.; Walsh, C.M.; Neylan, T.C.; Grinberg, L.T. The VLPO Analog in the Human Hypothalamus Shows Tau-Driven Extreme Loss of Sleep-Regulating Neurons in PSP and Alzheimer’s Disease: Unveiling the Basis of NREM Sleep Dysfunction in Tauopathies. Alzheimer’s Dement. 2024, 20, e084555. [Google Scholar] [CrossRef]
- Boeve, B.F.; Dickson, D.W.; Olson, E.J.; Shepard, J.W.; Silber, M.H.; Ferman, T.J.; Ahlskog, J.E.; Benarroch, E.E. Insights into REM Sleep Behavior Disorder Pathophysiology in Brainstem-Predominant Lewy Body Disease. Sleep Med. 2007, 8, 60–64. [Google Scholar] [CrossRef]
- García-Gomar, M.G.; Videnovic, A.; Singh, K.; Stauder, M.; Lewis, L.D.; Wald, L.L.; Rosen, B.R.; Bianciardi, M. Disruption of Brainstem Structural Connectivity in REM Sleep Behavior Disorder Using 7 Tesla Magnetic Resonance Imaging. Mov. Disord. 2022, 37, 847–853. [Google Scholar] [CrossRef]
- Vetrivelan, R.; Bandaru, S.S. Neural Control of REM Sleep and Motor Atonia: Current Perspectives. Curr. Neurol. Neurosci. Rep. 2023, 23, 907–923. [Google Scholar] [CrossRef]
- Kim, Y.E.; Yang, H.J.; Yun, J.Y.; Kim, H.-J.; Lee, J.-Y.; Jeon, B.S. REM Sleep Behavior Disorder in Parkinson Disease: Association with Abnormal Ocular Motor Findings. Park. Relat. Disord. 2014, 20, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-Y.; Siegel, J.M. Physiological and Anatomical Link between Parkinson-like Disease and REM Sleep Behavior Disorder. Mol. Neurobiol. 2003, 27, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Schmeichel, A.M.; Buchhalter, L.C.; Low, P.A.; Parisi, J.E.; Boeve, B.W.; Sandroni, P.; Benarroch, E.E. Mesopontine Cholinergic Neuron Involvement in Lewy Body Dementia and Multiple System Atrophy. Neurology 2008, 70, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s Disease: Separating the Wheat from the Chaff. J. Park. Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Beck, G.; Kanuka, M.; Arai, Y.; Tanaka, K.; Tatsuzawa, C.; Koga, Y.; Saito, Y.C.; Takagi, M.; Oishi, Y.; et al. A Pontine-Medullary Loop Crucial for REM Sleep and Its Deficit in Parkinson’s Disease. Cell 2024, 187, 6272–6289.e21. [Google Scholar] [CrossRef]
- Boucetta, S.; Salimi, A.; Dadar, M.; Jones, B.E.; Collins, D.L.; Dang-Vu, T.T. Structural Brain Alterations Associated with Rapid Eye Movement Sleep Behavior Disorder in Parkinson’s Disease. Sci. Rep. 2016, 6, 26782. [Google Scholar] [CrossRef]
- Halliday, G.M.; Blumbergs, P.C.; Cotton, R.G.H.; Blessing, W.W.; Geffen, L.B. Loss of Brainstem Serotonin- and Substance P-Containing Neurons in Parkinson’s Disease. Brain Res. 1990, 510, 104–107. [Google Scholar] [CrossRef]
- Thannickal, T.C.; Lai, Y.-Y.; Siegel, J.M. Hypocretin (Orexin) Cell Loss in Parkinson’s Disease. Brain 2007, 130, 1586–1595. [Google Scholar] [CrossRef]
- Happe, S.; Baier, P.C.; Helmschmied, K.; Meller, J.; Tatsch, K.; Paulus, W. Association of Daytime Sleepiness with Nigrostriatal Dopaminergic Degeneration in Early Parkinson’s Disease. J. Neurol. 2007, 254, 1037–1043. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoon, I.-Y.; Kim, J.-M.; Jeong, S.-H.; Kim, K.W.; Shin, Y.-K.; Kim, B.S.; Kim, S.E. The Implication of Nigrostriatal Dopaminergic Degeneration in the Pathogenesis of REM Sleep Behavior Disorder. Eur. J. Neurol. 2010, 17, 487–492. [Google Scholar] [CrossRef]
- Kalaitzakis, M.E.; Gentleman, S.M.; Pearce, R.K.B. Disturbed Sleep in Parkinson’s Disease: Anatomical and Pathological Correlates. Neuropathol. Appl. Neurobiol. 2013, 39, 644–653. [Google Scholar] [CrossRef]
- Chen, M.; Guo, G.; Liu, S.; Cai, J.; Tong, X.; Liu, X.; Zhang, Y.; Chen, Y.; Huo, J. Investigating the Relationship between Sleep Disturbances and Cortical Thickness, Brainstem Volume, Amyloid Accumulation, and Inflammatory Markers in Parkinson’s Disease Patients. Exp. Gerontol. 2025, 205, 112762. [Google Scholar] [CrossRef] [PubMed]
- Rosinvil, T.; Postuma, R.B.; Rahayel, S.; Bellavance, A.; Daneault, V.; Montplaisir, J.; Lina, J.-M.; Carrier, J.; Gagnon, J.-F. Clinical Symptoms and Neuroanatomical Substrates of Daytime Sleepiness in Parkinson’s Disease. NPJ Park. Dis. 2024, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.W.; Chahine, L.M.; Caspell-Garcia, C.; Long, J.D.; Coffey, C.; Högl, B.; Videnovic, A.; Iranzo, A.; Mayer, G.; Foldvary-Schaefer, N.; et al. Longitudinal Assessment of Excessive Daytime Sleepiness in Early Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 653–662. [Google Scholar] [CrossRef]
- Verbaan, D.; van Rooden, S.M.; Visser, M.; Marinus, J.; van Hilten, J.J. Nighttime Sleep Problems and Daytime Sleepiness in Parkinson’s Disease. Mov. Disord. 2008, 23, 35–41. [Google Scholar] [CrossRef]
- Zang, Q.L.; Zheng, J.H.; Ma, J.J.; Zhang, Q.; Huang, P.P.; Shen, N.N.; Miao, W. Neuroanatomy and Functional Connectivity in Patients with Parkinson’s Disease with or without Restless Legs Syndrome. Neurol. Ther. 2022, 11, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Kasanuki, K.; Ferman, T.J.; Murray, M.E.; Heckman, M.G.; Pedraza, O.; Hanna Al-Shaikh, F.S.; Mishima, T.; Diehl, N.N.; van Gerpen, J.A.; Uitti, R.J.; et al. Daytime Sleepiness in Dementia with Lewy Bodies Is Associated with Neuronal Depletion of the Nucleus Basalis of Meynert. Park. Relat. Disord. 2018, 50, 99–103. [Google Scholar] [CrossRef]
- Abbott, R.D.; Ross, G.W.; Duda, J.E.; Shin, C.; Uyehara-Lock, J.H.; Masaki, K.H.; Launer, L.J.; White, L.R.; Tanner, C.M.; Petrovitch, H. Excessive Daytime Sleepiness and Topographic Expansion of Lewy Pathology. Neurology 2019, 93, e1425–e1432. [Google Scholar] [CrossRef]
- Benarroch, E.E.; Schmeichel, A.M.; Dugger, B.N.; Sandroni, P.; Parisi, J.E.; Low, P.A. Dopamine Cell Loss in the Periaqueductal Gray in Multiple System Atrophy and Lewy Body Dementia. Neurology 2009, 73, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Presti, M.F.; Schmeichel, A.M.; Low, P.A.; Parisi, J.E.; Benarroch, E.E. Degeneration of Brainstem Respiratory Neurons in Dementia with Lewy Bodies. Sleep 2014, 37, 373–378. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Lue, L.; Sue, L.I.; Bachalakuri, J.; Henry-Watson, J.; Sasse, J.; Boyer, S.; Shirohi, S.; Brooks, R.; et al. Unified Staging System for Lewy Body Disorders: Correlation with Nigrostriatal Degeneration, Cognitive Impairment and Motor Dysfunction. Acta Neuropathol. 2009, 117, 613–634. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, L.; Marelli, S.; Iannaccone, S.; Magnani, G.; Ferini-Strambi, L.; Perani, D. Severe Brain Metabolic Decreases Associated with REM Sleep Behavior Disorder in Dementia with Lewy Bodies. J. Alzheimer’s Dis. 2016, 52, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.G.; Stopa, E.G.; McKee, A.C.; Satlin, A.; Harlan, P.C.; Goldstein, R.; Volicer, L. Differential Circadian Rhythm Disturbances in Men with Alzheimer Disease and Frontotemporal Degeneration. Arch. Gen. Psychiatry 2001, 58, 353–360. [Google Scholar] [CrossRef]
- Best, P.T.; Van Swieten, J.C.; Jiskoot, L.C.; Moreno, F.; Sánchez-Valle, R.; Laforce, R.; Graff, C.; Masellis, M.; Tartaglia, C.; Rowe, J.B.; et al. Association of Changes in Cerebral and Hypothalamic Structure with Sleep Dysfunction in Patients with Genetic Frontotemporal Dementia. Neurology 2024, 103, e209829. [Google Scholar] [CrossRef]
- Filardi, M.; Gnoni, V.; Tamburrino, L.; Nigro, S.; Urso, D.; Vilella, D.; Tafuri, B.; Giugno, A.; De Blasi, R.; Zoccolella, S.; et al. Sleep and Circadian Rhythm Disruptions in Behavioral Variant Frontotemporal Dementia. Alzheimer’s Dement. 2024, 20, 1966–1977. [Google Scholar] [CrossRef]
- Mayer, G.; Frohnhofen, H.; Jokisch, M.; Hermann, D.M.; Gronewold, J. Associations of Sleep Disorders with All-Cause MCI/Dementia and Different Types of Dementia—Clinical Evidence, Potential Pathomechanisms and Treatment Options: A Narrative Review. Front. Neurosci. 2024, 18, 1372326. [Google Scholar] [CrossRef]
- Wu, G.-R.; Baeken, C. Depression and Metabolic Connectivity: Insights into the Locus Coeruleus, HF-rTMS, and Anxiety. Transl. Psychiatry 2024, 14, 459. [Google Scholar] [CrossRef]
- Carrarini, C.; Calisi, D.; De Rosa, M.A.; Di Iorio, A.; D’Ardes, D.; Pellegrino, R.; Gazzina, S.; Pilotto, A.; Arighi, A.; Carandini, T.; et al. QEEG Abnormalities in Cognitively Unimpaired Patients with Delirium. J. Neurol. Neurosurg. Psychiatry 2023, 94, 251–254. [Google Scholar] [CrossRef]
- Jamieson, D.; Broadhouse, K.M.; Lagopoulos, J.; Hermens, D.F. Investigating the Links between Adolescent Sleep Deprivation, Fronto-Limbic Connectivity and the Onset of Mental Disorders: A Review of the Literature. Sleep Med. 2020, 66, 61–67. [Google Scholar] [CrossRef]
- Cotta Ramusino, M.; Imbimbo, C.; Capelli, M.; Cabini, R.F.; Bernini, S.; Lombardo, F.P.; Mazzocchi, L.; Farina, L.M.; Pichiecchio, A.; Perini, G.; et al. Role of Fronto-Limbic Circuit in Neuropsychiatric Symptoms of Dementia: Clinical Evidence from an Exploratory Study. Front. Psychiatry 2024, 15, 1231361. [Google Scholar] [CrossRef]
- Falup-Pecurariu, C.; Diaconu, Ș.; Țînț, D.; Falup-Pecurariu, O. Neurobiology of Sleep (Review). Exp. Ther. Med. 2021, 21, 272. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, H.; Li, A.; Sun, Q.; Jiang, T.; Li, X.; Gong, H. The Mesoscopic Connectome of the Cholinergic Pontomesencephalic Tegmentum. Front. Neuroanat. 2022, 16, 843303. [Google Scholar] [CrossRef]
- Fraigne, J.J.; Luppi, P.H.; Mahoney, C.E.; De Luca, R.; Shiromani, P.J.; Weber, F.; Adamantidis, A.; Peever, J. Dopamine Neurons in the Ventral Tegmental Area Modulate Rapid Eye Movement Sleep. Sleep 2023, 46, zsad024. [Google Scholar] [CrossRef] [PubMed]
- Van Erum, J.; Van Dam, D.; De Deyn, P.P. Alzheimer’s Disease: Neurotransmitters of the Sleep-Wake Cycle. Neurosci. Biobehav. Rev. 2019, 105, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kilduff, T.S.; Cauli, B.; Gerashchenko, D. Activation of Cortical Interneurons during Sleep: An Anatomical Link to Homeostatic Sleep Regulation? Trends Neurosci. 2011, 34, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sani, T.P.; Bond, R.L.; Marshall, C.R.; Hardy, C.J.D.; Russell, L.L.; Moore, K.M.; Slattery, C.F.; Paterson, R.W.; Woollacott, I.O.C.; Wendi, I.P.; et al. Sleep Symptoms in Syndromes of Frontotemporal Dementia and Alzheimer’s Disease: A Proof-of-Principle Behavioural Study. eNeurologicalSci 2019, 17, 100212. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Zinchenko, V.P.; Nadeev, A.D.; Goncharov, N.V. Serotonergic Regulation in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5218. [Google Scholar] [CrossRef]
- Hu, K.; Harper, D.G.; Shea, S.A.; Stopa, E.G.; Scheer, F.A.J.L. Noninvasive Fractal Biomarker of Clock Neurotransmitter Disturbance in Humans with Dementia. Sci. Rep. 2013, 3, 2229. [Google Scholar] [CrossRef]
- Satpati, A.; Neylan, T.; Grinberg, L.T. Histaminergic Neurotransmission in Aging and Alzheimer’s Disease: A Review of Therapeutic Opportunities and Gaps. Alzheimer’s Dement. 2023, 9, e12379. [Google Scholar] [CrossRef]
- Imbesi, M.; Yildiz, S.; Dirim Arslan, A.; Sharma, R.; Manev, H.; Uz, T. Dopamine Receptor-Mediated Regulation of Neuronal “Clock” Gene Expression. Neuroscience 2009, 158, 537–544. [Google Scholar] [CrossRef]
- Rothman, S.M.; Mattson, M.P. Sleep Disturbances in Alzheimer’s and Parkinson’s Diseases. Neuromol. Med. 2012, 14, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; Giordano, B.; Turkheimer, F.E.; Chaudhuri, K.R.; Politis, M. Serotonergic Dysregulation Is Linked to Sleep Problems in Parkinson’s Disease. Neuroimage Clin. 2018, 18, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-H.; Ren, N.; Xu, J.; Chen, L. Clinical Features, Plasma Neurotransmitter Levels and Plasma Neurohormone Levels among Patients with Early-Stage Parkinson’s Disease with Sleep Disorders. Cell Commun. Signal. 2025, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Bollu, P.C.; Sahota, P. Sleep and Parkinson Disease. Mo. Med. 2017, 114, 381–386. [Google Scholar]
- Massironi, G.; Galluzzi, S.; Frisoni, G.B. Drug Treatment of REM Sleep Behavior Disorders in Dementia with Lewy Bodies. Int. Psychogeriatr. 2003, 15, 377–383. [Google Scholar] [CrossRef]
- Benussi, A.; Alberici, A.; Buratti, E.; Ghidoni, R.; Gardoni, F.; Di Luca, M.; Padovani, A.; Borroni, B. Toward a Glutamate Hypothesis of Frontotemporal Dementia. Front. Neurosci. 2019, 13, 304. [Google Scholar] [CrossRef]
- Izawa, S.; Fusca, D.; Jiang, H.; Heilinger, C.; Hausen, A.C.; Wunderlich, F.T.; Steuernagel, L.; Kloppenburg, P.; Brüning, J.C. Orexin/Hypocretin Receptor 2 Signaling in MCH Neurons Regulates REM Sleep and Insulin Sensitivity. Cell Rep. 2025, 44, 115277. [Google Scholar] [CrossRef]
- Hwang, Y.T.; Piguet, O.; Hodges, J.R.; Grunstein, R.; Burrell, J.R. Sleep and Orexin: A New Paradigm for Understanding Behavioural-Variant Frontotemporal Dementia? Sleep Med. Rev. 2020, 54, 101361. [Google Scholar] [CrossRef]
- Andrade, M.K.; Souza, L.C.; Azevedo, E.M.; Bail, E.L.; Zanata, S.M.; Andreatini, R.; Vital, M.A.B.F. Melatonin Reduces β-Amyloid Accumulation and Improves Short-Term Memory in Streptozotocin-Induced Sporadic Alzheimer’s Disease Model. IBRO Neurosci. Rep. 2023, 14, 264–272. [Google Scholar] [CrossRef]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Effect of Melatonin on Neuroinflammation and Acetylcholinesterase Activity Induced by LPS in Rat Brain. Eur. J. Pharmacol. 2010, 640, 206–210. [Google Scholar] [CrossRef]
- Escames, G.; Macías, M.; León, J.; García, J.; Khaldy, H.; Martín, M.; Vives, F.; Acuña-Castroviejo, D. Calcium-Dependent Effects of Melatonin Inhibition of Glutamatergic Response in Rat Striatum. J. Neuroendocrinol. 2001, 13, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Sochal, M.; Ditmer, M.; Binienda, A.; Tarasiuk, A.; Białasiewicz, P.; Turkiewicz, S.; Karuga, F.F.; Jakub, F.; Gabryelska, A. Interactions between Neurotrophins, Mood, and Physical Activity under the Conditions of Sleep Deprivation. Transl. Psychiatry 2024, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.E.; Miyanishi, K.; Takeda, H.; Islam, A.; Matsuoka, N.; Kubo, M.; Matsumoto, S.; Kunieda, T.; Nomoto, M.; Yano, H.; et al. Phagocytic Elimination of Synapses by Microglia during Sleep. Glia 2020, 68, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Sanford, L.D.; Yang, L.; Zhou, J.; Tan, L.; Li, T.; Zhang, J.; Wing, Y.-K.; Shi, J.; et al. Sleep in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Polysomnographic Findings. Sleep Med. Rev. 2020, 51, 101281. [Google Scholar] [CrossRef]
- Cao, D.; Zhao, Y.; Wang, Y.; Wei, D.; Yan, M.; Su, S.; Pan, H.; Wang, Q. Effects of Sleep Deprivation on Anxiety-Depressive-like Behavior and Neuroinflammation. Brain Res. 2024, 1836, 148916. [Google Scholar] [CrossRef]
- Xiao, S.-Y.; Liu, Y.-J.; Lu, W.; Sha, Z.-W.; Xu, C.; Yu, Z.-H.; Lee, S.-D. Possible Neuropathology of Sleep Disturbance Linking to Alzheimer’s Disease: Astrocytic and Microglial Roles. Front. Cell. Neurosci. 2022, 16, 875138. [Google Scholar] [CrossRef]
- Yong, V.W. Microglia in Multiple Sclerosis: Protectors Turn Destroyers. Neuron 2022, 110, 3534–3548. [Google Scholar] [CrossRef]
- Niedziela, N.; Kalinowska, A.; Kułakowska, A.; Mirowska-Guzel, D.; Rejdak, K.; Siger, M.; Stasiołek, M.; Adamczyk-Sowa, M. Clinical and Therapeutic Challenges of Smouldering Multiple Sclerosis. Neurol. Neurochir. Pol. 2024, 58, 245–255. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef]
- Baril, A.-A.; Beiser, A.S.; Redline, S.; McGrath, E.R.; Aparicio, H.J.; Gottlieb, D.J.; Seshadri, S.; Pase, M.P.; Himali, J.J. Systemic Inflammation as a Moderator between Sleep and Incident Dementia. Sleep 2021, 44, zsaa164. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef] [PubMed]
- Puech, C.; Badran, M.; Runion, A.R.; Barrow, M.B.; Cataldo, K.; Gozal, D. Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period. Int. J. Mol. Sci. 2023, 24, 9880. [Google Scholar] [CrossRef] [PubMed]
- Rábago-Monzón, Á.R.; Osuna-Ramos, J.F.; Armienta-Rojas, D.A.; Camberos-Barraza, J.; Camacho-Zamora, A.; Magaña-Gómez, J.A.; De la Herrán-Arita, A.K. Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders. Biomedicines 2025, 13, 1121. [Google Scholar] [CrossRef]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-Grade Neuroinflammation Due to Chronic Sleep Deprivation Results in Anxiety and Learning and Memory Impairments. Mol. Cell. Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Dong, Y.; Xu, Z.; Gompf, H.S.; Ward, S.A.P.; Xue, Z.; Miao, C.; Zhang, Y.; Chamberlin, N.L.; Xie, Z. Sleep Disturbance Induces Neuroinflammation and Impairment of Learning and Memory. Neurobiol. Dis. 2012, 48, 348–355. [Google Scholar] [CrossRef]
- Baril, A.-A.; Picard, C.; Labonté, A.; Sanchez, E.; Duclos, C.; Mohammediyan, B.; Breitner, J.C.S.; Villeneuve, S.; Poirier, J.; PREVENT-AD Research Group. Longer Sleep Duration and Neuroinflammation in At-Risk Elderly with a Parental History of Alzheimer’s Disease. Sleep 2024, 47, zsae081. [Google Scholar] [CrossRef]
- Baril, A.-A.; Labonté, A.; Sanchez, E.; Picard, C.; Duclos, C.; Rosa-Neto, P.; Breitner, J.C.S.; Villeneuve, S.; Poirier, J.; Group, P.-A.R. Actigraphy Sleep Patterns in Presymptomatic Alzheimer’s Disease: The Potential Role of Neuroinflammation. Alzheimer’s Dement. 2023, 19, e067964. [Google Scholar] [CrossRef]
- Baril, A.-A.; Beiser, A.S.; Redline, S.; McGrath, E.R.; Gottlieb, D.J.; Aparicio, H.; Seshadri, S.; Himali, J.J.; Pase, M.P. Interleukin-6 Interacts with Sleep Apnea Severity When Predicting Incident Alzheimer’s Disease Dementia. J. Alzheimer’s Dis. 2021, 79, 1451–1457. [Google Scholar] [CrossRef]
- Mander, B.A.; Dave, A.; Lui, K.K.; Sprecher, K.E.; Berisha, D.; Chappel-Farley, M.G.; Chen, I.Y.; Riedner, B.A.; Heston, M.; Suridjan, I.; et al. Inflammation, Tau Pathology, and Synaptic Integrity Associated with Sleep Spindles and Memory Prior to β-Amyloid Positivity. Sleep 2022, 45, zsac135. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Alshammari, M.A.; Alasmari, A.F.; Alanazi, W.A.; Alhazzani, K. Neuroinflammatory Cytokines Induce Amyloid Beta Neurotoxicity through Modulating Amyloid Precursor Protein Levels/Metabolism. BioMed Res. Int. 2018, 2018, 3087475. [Google Scholar] [CrossRef]
- Tissot, C.; Blais, H.; Thompson, C.; Benedet, A.L.; Pascoal, T.A.; Therriault, J.; Chamoun, M.; Lussier, F.Z.; Savard, M.; Rahmouni, N.; et al. Neuroinflammation Is Associated with Non-REM Sleep Reduction in Individuals without Dementia. Alzheimer’s Dement. 2020, 16, e046636. [Google Scholar] [CrossRef]
- Zhang, W.; Lian, T.; He, M.; Guo, P.; Guan, H.; Li, J.; Qi, J.; Luo, D.; Li, J.; Zhang, Y.; et al. Sleep Disorders and Alzheimer’s Disease: Relationship and Mechanisms Involving Neuroinflammation, Orexin and Aβ. Fluids Barriers CNS 2025, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Beiyu, Z.; Rong, Z.; Yi, Z.; Shan, W.; Peng, L.; Meng, W.; Wei, P.; Ye, Y.; Qiumin, Q. Oxidative Stress Is Associated with Aβ Accumulation in Chronic Sleep Deprivation Model. Brain Res. 2024, 1829, 148776. [Google Scholar] [CrossRef]
- Deutsch, S.; Malik, B.R. Impact of Sleep on Autophagy and Neurodegenerative Disease: Sleeping Your Mind Clear. Arch. Mol. Biol. Genet. 2022, 1, 43–56. [Google Scholar] [CrossRef]
- Lind-Holm Mogensen, F.; Seibler, P.; Grünewald, A.; Michelucci, A. Microglial Dynamics and Neuroinflammation in Prodromal and Early Parkinson’s Disease. J. Neuroinflamm. 2025, 22, 136. [Google Scholar] [CrossRef]
- Käufer, C.; Stanojlović, M.; Schidlitzki, A.; Bonsberger, J.; Storch, A.; Richter, F. Alterations in Non-REM Sleep and EEG Spectra Precede REM-Sleep Deficits in a Model of Synucleinopathy. J. Park. Dis. 2025, 15, 311–328. [Google Scholar] [CrossRef]
- Stokholm, M.G.; Iranzo, A.; Østergaard, K.; Serradell, M.; Otto, M.; Svendsen, K.B.; Garrido, A.; Vilas, D.; Borghammer, P.; Santamaria, J.; et al. Assessment of Neuroinflammation in Patients with Idiopathic Rapid-Eye-Movement Sleep Behaviour Disorder: A Case-Control Study. Lancet Neurol. 2017, 16, 789–796. [Google Scholar] [CrossRef]
- Carvalho, D.Z.; Schönwald, S.V.; Schumacher-Schuh, A.F.; Braga, C.W.; Souza, D.O.; Oses, J.P.; Donis, K.C.; Rieder, C.R. Overnight S100B in Parkinson’s Disease: A Glimpse into Sleep-Related Neuroinflammation. Neurosci. Lett. 2015, 608, 57–63. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, Y.; Shi, Y.; Bao, H.; Cheng, X.; Jiang, M.; Peng, Z.; Song, J.; Fang, F.; Jian, C.; et al. Sleep Deprivation Accelerates Parkinson’s Disease via Modulating Gut Microbiota Associated Microglial Activation and Oxidative Stress. Microbiol. Res. 2025, 293, 128077. [Google Scholar] [CrossRef]
- Duan, W.-X.; Xie, W.-Y.; Ying, C.; Fen, W.; Cheng, X.-Y.; Mao, C.-J.; Liu, J.-Y.; Liu, C.-F. Butyrate Improves Abnormal Sleep Architecture in a Parkinson’s Disease Mouse Model via BDNF/TrkB Signaling. NPJ Park. Dis. 2025, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J. Brain Circadian Clocks Timing the 24h Rhythms of Behavior. npj Biol. Timing Sleep 2025, 2, 13. [Google Scholar] [CrossRef]
- Mao, W.; Ge, X.; Chen, Q.; Li, J.-D. Epigenetic Mechanisms in the Transcriptional Regulation of Circadian Rhythm in Mammals. Biology 2025, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Ahmed, R.; Nakahata, Y.; Shinohara, K.; Bessho, Y. Cellular Senescence Triggers Altered Circadian Clocks with a Prolonged Period and Delayed Phases. Front. Neurosci. 2021, 15, 638122. [Google Scholar] [CrossRef]
- Manni, R.; Cremascoli, R.; Perretti, C.; De Icco, R.; Picascia, M.; Ghezzi, C.; Cerri, S.; Sinforiani, E.; Terzaghi, M. Evening Melatonin Timing Secretion in Real Life Conditions in Patients with Alzheimer Disease of Mild to Moderate Severity. Sleep Med. 2019, 63, 122–126. [Google Scholar] [CrossRef]
- Carpi, M.; Fernandes, M.; Risino, I.; Benedetti, R.; Testone, G.; Cirillo, F.; Nuccetelli, M.; Bernardini, S.; Mercuri, N.B.; Liguori, C. Alteration of Circadian Sleep-Wake Rhythm and Salivary Melatonin Secretion in Idiopathic/Isolated REM Sleep Behavior Disorder: Preliminary Evidence. Sleep Med. 2024, 119, 135–138. [Google Scholar] [CrossRef]
- Mishima, K.; Tozawa, T.; Satoh, K.; Matsumoto, Y.; Hishikawa, Y.; Okawa, M. Melatonin Secretion Rhythm Disorders in Patients with Senile Dementia of Alzheimer’s Type with Disturbed Sleep-Waking. Biol. Psychiatry 1999, 45, 417–421. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Swaab, D.F.; Zhou, J.-N. Sleep-Wake Modulation and Pathogenesis of Alzheimer Disease: Suggestions for Postponement and Treatment. Handb. Clin. Neurol. 2025, 206, 211–229. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Zhou, J.-N.; Van Heerikhuize, J.; Jockers, R.; Swaab, D.F. Decreased MT1 Melatonin Receptor Expression in the Suprachiasmatic Nucleus in Aging and Alzheimer’s Disease. Neurobiol. Aging 2007, 28, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Feenstra, M.G.P.; Zhou, J.-N.; Liu, R.-Y.; Toranõ, J.S.; Van Kan, H.J.M.; Fischer, D.F.; Ravid, R.; Swaab, D.F. Molecular Changes Underlying Reduced Pineal Melatonin Levels in Alzheimer Disease: Alterations in Preclinical and Clinical Stages. J. Clin. Endocrinol. Metab. 2003, 88, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, C.-Q.; Hu, X.-Y.; Li, S.-B.; Zhang, X.-M. Functional CLOCK Gene Rs1554483 G/C Polymorphism Is Associated with Susceptibility to Alzheimer’s Disease in the Chinese Population. J. Int. Med. Res. 2013, 41, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sulkava, S.; Muggalla, P.; Sulkava, R.; Ollila, H.M.; Peuralinna, T.; Myllykangas, L.; Kaivola, K.; Stone, D.J.; Traynor, B.J.; Renton, A.E.; et al. Melatonin Receptor Type 1A Gene Linked to Alzheimer’s Disease in Old Age. Sleep 2018, 41, zsy103. [Google Scholar] [CrossRef]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, Circadian Rhythms, and the Pathogenesis of Alzheimer Disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef]
- Kataoka, H.; Saeki, K.; Kurumatani, N.; Sugie, K.; Obayashi, K. Melatonin Secretion in Patients with Parkinson’s Disease Receiving Different-Dose Levodopa Therapy. Sleep Med. 2020, 75, 309–314. [Google Scholar] [CrossRef]
- Videnovic, A.; Noble, C.; Reid, K.J.; Peng, J.; Turek, F.W.; Marconi, A.; Rademaker, A.W.; Simuni, T.; Zadikoff, C.; Zee, P.C. Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurol. 2014, 71, 463–469. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, E.; Ueda, M.; Suzuki, M.; Tohnai, G.; Katsuno, M. Association of Impaired Melatonin Secretion with Sleep Disturbance in Parkinson’s Disease. J. Neurol. Sci. 2017, 381, 296. [Google Scholar] [CrossRef]
- Milanowski, J.; Kozerawski, K.; Falęcka, W.; Dudek, D.; Lisewska, B.; Lisewski, P.; Nuszkiewicz, J.; Wesołowski, R.; Wojtasik, J.; Mila-Kierzenkowska, C.; et al. Changes in the Secretion of Melatonin and Selected Adipokines during the Progression of Parkinson’s Disease-Preliminary Studies. Metabolites 2023, 13, 668. [Google Scholar] [CrossRef]
- Hunt, J.; Coulson, E.J.; Rajnarayanan, R.; Oster, H.; Videnovic, A.; Rawashdeh, O. Sleep and Circadian Rhythms in Parkinson’s Disease and Preclinical Models. Mol. Neurodegener. 2022, 17, 2. [Google Scholar] [CrossRef]
- Bolitho, S.J.; Naismith, S.L.; Rajaratnam, S.M.W.; Grunstein, R.R.; Hodges, J.R.; Terpening, Z.; Rogers, N.; Lewis, S.J.G. Disturbances in Melatonin Secretion and Circadian Sleep-Wake Regulation in Parkinson Disease. Sleep Med. 2014, 15, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Adi, N.; Mash, D.C.; Ali, Y.; Singer, C.; Shehadeh, L.; Papapetropoulos, S. Melatonin MT1 and MT2 Receptor Expression in Parkinson’s Disease. Med. Sci. Monit. 2010, 16, BR61–BR67. [Google Scholar] [PubMed]
- De Pablo-Fernández, E.; Courtney, R.; Warner, T.T.; Holton, J.L. A Histologic Study of the Circadian System in Parkinson Disease, Multiple System Atrophy, and Progressive Supranuclear Palsy. JAMA Neurol. 2018, 75, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.M.; Pereira, G.M.; Altmann, V.; de Almeida, R.M.M.; Rieder, C.R.M. Cortisol Levels, Motor, Cognitive and Behavioral Symptoms in Parkinson’s Disease: A Systematic Review. J. Neural Transm. 2019, 126, 219–232. [Google Scholar] [CrossRef]
- Comas, M.; Vidal, X.; Rawashdeh, O.; Grunstein, R.R.; Lewis, S.J.G.; Matar, E. Alterations in Sleep-Activity Cycles and Clock Gene Expression across the Synucleinopathy Spectrum. Transl. Neurodegener. 2025, 14, 28. [Google Scholar] [CrossRef]
- Anderson, K.N.; Hatfield, C.; Kipps, C.; Hastings, M.; Hodges, J.R. Disrupted Sleep and Circadian Patterns in Frontotemporal Dementia. Eur. J. Neurol. 2009, 16, 317–323. [Google Scholar] [CrossRef]
- Parekh, P.K.; Ozburn, A.R.; McClung, C.A. Circadian Clock Genes: Effects on Dopamine, Reward and Addiction. Alcohol 2015, 49, 341–349. [Google Scholar] [CrossRef]
- Rongve, A.; Boeve, B.F.; Aarsland, D. Frequency and Correlates of Caregiver-Reported Sleep Disturbances in a Sample of Persons with Early Dementia. J. Am. Geriatr. Soc. 2010, 58, 480–486. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, J.k.; Qi, R.b. Cognitive Function, Sleep Characteristics and Their Relationship in Older Adults with Insomnia and Anxiety. Sci. Rep. 2025, 15, 30284. [Google Scholar] [CrossRef]
- Pat-Horenczyk, R.; Klauber, M.R.; Shochat, T.; Ancoli-Israel, S. Hourly Profiles of Sleep and Wakefulness in Severely versus Mild-Moderately Demented Nursing Home Patients. Aging 1998, 10, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhan, S.; Zhou, J.; Liu, X.; Liang, H. Editorial: Interaction between Neuropsychiatry and Sleep Disorders: From Mechanism to Clinical Practice. Front. Neurol. 2022, 13, 1070040. [Google Scholar] [CrossRef]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, Insomnia and Mental Health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.M.; Lorenz, R. Sleep Disturbances in Dementia. J. Gerontol. Nurs. 2010, 36, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef]
- Mattos, M.K.; Bernacchi, V.; Shaffer, K.M.; Gallagher, V.; Seo, S.; Jepson, L.; Manning, C. Sleep and Caregiver Burden Among Caregivers of Persons Living with Dementia: A Scoping Review. Innov. Aging 2024, 8, igae005. [Google Scholar] [CrossRef]
- Tractenberg, R.E.; Singer, C.M.; Cummings, J.L.; Thal, L.J. The Sleep Disorders Inventory: An Instrument for Studies of Sleep Disturbance in Persons with Alzheimer’s Disease. J. Sleep Res. 2003, 12, 331–337. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh Sleep Quality Index as a Screening Tool for Sleep Dysfunction in Clinical and Non-Clinical Samples: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Walker, J.; Muench, A.; Perlis, M.L.; Vargas, I. Cognitive Behavioral Therapy for Insomnia (CBT-I): A Primer. Klin. Spec. Psihol. 2022, 11, 123–137. [Google Scholar] [CrossRef]
- Irish, L.A.; Kline, C.E.; Gunn, H.E.; Buysse, D.J.; Hall, M.H. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence. Sleep Med. Rev. 2015, 22, 23–36. [Google Scholar] [CrossRef]
- Markwald, R.R.; Iftikhar, I.; Youngstedt, S.D. Behavioral Strategies, Including Exercise, for Addressing Insomnia. ACSMs Health Fit. J. 2018, 22, 23–29. [Google Scholar] [CrossRef] [PubMed]
- McMahon, W.R.; Ftouni, S.; Phillips, A.J.K.; Beatty, C.; Lockley, S.W.; Rajaratnam, S.M.W.; Maruff, P.; Drummond, S.P.A.; Anderson, C. The Impact of Structured Sleep Schedules Prior to an In-Laboratory Study: Individual Differences in Sleep and Circadian Timing. PLoS ONE 2020, 15, e0236566. [Google Scholar] [CrossRef]
- Crowley, P.; O’Donovan, M.R.; Leahy, P.; Flanagan, E.; O’Caoimh, R. Pharmacological and Non-Pharmacological Interventions to Improve Sleep in People with Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2025, 22, 956. [Google Scholar] [CrossRef] [PubMed]
- Sloane, P.D.; Figueiro, M.; Garg, S.; Cohen, L.W.; Reed, D.; Williams, C.S.; Preisser, J.; Zimmerman, S. Effect of Home-Based Light Treatment on Persons with Dementia and Their Caregivers. Light. Res. Technol. 2015, 47, 161–176. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Sharpley, A.L. Pharmacotherapies for Sleep Disturbances in Dementia. Cochrane Database Syst. Rev. 2020, 11, CD009178. [Google Scholar] [CrossRef]
- National Institutes of Health National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep 2005, 28, 1049–1057. [CrossRef]
- Yesavage, J.A.; Friedman, L.; Ancoli-Israel, S.; Bliwise, D.; Singer, C.; Vitiello, M.V.; Monjan, A.A.; Lebowitz, B. Development of Diagnostic Criteria for Defining Sleep Disturbance in Alzheimer’s Disease. J. Geriatr. Psychiatry Neurol. 2003, 16, 131–139. [Google Scholar] [CrossRef]
- Richardson, K.; Loke, Y.K.; Fox, C.; Maidment, I.; Howard, R.; Steel, N.; Arthur, A.; Boyd, P.J.; Aldus, C.; Ballard, C.; et al. Adverse Effects of Z-Drugs for Sleep Disturbance in People Living with Dementia: A Population-Based Cohort Study. BMC Med. 2020, 18, 351. [Google Scholar] [CrossRef]
- Liang, L.; Huang, Y.; Xu, R.; Wei, Y.; Xiao, L.; Wang, G. Eszopiclone for the Treatment of Primary Insomnia: A Systematic Review and Meta-Analysis of Double-Blind, Randomized, Placebo-Controlled Trials. Sleep Med. 2019, 62, 6–13. [Google Scholar] [CrossRef]
- Dowling, G.A.; Burr, R.L.; Van Someren, E.J.W.; Hubbard, E.M.; Luxenberg, J.S.; Mastick, J.; Cooper, B.A. Melatonin and Bright-Light Treatment for Rest-Activity Disruption in Institutionalized Patients with Alzheimer’s Disease. J. Am. Geriatr. Soc. 2008, 56, 239–246. [Google Scholar] [CrossRef]
- Morales-Delgado, R.; Cámara-Lemarroy, C.R.; Salinas-Martínez, R.; Gámez-Treviño, D.; Arredondo-Jaime, A.; Hernández-Maldonado, E.; Guajardo-Álvarez, G. A Randomized Placebo-Controlled Trial Evaluating the Effect of Melatonin on Sleep Quality in Patients with Mild-Moderate Dementia. Eur. Geriatr. Med. 2018, 9, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, M.; Kennell-Webb, S.; Warner, J.; Blizard, R.; Raven, P. Double Blind Randomised Placebo Controlled Trial of Low Dose Melatonin for Sleep Disorders in Dementia. Int. J. Geriatr. Psychiatry 2002, 17, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.; Tractenberg, R.E.; Kaye, J.; Schafer, K.; Gamst, A.; Grundman, M.; Thomas, R.; Thal, L.J. Alzheimer’s Disease Cooperative Study A Multicenter, Placebo-Controlled Trial of Melatonin for Sleep Disturbance in Alzheimer’s Disease. Sleep 2003, 26, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on Prolonged-Release Melatonin for Cognitive Function and Sleep in Mild to Moderate Alzheimer’s Disease: A 6-Month, Randomized, Placebo-Controlled, Multicenter Trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar] [CrossRef]
- Crocco, E.A.; Jaramillo, S.; Cruz-Ortiz, C.; Camfield, K. Pharmacological Management of Anxiety Disorders in the Elderly. Curr. Treat. Options Psychiatry 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Camargos, E.F.; Louzada, L.L.; Quintas, J.L.; Naves, J.O.S.; Louzada, F.M.; Nóbrega, O.T. Trazodone Improves Sleep Parameters in Alzheimer Disease Patients: A Randomized, Double-Blind, and Placebo-Controlled Study. Am. J. Geriatr. Psychiatry 2014, 22, 1565–1574. [Google Scholar] [CrossRef]
- Camargos, E.F.; Quintas, J.L.; Louzada, L.L.; Naves, J.O.S.; Furioso, A.C.T.; Nóbrega, O.T. Trazodone and Cognitive Performance in Alzheimer Disease. J. Clin. Psychopharmacol. 2015, 35, 88–89. [Google Scholar] [CrossRef]
- Herring, W.J.; Ceesay, P.; Snyder, E.; Bliwise, D.; Budd, K.; Hutzelmann, J.; Stevens, J.; Lines, C.; Michelson, D. Polysomnographic Assessment of Suvorexant in Patients with Probable Alzheimer’s Disease Dementia and Insomnia: A Randomized Trial. Alzheimer’s Dement. 2020, 16, 541–551. [Google Scholar] [CrossRef]
- Thein, S.G.; Bsharat, M.; Kemethofer, M.; Filippov, G.; Kubota, N.; Moline, M. F1-06-04: Response to Treatment with Lemborexant: Subjects with Irregular Sleep-Wake Rhythm Disorder and Alzheimer’s Disease Dementia. Alzheimer’s Dement. 2019, 15, P187. [Google Scholar] [CrossRef]
- Rémi, J.; Pollmächer, T.; Spiegelhalder, K.; Trenkwalder, C.; Young, P. Sleep-Related Disorders in Neurology and Psychiatry. Dtsch. Arztebl. Int. 2019, 116, 681–688. [Google Scholar] [CrossRef]
- Kinnunen, K.M.; Vikhanova, A.; Livingston, G. The Management of Sleep Disorders in Dementia: An Update. Curr. Opin. Psychiatry 2017, 30, 491–497. [Google Scholar] [CrossRef]
- Ooms, S.; Ju, Y.-E. Treatment of Sleep Disorders in Dementia. Curr. Treat. Options Neurol. 2016, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Cicolin, A.; Ferini-Strambi, L. Focus on Multidisciplinary Aspects of Sleep Medicine. Brain Sci. 2023, 13, 1327. [Google Scholar] [CrossRef]
- Gong, L.; Xu, R.; Qin, M.; Liu, D.; Zhang, B.; Bi, Y.; Xi, C. New Potential Stimulation Targets for Noninvasive Brain Stimulation Treatment of Chronic Insomnia. Sleep Med. 2020, 75, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, H.; Wei, Y.; Ye, S.; Jiang, J.; Mak, M.K.Y.; Pang, M.Y.C.; Gao, Q.; Huang, M. Effects of Non-Invasive Brain Stimulation over the Supplementary Motor Area on Motor Function in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Stimul. 2025, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Guo, C. Assessment of Noninvasive Brain Stimulation Interventions in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis. Sci. Rep. 2024, 14, 14219. [Google Scholar] [CrossRef]
- Cristini, J.; Medina-Rincon, A.; Van Roy, A.; Seo, F.; Moncion, K.; Carrier, J.; Paquette, C.; Dagher, A.; Steib, S.; Roig, M. Non-Invasive Brain Stimulation to Enhance Sleep Quality and Architecture in Parkinson’s Disease: A Systematic Review and Bayesian Network Meta-Analysis. Sleep Med. Rev. 2025, 82, 102117. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Ling, Z.; Yu, X. Repetitive Transcranial Magnetic Stimulation for Cognitive Impairment in Alzheimer’s Disease: A Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2020, 267, 791–801. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y. The Repetitive Transcranial Magnetic Stimulation in Alzheimer’s Disease Patients with Behavioral and Psychological Symptoms of Dementia: A Case Report. BMC Psychiatry 2023, 23, 354. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Di Lorenzo, F.; Manzoli, L.; Flacco, M.E.; Koch, G. Blood Phosphorylated Tau217 Distinguishes Amyloid-Positive from Amyloid-Negative Subjects in the Alzheimer’s Disease Continuum. A Systematic Review and Meta-Analysis. J. Neurol. 2025, 272, 252. [Google Scholar] [CrossRef]
- Siderowf, A.; Concha-Marambio, L.; Lafontant, D.-E.; Farris, C.M.; Ma, Y.; Urenia, P.A.; Nguyen, H.; Alcalay, R.N.; Chahine, L.M.; Foroud, T.; et al. Assessment of Heterogeneity among Participants in the Parkinson’s Progression Markers Initiative Cohort Using α-Synuclein Seed Amplification: A Cross-Sectional Study. Lancet Neurol. 2023, 22, 407–417. [Google Scholar] [CrossRef]

| Disease | Pathogenic Protein Aggregates | Pathogenic Mutations | Affected Brain Regions | Affected Neurotransmitters | Clinical Symptoms |
|---|---|---|---|---|---|
| AD | Aβ (extracellular senile plaques), tau (intracellular NFT) [19] | APP, PSEN1, PSEN2, APOEε4 [19] | entorhinal cortex, hippocampus, temporal, parietal, and frontal lobes [20] | acetylcholine [21] | memory loss, executive dysfunction, confusion, language problems, and mood changes [20] |
| PD | α-synuclein (Lewy bodies, Lewy neurites) [22] | SNCA, PRKN, PINK1, DJ-1, LRRK2, GBA [19,23] | midbrain, basal ganglia [22] | dopamine [21] | Parkinsonism (tremor, bradykinesia, rigidity, and imbalance) [19] |
| DLB | α-synuclein (Lewy bodies, Lewy neurites) [22] | SNCA, GBA, APOEε4 [24] | basal ganglia, limbic system, brainstem, temporal, parietal, and frontal lobes [21] | acetylcholine, dopamine [21] | fluctuating cognition, Parkinsonism, visual hallucinations, and RBD [19,25] |
| FTD | tau, TDP-43, FUS [26] | C9orf72, MAPT, GRN [27] | frontal and temporal lobes [27] | serotonin, dopamine, glutamate, GABA [28] | behavioral and personality changes, and language difficulties [27] |
| MSA | α-synuclein (GCIs) [29] | SNCA, COQ2 [22] | basal ganglia, brainstem, cerebellum [29] | dopamine, serotonin, and acetylcholine [30] | Parkinsonism, autonomic dysfunction, ataxia, and pyramidal dysfunction [29] |
| PSP | 4R tau (intracellular NFT, neuropil threads, tufted astrocytes, and oligodendroglial coiled bodies) [31] | MAPT, LRRK2, DCTN1 [31] | basal ganglia, brainstem [31] | various [28] | Parkinsonism, frequent falls, supranuclear gaze palsy, and bulbar dysfunction [31] |
| CBD | 4R tau (astrocytic plaques, oligodendroglial coiled bodies) [32] | MAPT, GRN, C9orf72, PRNP [33] | cerebral cortex, and basal ganglia [32] | various [28] | asymmetric Parkinsonism, dystonia, apraxia, and sensory deficits [33] |
| VaD | - | NOTCH3, APOEε4, MTHFR [34] | cerebral cortex, white matter, basal ganglia, and thalamus [35] | acetylcholine, serotonin, and dopamine [36] | reduced mental speed, executive dysfunctions, confusion, memory loss, and language problems [35] |
| Disease | Insomnia | EDS | RBD | Nightmares | SDB | RLS |
|---|---|---|---|---|---|---|
| PD [53,54,55] | 32–44% | 21–76% | 39–46% | 17–30% | 27–48% | 14% |
| MSA [56,57,58] | 19% | 28% | 88% | - | Stridor 30–42% OSA 15–37% | 4.7–28% |
| DLB [59] | 26–75% | 11–100% | 76% | 83% | 34–60% | - |
| FTD [60] | 48% | 64% | Rare | Rare | 68% | 8% |
| CBD [61] | Rare | - | 14% | - | Rare | Rare |
| PSP [62] | 60% | 60% | 11–28% | - | 55% | 3.7–58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwecka, N.; Golberg, M.; Świerczewska, D.; Filipek, B.; Pendrasik, K.; Bączek-Grzegorzewska, A.; Stasiołek, M.; Świderek-Matysiak, M. Sleep Disorders in Neurodegenerative Diseases with Dementia: A Comprehensive Review. J. Clin. Med. 2025, 14, 7119. https://doi.org/10.3390/jcm14197119
Siwecka N, Golberg M, Świerczewska D, Filipek B, Pendrasik K, Bączek-Grzegorzewska A, Stasiołek M, Świderek-Matysiak M. Sleep Disorders in Neurodegenerative Diseases with Dementia: A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(19):7119. https://doi.org/10.3390/jcm14197119
Chicago/Turabian StyleSiwecka, Natalia, Michał Golberg, Dominika Świerczewska, Beata Filipek, Karolina Pendrasik, Adrianna Bączek-Grzegorzewska, Mariusz Stasiołek, and Mariola Świderek-Matysiak. 2025. "Sleep Disorders in Neurodegenerative Diseases with Dementia: A Comprehensive Review" Journal of Clinical Medicine 14, no. 19: 7119. https://doi.org/10.3390/jcm14197119
APA StyleSiwecka, N., Golberg, M., Świerczewska, D., Filipek, B., Pendrasik, K., Bączek-Grzegorzewska, A., Stasiołek, M., & Świderek-Matysiak, M. (2025). Sleep Disorders in Neurodegenerative Diseases with Dementia: A Comprehensive Review. Journal of Clinical Medicine, 14(19), 7119. https://doi.org/10.3390/jcm14197119






