Enhancing Surgical Efficiency and Radiological Outcomes Through Advances in Patient-Specific Instrument Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
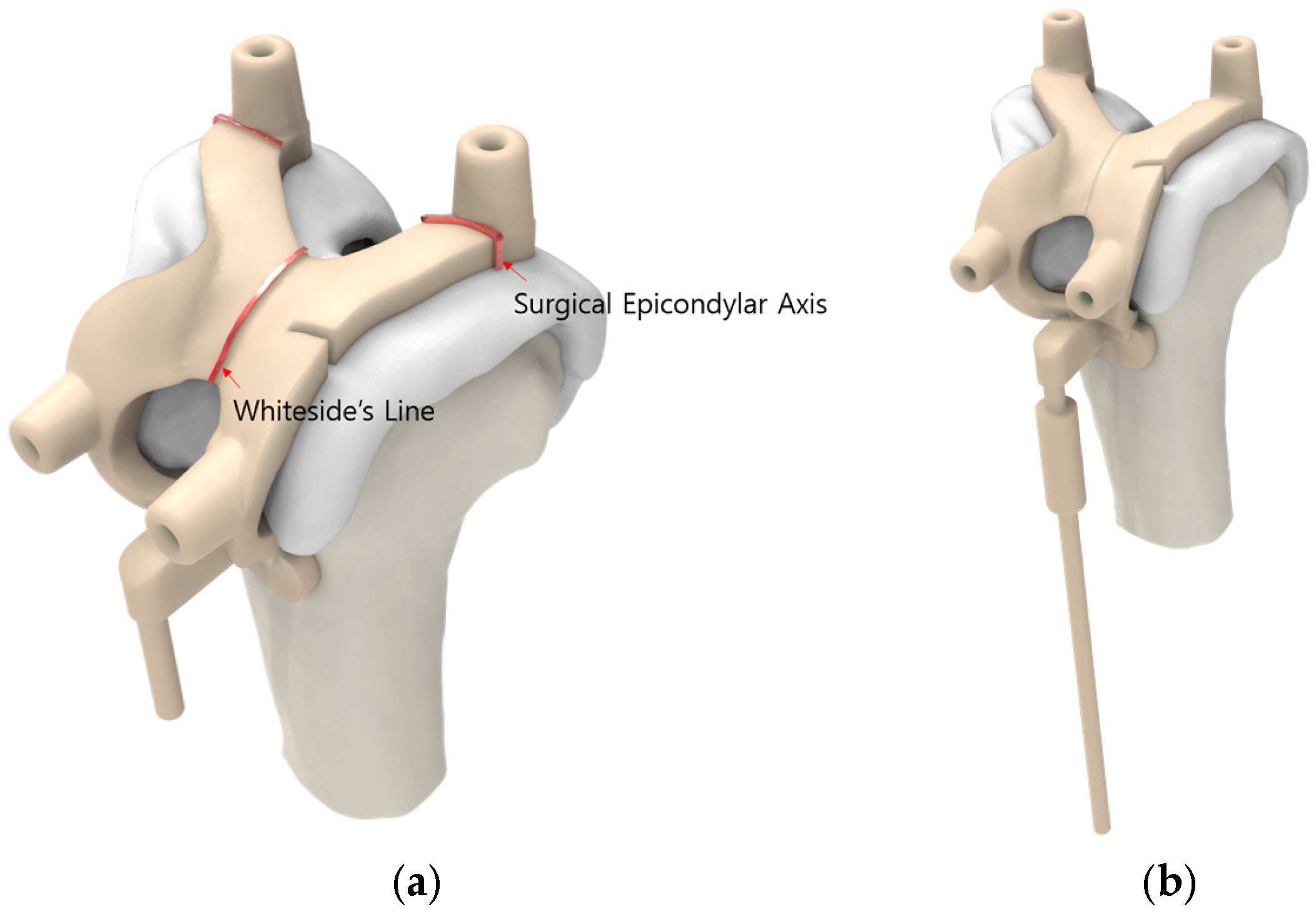
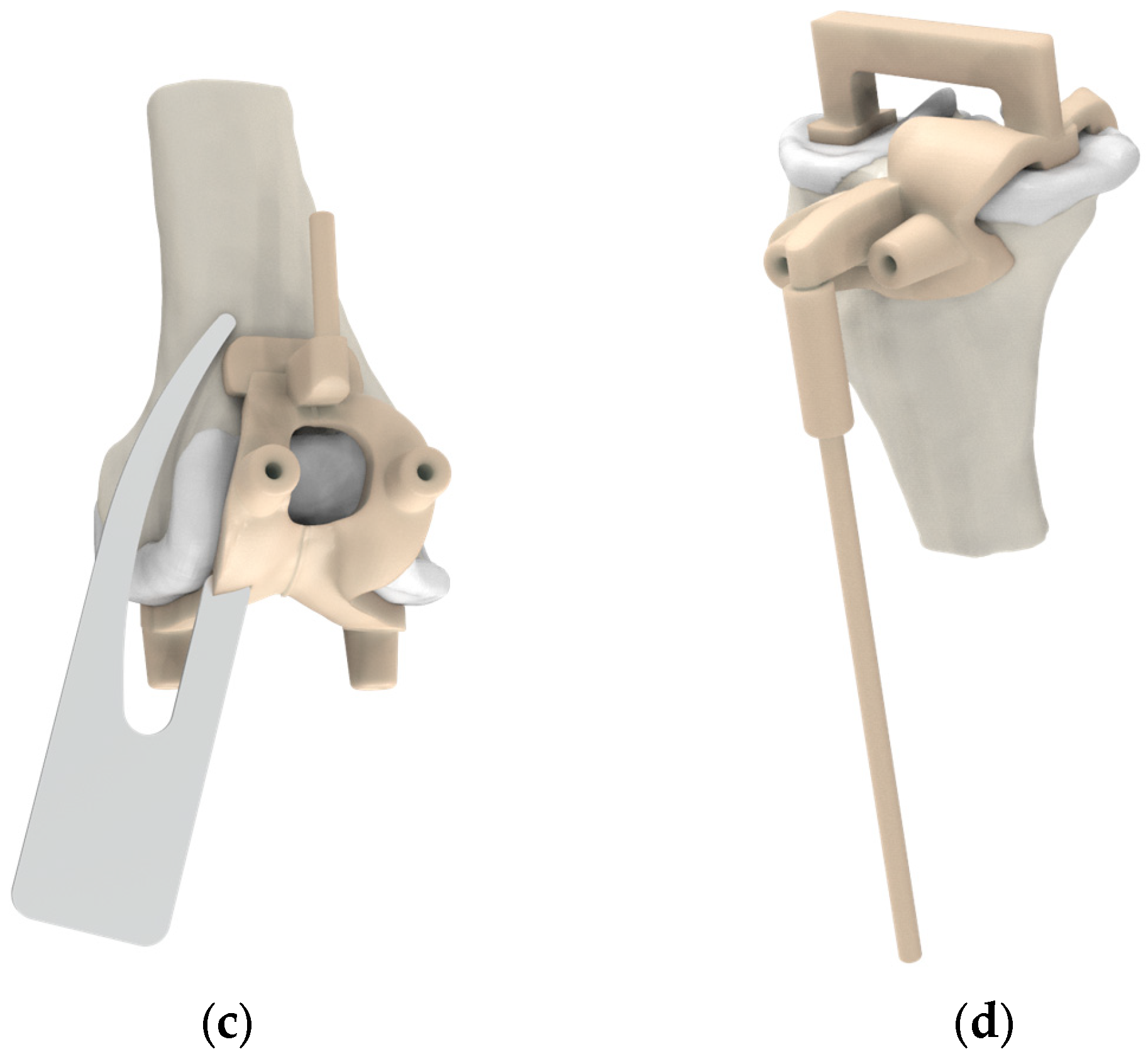
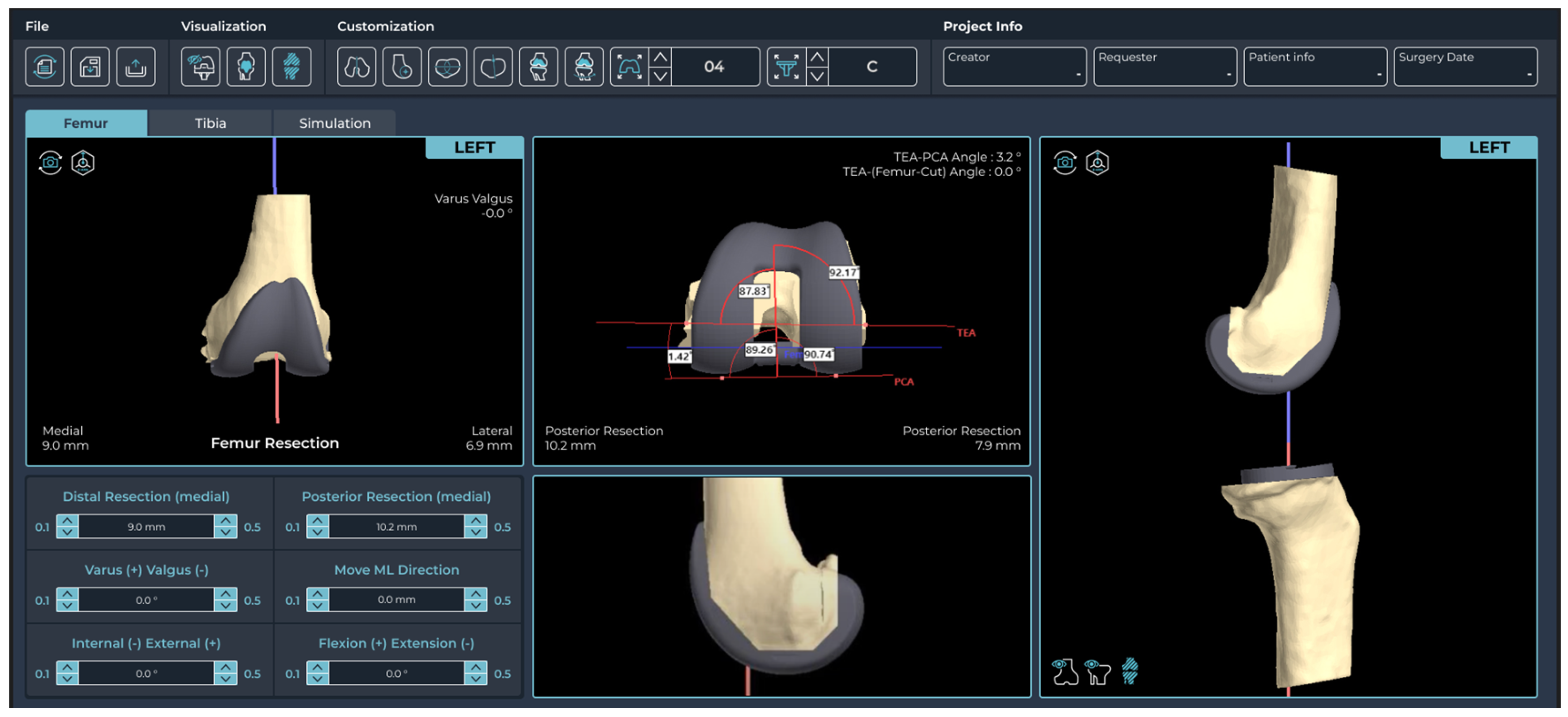
2.2. Pre-Planning and PSI Design Methods
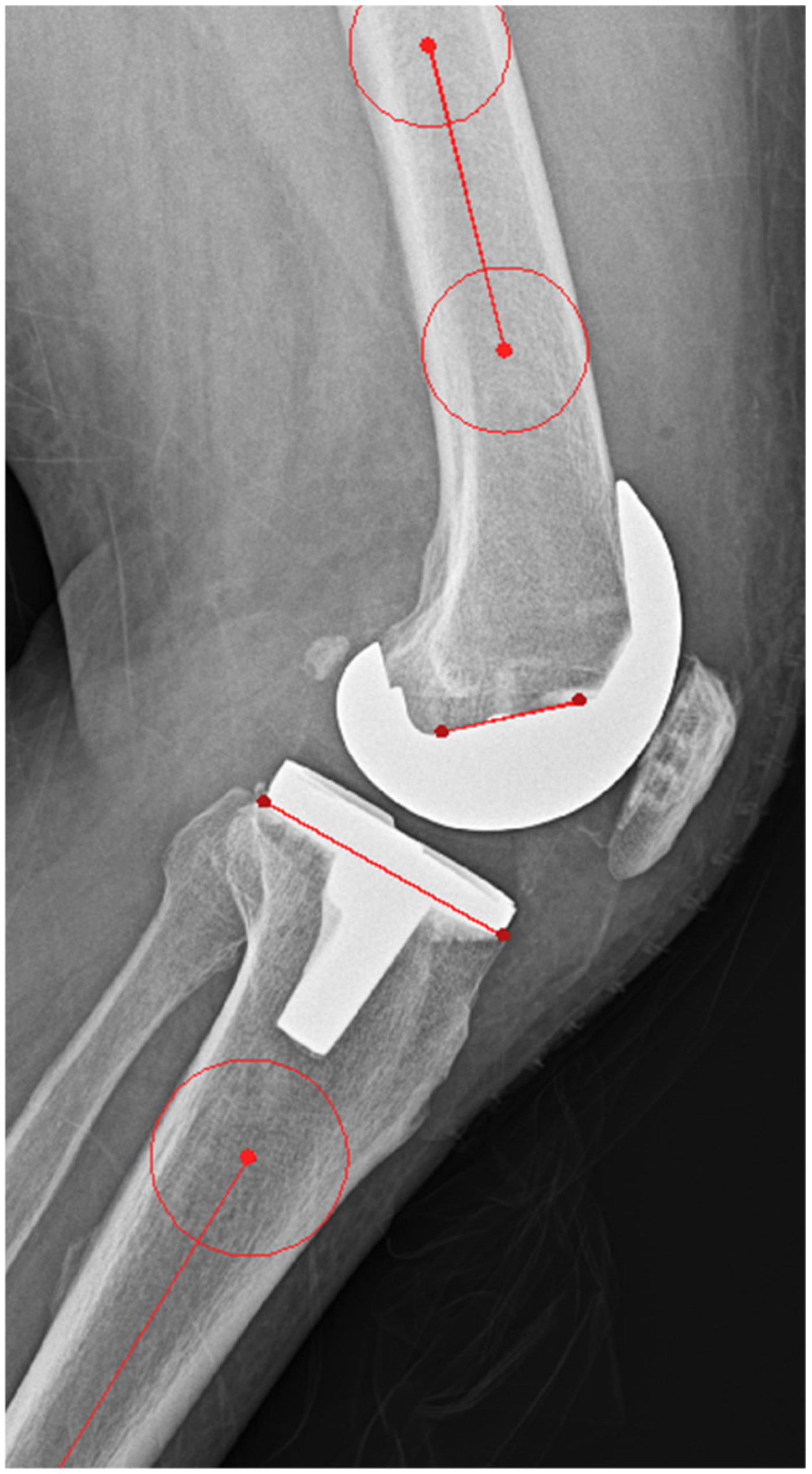
2.3. Outcome Measurements
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, F.; Patel, S.; Haddad, F.S. Midterm assessment of causes and results of revision total knee arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.; Ghijselings, S.; Tajdar, F.; Van Damme, G.; Deprez, P.; Arnout, N.; Van Der Straeten, C. Total knee arthroplasty at 15–17 years: Does implant design affect outcome? Int. Orthop. 2014, 38, 235–241. [Google Scholar] [CrossRef]
- Barrack, R.L.; Schrader, T.; Bertot, A.J.; Wolfe, M.W.; Myers, L. Component rotation and anterior knee pain after total knee arthroplasty. Clin. Orthop. Relat. Res. 2001, 392, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, J.M. Alignment in total knee replacement. J. Bone Jt. Surg. Br. 2008, 90, 1121–1127. [Google Scholar] [CrossRef]
- Jones, C.W.; Jerabek, S.A. Current role of computer navigation in total knee arthroplasty. J. Arthroplast. 2018, 33, 1989–1993. [Google Scholar] [CrossRef]
- Lombardi, A.V., Jr.; Frye, B.M. Customization of cutting blocks: Can this address the problem? Curr. Rev. Musculoskelet. Med. 2012, 5, 309–314. [Google Scholar] [CrossRef]
- Ng, V.Y.; DeClaire, J.H.; Berend, K.R.; Gulick, B.C.; Lombardi, A.V., Jr. Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in tka. Clin. Orthop. Relat. Res. 2012, 470, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Maher, P.A.; Rebolledo, B.J.; Nawabi, D.H.; McLawhorn, A.S.; Pearle, A.D. Patient specific cutting guides versus an imageless, computer-assisted surgery system in total knee arthroplasty. Knee 2013, 20, 263–267. [Google Scholar] [CrossRef]
- Huijbregts, H.J.; Khan, R.J.; Fick, D.P.; Hall, M.J.; Punwar, S.A.; Sorensen, E.; Reid, M.J.; Vedove, S.D.; Haebich, S. Component alignment and clinical outcome following total knee arthroplasty: A randomised controlled trial comparing an intramedullary alignment system with patient-specific instrumentation. Bone Jt. J. 2016, 98-b, 1043–1049. [Google Scholar] [CrossRef]
- Woolson, S.T.; Harris, A.H.; Wagner, D.W.; Giori, N.J. Component alignment during total knee arthroplasty with use of standard or custom instrumentation: A randomized clinical trial using computed tomography for postoperative alignment measurement. J. Bone Jt. Surg. Am. 2014, 96, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.R.; Kang, K.T.; Son, J.; Choi, Y.J.; Suh, D.S.; Koh, Y.G. The effect of femoral cutting guide design improvements for patient-specific instruments. Biomed. Res. Int. 2015, 2015, 978686. [Google Scholar] [CrossRef] [PubMed]
- Asada, S.; Mori, S.; Matsushita, T.; Nakagawa, K.; Tsukamoto, I.; Akagi, M. Comparison of mri- and ct-based patient-specific guides for total knee arthroplasty. Knee 2014, 21, 1238–1243. [Google Scholar] [CrossRef]
- Kwon, O.R.; Kang, K.T.; Son, J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Patient-specific instrumentation development in tka: 1st and 2nd generation designs in comparison with conventional instrumentation. Arch. Orthop. Trauma Surg. 2017, 137, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.R.; Kang, K.T.; Son, J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. The effect of patient-specific instrumentation incorporating an extramedullary tibial guide on operative efficiency for total knee arthroplasty. Biomed. Res. Int. 2017, 2017, 2034782. [Google Scholar] [CrossRef] [PubMed]
- Lionberger, D.R.; Crocker, C.L.; Chen, V. Patient specific instrumentation. J. Arthroplast. 2014, 29, 1699–1704. [Google Scholar] [CrossRef]
- Voleti, P.B.; Hamula, M.J.; Baldwin, K.D.; Lee, G.C. Current data do not support routine use of patient-specific instrumentation in total knee arthroplasty. J. Arthroplast. 2014, 29, 1709–1712. [Google Scholar] [CrossRef]
- Radermacher, K.; Portheine, F.; Anton, M.; Zimolong, A.; Kaspers, G.; Rau, G.; Staudte, H.W. Computer assisted orthopaedic surgery with image based individual templates. Clin. Orthop. Relat. Res. 1998, 354, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.V., Jr.; Berend, K.R.; Adams, J.B. Patient-specific approach in total knee arthroplasty. Orthopedics 2008, 31, 927–930. [Google Scholar] [PubMed]
- Hong, H.T.; Koh, Y.G.; Cho, B.W.; Kwon, H.M.; Park, K.K.; Kang, K.T. An image-based augmented reality system for achieving accurate bone resection in total knee arthroplasty. Cureus 2024, 16, e58281. [Google Scholar] [CrossRef]
- Pfitzner, T.; Abdel, M.P.; von Roth, P.; Perka, C.; Hommel, H. Small improvements in mechanical axis alignment achieved with mri versus ct-based patient-specific instruments in tka: A randomized clinical trial. Clin. Orthop. Relat. Res. 2014, 472, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.J.; Blakey, C.M.; Anderson, A.A.; Tomouk, W.M.; Buckley, S.C.; Hamer, A.J.; Sutton, P.M. Minimum 5-year outcomes of a multicenter, prospective, randomized control trial assessing clinical and radiological outcomes of patient-specific instrumentation in total knee arthroplasty. J. Arthroplast. 2022, 37, 1579–1585. [Google Scholar] [CrossRef]
- Tammachote, N.; Panichkul, P.; Kanitnate, S. Comparison of customized cutting block and conventional cutting instrument in total knee arthroplasty: A randomized controlled trial. J. Arthroplast. 2018, 33, 746–751.e3. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, J.; Snorrason, F.; Röhrl, S.M. No radiological and clinical advantages with patient-specific positioning guides in total knee replacement. Acta Orthop. 2018, 89, 89–94. [Google Scholar] [CrossRef]
- Pourgiezis, N.; Reddy, S.P.; Nankivell, M.; Morrison, G.; VanEssen, J. Alignment and component position after patient-matched instrumentation versus conventional total knee arthroplasty. J. Orthop. Surg. (Hong Kong) 2016, 24, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Abane, L.; Anract, P.; Boisgard, S.; Descamps, S.; Courpied, J.P.; Hamadouche, M. A comparison of patient-specific and conventional instrumentation for total knee arthroplasty: A multicentre randomised controlled trial. Bone Jt. J. 2015, 97, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ivie, C.B.; Probst, P.J.; Bal, A.K.; Stannard, J.T.; Crist, B.D.; Sonny Bal, B. Improved radiographic outcomes with patient-specific total knee arthroplasty. J. Arthroplast. 2014, 29, 2100–2103. [Google Scholar] [CrossRef]
- Lombardi, A.V., Jr.; Berend, K.R.; Ng, V.Y. Neutral mechanical alignment: A requirement for successful tka: Affirms. Orthopedics 2011, 34, e504–e509. [Google Scholar] [CrossRef]
- Minoda, Y.; Kobayashi, A.; Iwaki, H.; Ohashi, H.; Takaoka, K. Tka sagittal alignment with navigation systems and conventional techniques vary only a few degrees. Clin. Orthop. Relat. Res. 2009, 467, 1000–1006. [Google Scholar] [CrossRef]
- Renson, L.; Poilvache, P.; Van den Wyngaert, H. Improved alignment and operating room efficiency with patient-specific instrumentation for tka. Knee 2014, 21, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, M.; Djahani, O.; Hochegger, M.; Plattner, F.; Hofmann, S. Patient-specific total knee arthroplasty: The importance of planning by the surgeon. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2220–2226. [Google Scholar] [CrossRef] [PubMed]

| Variables | Conventional Group | PSI Group | p-Value |
|---|---|---|---|
| Age (yrs) | 69.7 ± 7.6 | 70.5 ± 7.0 | 0.07 |
| BMI (kg/m2) | 26.8 ± 15.6 | 26.8 ± 10.1 | 0.99 |
| Variables | Conventional Group | PSI Group | p-Value |
|---|---|---|---|
| Surgical time (min) | 59.2 ± 14.2 | 47.6 ± 12.4 | <0.001 |
| Variables | Conventional Group | PSI Group | p-Value |
|---|---|---|---|
| HKA angle | |||
| Value (°) | 59.2 ± 14.2 | 47.6 ± 12.4 | 0.004 |
| Outlier (n, %) | 119 (36.3) | 23 (7) | <0.001 |
| FCA | |||
| Value (°) | 89.7 ± 1.9 | 89.8 ± 1.5 | 0.453 |
| Outlier (n, %) | 39 (11.9) | 14 (4.3) | 0.002 |
| TCA | |||
| Value (°) | 89.1 ± 1.9 | 89.7 ± 1.6 | <0.001 |
| Outlier (n, %) | 42 (12.8) | 23 (7) | 0.03 |
| FSA | |||
| Value (°) | 85.0 ± 4.4 | 86.9 ± 3.0 | <0.001 |
| Outlier (n, %) | 189 (57.6) | 108 (32.9) | <0.001 |
| TSA | |||
| Value (°) | 85.0 ± 4.4 | 86.9 ± 3.0 | <0.001 |
| Outlier (n, %) | 95 (29) | 123 (37.5) | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, Y.-G.; Nam, J.-H.; Kim, J.-K.; Suh, D.-S.; Chung, J.H.; Park, K.K.; Kang, K.-T. Enhancing Surgical Efficiency and Radiological Outcomes Through Advances in Patient-Specific Instrument Design. J. Clin. Med. 2025, 14, 307. https://doi.org/10.3390/jcm14020307
Koh Y-G, Nam J-H, Kim J-K, Suh D-S, Chung JH, Park KK, Kang K-T. Enhancing Surgical Efficiency and Radiological Outcomes Through Advances in Patient-Specific Instrument Design. Journal of Clinical Medicine. 2025; 14(2):307. https://doi.org/10.3390/jcm14020307
Chicago/Turabian StyleKoh, Yong-Gon, Ji-Hoon Nam, Jong-Keun Kim, Dong-Suk Suh, Jai Hyun Chung, Kwan Kyu Park, and Kyoung-Tak Kang. 2025. "Enhancing Surgical Efficiency and Radiological Outcomes Through Advances in Patient-Specific Instrument Design" Journal of Clinical Medicine 14, no. 2: 307. https://doi.org/10.3390/jcm14020307
APA StyleKoh, Y.-G., Nam, J.-H., Kim, J.-K., Suh, D.-S., Chung, J. H., Park, K. K., & Kang, K.-T. (2025). Enhancing Surgical Efficiency and Radiological Outcomes Through Advances in Patient-Specific Instrument Design. Journal of Clinical Medicine, 14(2), 307. https://doi.org/10.3390/jcm14020307






