Retrospective Comparison of Stiff Wire-Based 2D3D, Traditional 3D3D Image Fusion, and Non-Image Fusion Techniques and Their Role in Thoracic Aortic Endovascular Repair
Abstract
1. Introduction
2. Methods and Materials
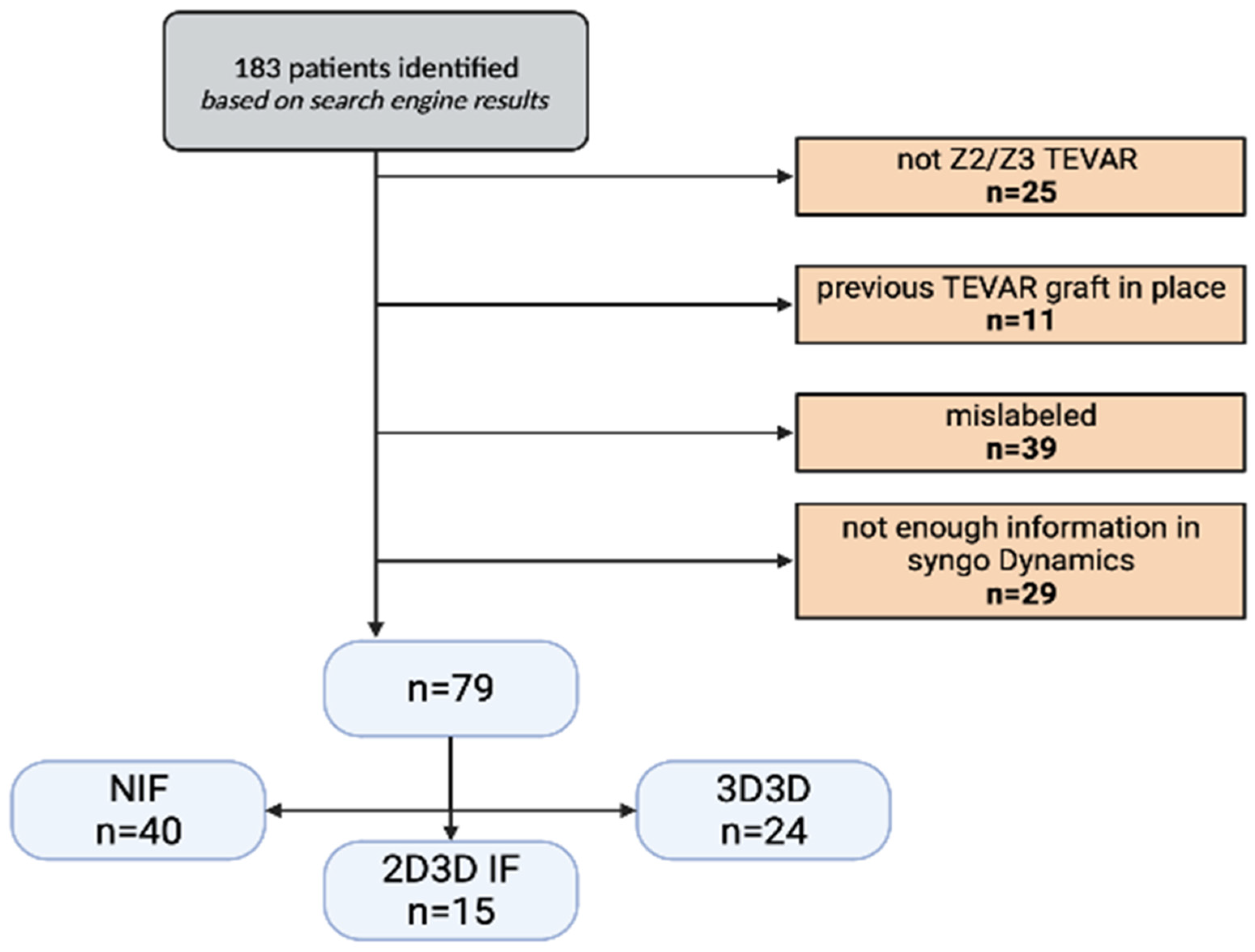
2.1. Study Design
2.2. Ethics
2.3. Outcomes
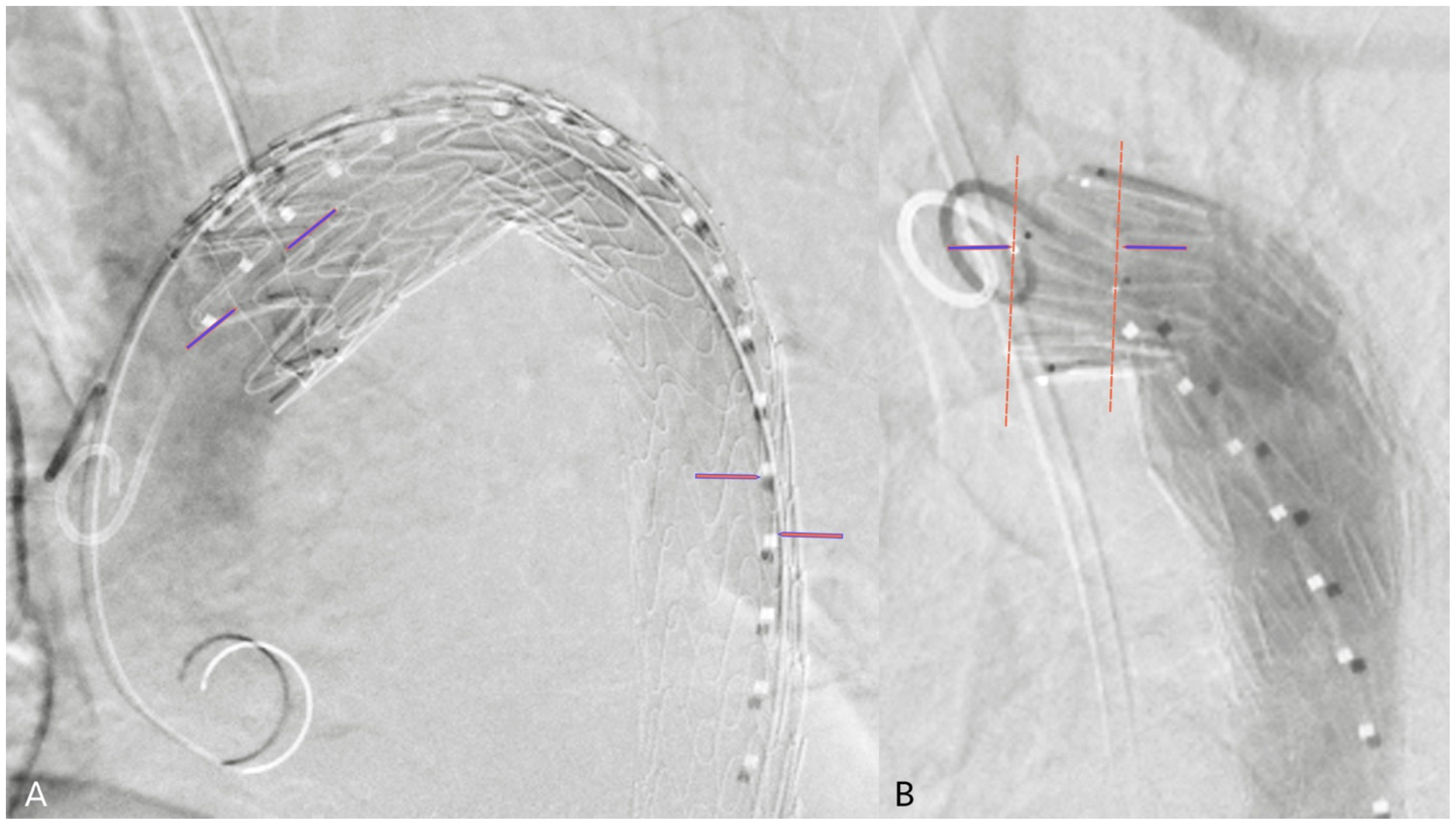
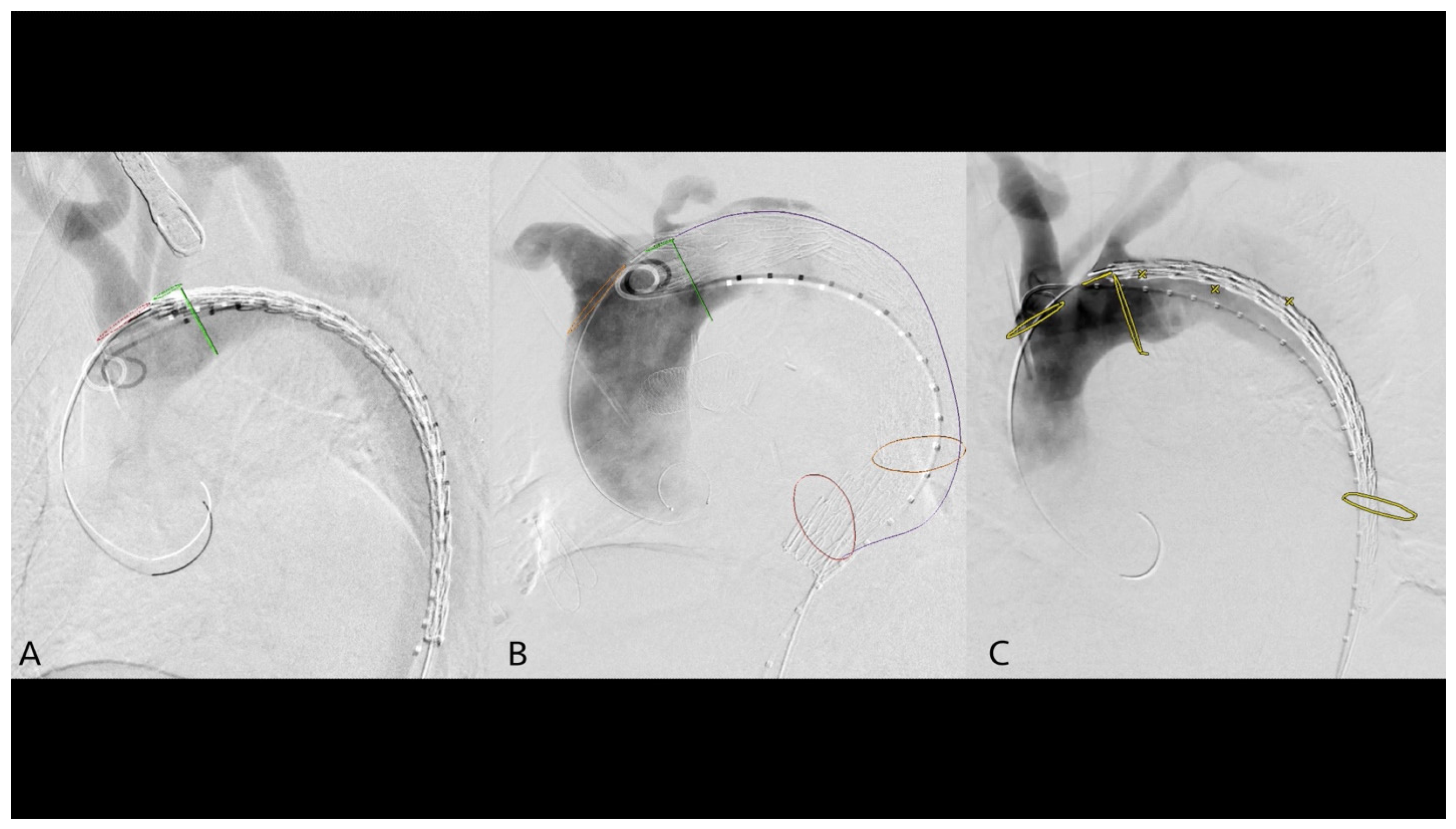
2.4. 2D3D Fusion Technique
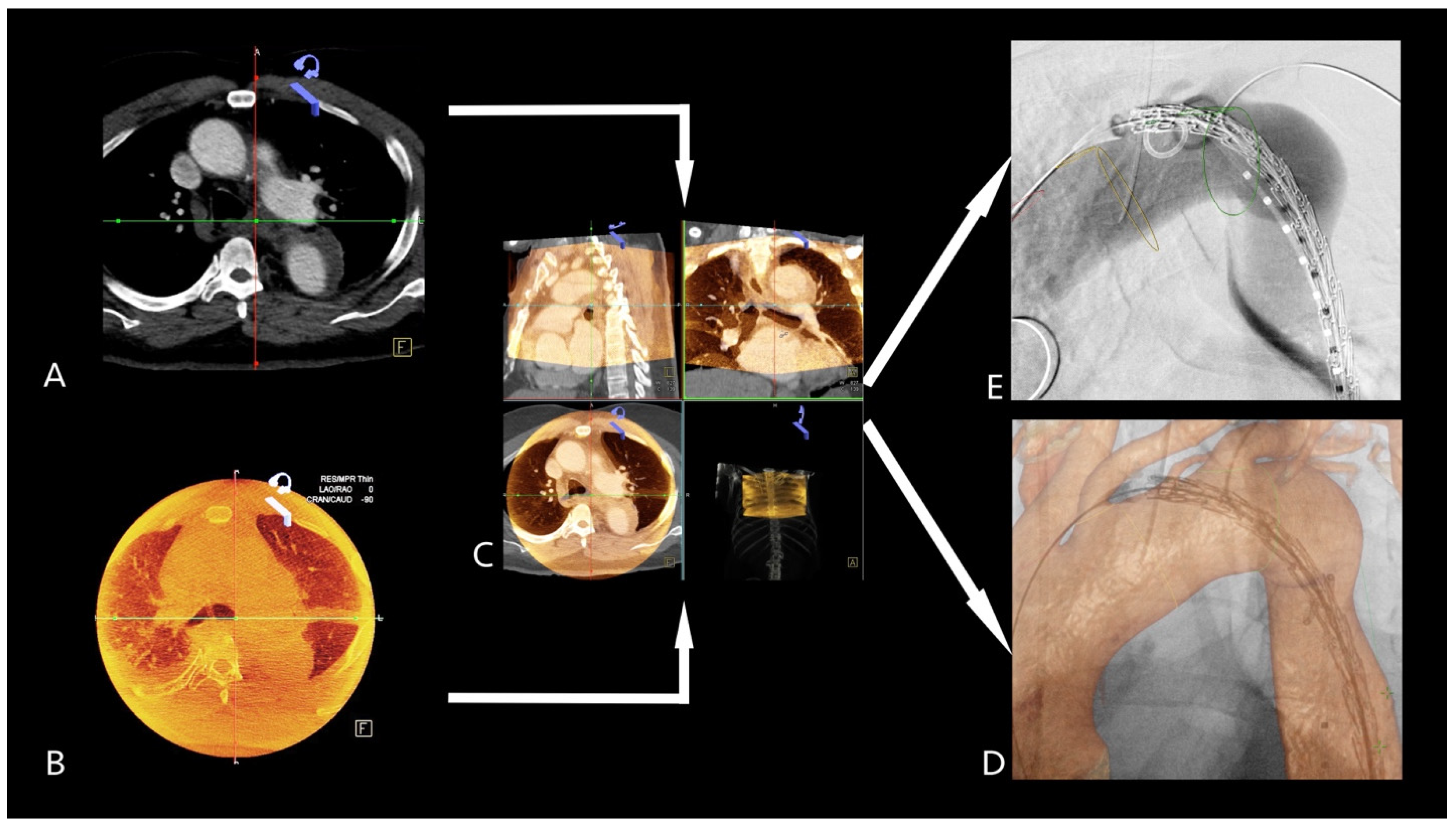
2.5. 3D3D Fusion Technique
2.6. Statistical Analysis
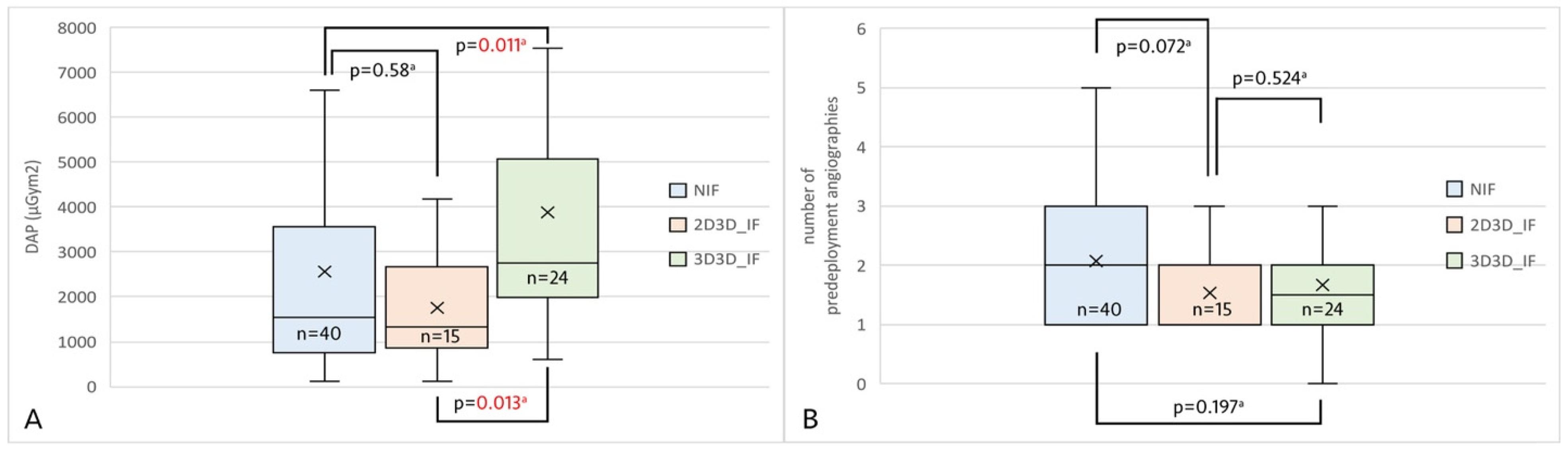
3. Results
Z3 TEVAR Subgroup
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALARA | As low as reasonably achievable |
| BMI | Body mass index |
| CBCT | Cone-beam CT |
| DAP | Dose area product |
| IF group | Image fusion group |
| NIF group | Non-image fusion group |
| OSR | Open surgical repair |
| TEVAR | Thoracic endovascular aortic repair |
References
- Harky, A.; Kai Chan, J.S.; Ming Wong, C.H.; Bashir, M. Open versus Endovascular Repair of Descending Thoracic Aortic Aneurysm Disease: A Systematic Review and Meta-analysis. Ann. Vasc. Surg. 2019, 54, 304–315.e5. [Google Scholar] [CrossRef] [PubMed]
- Treffalls, J.A.; Sylvester, C.B.; Parikh, U.; Zea-Vera, R.; Ryan, C.T.; Zhang, Q.; Rosengart, T.K.; Wall, M.J.; Coselli, J.S.; Chatterjee, S.; et al. Nationwide database analysis of one-year readmission rates after open surgical or thoracic endovascular repair of Stanford Type B aortic dissection. JTCVS Open 2022, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mussa, F.F.; Horton, J.D.; Moridzadeh, R.; Nicholson, J.; Trimarchi, S.; Eagle, K.A. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA 2016, 316, 754–763. [Google Scholar] [CrossRef]
- Upchurch, G.R.; Escobar, G.A.; Azizzadeh, A.; Beck, A.W.; Conrad, M.F.; Matsumura, J.S.; Murad, M.H.; Perry, R.J.; Singh, M.J.; Veeraswamy, R.K.; et al. Society for Vascular Surgery clinical practice guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J. Vasc. Surg. 2021, 73, 55s–83s. [Google Scholar] [CrossRef] [PubMed]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Gray, D.E.; Eisenack, M.; Gawenda, M.; Torsello, G.; Majd, P.; Brunkwall, J.; Osada, N.; Donas, K.P. Repeated contrast medium application after endovascular aneurysm repair and not the type of endograft fixation seems to have deleterious effect on the renal function. J. Vasc. Surg. 2017, 65, 46–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kougias, P.; Sharath, S.; Barshes, N.R.; Lowery, B.; Garcia, A.; Pak, T.; Bechara, C.F.; Pisimisis, G. Impact of cumulative intravascular contrast exposure on renal function in patients with occlusive and aneurysmal vascular disease. J. Vasc. Surg. 2014, 59, 1644–1650. [Google Scholar] [CrossRef]
- Kawatani, Y.; Kurobe, H.; Nakamura, Y.; Hori, T.; Kitagawa, T. The ratio of contrast medium volume to estimated glomerular filtration rate as a predictor of contrast-induced nephropathy after endovascular aortic repair. J. Med. Investig. 2018, 65, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kawatani, Y.; Nakamura, Y.; Mochida, Y.; Yamauchi, N.; Hayashi, Y.; Taneichi, T.; Ito, Y.; Kurobe, H.; Suda, Y.; Hori, T. Contrast Medium Induced Nephropathy after Endovascular Stent Graft Placement: An Examination of Its Prevalence and Risk Factors. Radiol. Res. Pract. 2016, 2016, 5950986. [Google Scholar] [CrossRef]
- Morcos, S.K.; Epstein, F.H.; Haylor, J.; Dobrota, M. Aspects of contrast media nephrotoxicity. Eur. J. Radiol. 1996, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-C.; Lin, H.-Y.; Hung, S.-K.; Huang, Y.-T.; Lee, M.-S.; Wang, W.-H.; Wu, C.-S.; Su, Y.-C.; Shen, B.-J.; Tsai, S.-J.; et al. Leukemia Risk After Cardiac Fluoroscopic Interventions Stratified by Procedure Number, Exposure Latent Time, and Sex: A Nationwide Population-Based Case-Control Study. Medicine 2016, 95, e2953. [Google Scholar] [CrossRef]
- Schwartz, R.A.; Burgess, G.H.; Milgrom, H. Breast carcinoma and basal cell epithelioma after x-ray therapy for hirsutism. Cancer 1979, 44, 1601–1605. [Google Scholar] [CrossRef]
- Miller, A.B.; Howe, G.R.; Sherman, G.J.; Lindsay, J.P.; Yaffe, M.J.; Dinner, P.J.; Risch, H.A.; Preston, D.L. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N. Engl. J. Med. 1989, 321, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Koutouzi, G.; Henrikson, O.; Roos, H.; Zachrisson, K.; Falkenberg, M. EVAR Guided by 3D Image Fusion and CO2 DSA: A New Imaging Combination for Patients With Renal Insufficiency. J. Endovasc. Ther. 2015, 22, 912–917. [Google Scholar] [CrossRef]
- Kaladji, A.; Dumenil, A.; Mahé, G.; Castro, M.; Cardon, A.; Lucas, A.; Haigron, P. Safety and accuracy of endovascular aneurysm repair without pre-operative and intra-operative contrast agent. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 255–261. [Google Scholar] [CrossRef]
- Hertault, A.; Maurel, B.; Sobocinski, J.; Gonzalez, T.M.; Le Roux, M.; Azzaoui, R.; Midulla, M.; Haulon, S. Impact of hybrid rooms with image fusion on radiation exposure during endovascular aortic repair. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 382–390. [Google Scholar] [CrossRef]
- Kobeiter, H.; Nahum, J.; Becquemin, J.P. Zero-contrast thoracic endovascular aortic repair using image fusion. Circulation 2011, 124, e280–e282. [Google Scholar] [CrossRef]
- Tacher, V.; Lin, M.; Desgranges, P.; Deux, J.-F.; Grünhagen, T.; Becquemin, J.-P.; Luciani, A.; Rahmouni, A.; Kobeiter, H. Image guidance for endovascular repair of complex aortic aneurysms: Comparison of two-dimensional and three-dimensional angiography and image fusion. J. Vasc. Interv. Radiol. 2013, 24, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Obeidi, Y.; Majd, P.; Brunkwall, J.S. The 2D-3D Registration Method in Image Fusion Is Accurate and Helps to Reduce the Used Contrast Medium, Radiation, and Procedural Time in Standard EVAR Procedures. Ann. Vasc. Surg. 2018, 51, 177–186. [Google Scholar] [CrossRef]
- Barral, P.-A.; Demasi-Jacquier, M.A.; Bal, L.; Omnes, V.; Bartoli, A.; Piquet, P.; Jacquier, A.; Gaudry, M. Fusion Imaging to Guide Thoracic Endovascular Aortic Repair (TEVAR): A Randomized Comparison of Two Methods, 2D/3D Versus 3D/3D Image Fusion. Cardiovasc. Interv. Radiol. 2019, 42, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Hasselmann, H.C.; Galas, N.; Majd, P.; Brunkwall, S.; Brunkwall, J.S. Image fusion using the two-dimensional-three-dimensional registration method helps reduce contrast medium volume, fluoroscopy time, and procedure time in hybrid thoracic endovascular aortic repairs. J. Vasc. Surg. 2019, 69, 1003–1010. [Google Scholar] [CrossRef] [PubMed]


| NIF (n = 40) | IF (n = 39) | p-Value | ||
|---|---|---|---|---|
| 2D3D IF (n = 15) | 3D3D IF (n = 24) | |||
| Age (year) | 67.5 (59.75–76) | 64 (54.5–72) | 72 (67–78) | 0.134 a |
| Male/Female | 28/12 | 25/14 | 0.105 b | |
| 9/6 | 16/8 | - | ||
| BMI (kg/m2) | 28.08 (25.49–32.92) | 27.83 (23.47–31.67) | 24.89 (22.59–27.73) | 0.069 a |
| NIF vs. 2D3D IF | 28.08 (25.49–32.92) | 27.83 (23.47–31.67) | - | 0.547 c |
| NIF vs. 3D3D IF | 28.08 (25.49–32.92) | - | 24.89 (22.59–27.73) | 0.021 c |
| 2D3D IF vs. 3D3D IF | - | 27.83 (23.47–31.67) | 24.89 (22.59–27.73) | 0.209 c |
| Past medical history | ||||
| Atrial fibrillation | 6 (15) | 6 (15.38) | 1.00 b | |
| 1 (6.7) | 5 (20.8) | |||
| Arterial hypertension | 33 (82.5) | 34 (87.18) | 1.00 b | |
| 11 (73.33) | 23 (92) | |||
| CAD | 4 (10) | 3 (7.69) | 1.00 b | |
| 0 (0) | 3 (12) | |||
| DM | 7 (17.5) | 5 (12.82) | 1.00 b | |
| 3 (2) | 2 (0.8) | |||
| CKD | 5 (12.5) | 6 (15.38) | 0.57 b | |
| 1 (6.67) | 5 (2) | |||
| Indication for TEVAR | ||||
| Aneurysm/pseudoaneurysm | 9 (22.5) | 13 (33.33) | 0.32 b | |
| aortic dissection | 26 (65) | 20 (51.28) | 0.258 b | |
| acute TBAD | 15 (37.5) | 13 (33.33) | ||
| chronic TBAD | 5 (12.5) | 5 (12.82) | ||
| residual TAAD | 6 (15) | 2 (5.13) | ||
| Other | 5 (12.5) | 6 (15.38) | 0.756 b | |
| PAU, IMH | 5 (12.5) | 5 (12.82) | ||
| protective TEVAR before LA mass removal | 0 (0) | 1 (2.56) | ||
| Emergency cases | 19 (47.5) | 10 (25.64) | 0.062 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osztrogonacz, P.; Garami, Z.; Lumsden, A.B.; Csobay-Novák, C.; Chinnadurai, P. Retrospective Comparison of Stiff Wire-Based 2D3D, Traditional 3D3D Image Fusion, and Non-Image Fusion Techniques and Their Role in Thoracic Aortic Endovascular Repair. J. Clin. Med. 2025, 14, 301. https://doi.org/10.3390/jcm14020301
Osztrogonacz P, Garami Z, Lumsden AB, Csobay-Novák C, Chinnadurai P. Retrospective Comparison of Stiff Wire-Based 2D3D, Traditional 3D3D Image Fusion, and Non-Image Fusion Techniques and Their Role in Thoracic Aortic Endovascular Repair. Journal of Clinical Medicine. 2025; 14(2):301. https://doi.org/10.3390/jcm14020301
Chicago/Turabian StyleOsztrogonacz, Peter, Zsolt Garami, Alan B. Lumsden, Csaba Csobay-Novák, and Ponraj Chinnadurai. 2025. "Retrospective Comparison of Stiff Wire-Based 2D3D, Traditional 3D3D Image Fusion, and Non-Image Fusion Techniques and Their Role in Thoracic Aortic Endovascular Repair" Journal of Clinical Medicine 14, no. 2: 301. https://doi.org/10.3390/jcm14020301
APA StyleOsztrogonacz, P., Garami, Z., Lumsden, A. B., Csobay-Novák, C., & Chinnadurai, P. (2025). Retrospective Comparison of Stiff Wire-Based 2D3D, Traditional 3D3D Image Fusion, and Non-Image Fusion Techniques and Their Role in Thoracic Aortic Endovascular Repair. Journal of Clinical Medicine, 14(2), 301. https://doi.org/10.3390/jcm14020301







