Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review
Abstract
:1. Introduction
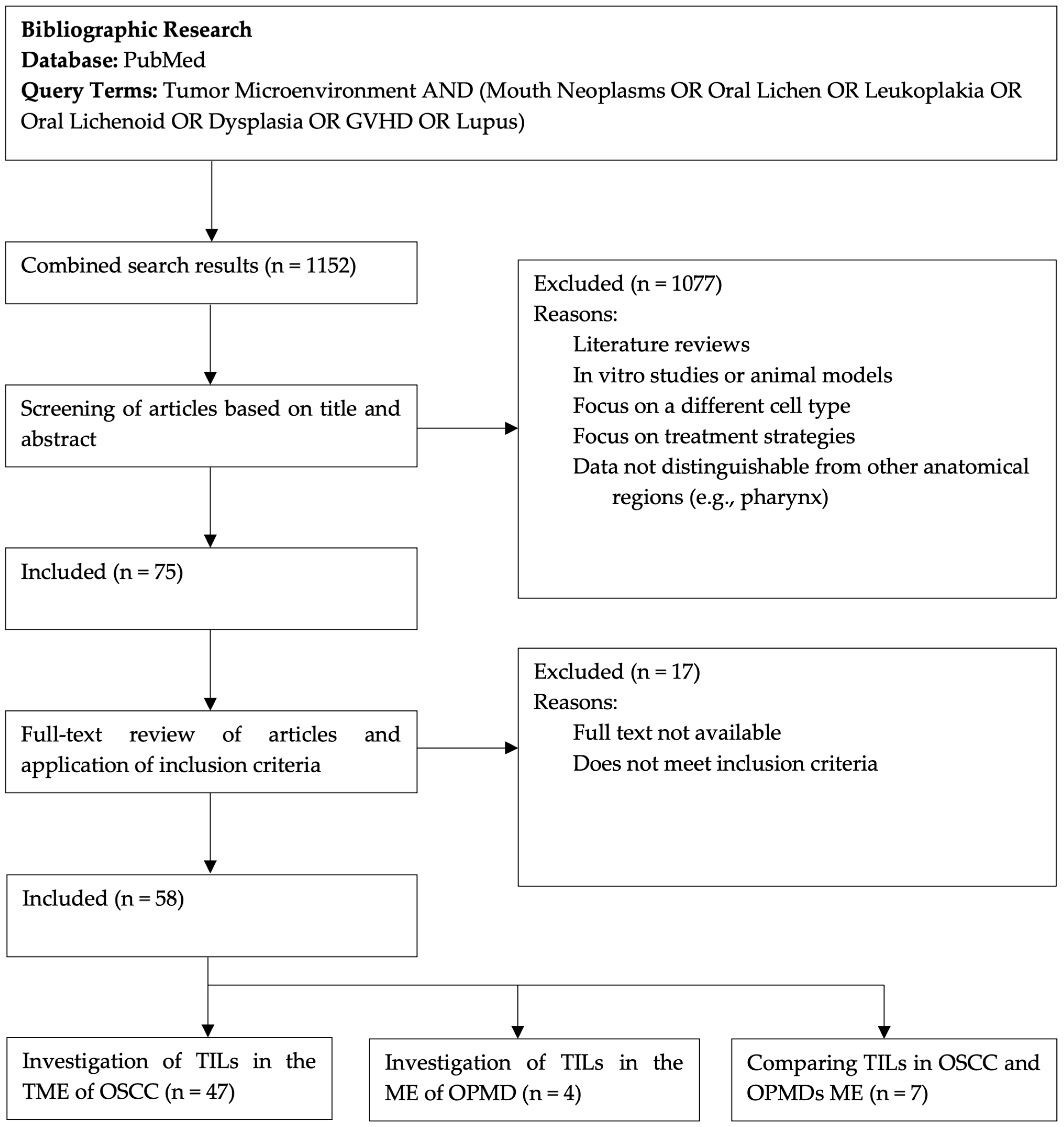
2. Materials and Methods
3. Results
4. Discussion
4.1. T Lymphocytes
4.2. B Lymphocytes
4.3. Natural Killer
4.4. TME Composition and Distribution of the Immune Cells
4.5. Mediators
4.6. Infiltrating Lymphocytes in OPMDs
4.6.1. T Lymphocytes
4.6.2. Natural Killer
4.7. Infiltrating Lymphocytes Recap
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral. Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Pentenero, M.; Sutera, S.; Lodi, G.; Bagan, J.V.; Farah, C.S. Oral medicine practice in Europe and Australia: Identifying practitioner characteristics and their clinical activity. Oral. Dis. 2021, 28, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Pentenero, M.; Sutera, S.; Lodi, G.; Bagan, J.V.; Farah, C.S. Oral leukoplakia diagnosis and treatment in Europe and Australia: Oral Medicine Practitioners’ attitudes and practice. Oral. Dis. 2022, 29, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.G.; de Lourdes Carvalho, A.; Miranda, C.S.S.; Cardoso, L.P.V. Potential Histopathological and Immune Biomarkers in Malignant and Non-Malignant Oral Lesions. J. Oral. Maxillofac. Res. 2022, 13, e3. [Google Scholar] [CrossRef]
- William, W.N., Jr.; Zhang, J.; Zhao, X.; Parra, E.R.; Uraoka, N.; Lin, H.Y.; Peng, S.A.; El-Naggar, A.K.; Rodriguez-Canales, J.; Song, J.; et al. Spatial PD-L1, immune-cell microenvironment, and genomic copy-number alteration patterns and drivers of invasive-disease transition in prospective oral precancer cohort. Cancer 2023, 129, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Hum, L.; Bethmann, D.; Feng, Z.; Chang, S.C.; Eckert, A.; Ballesteros-Merino, C.; Keschke, C.; Kappler, M.; Bifulco, C.B.; Wickenhauser, C.; et al. Cumulative suppressive index as a predictor of relapse free survival and overall survival in Human Papilloma Virus-negative oral squamous cell carcinomas with negative resection margins. Head. Neck 2021, 43, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Chepeha, D.B.; Bellile, E.; Nguyen, A.; Thomas, D.; McHugh, J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral. Oncol. 2015, 51, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Dayan, D.; Salo, T.; Salo, S.; Nyberg, P.; Nurmenniemi, S.; Costea, D.E.; Vered, M. Molecular crosstalk between cancer cells and tumor microenvironment components suggests potential targets for new therapeutic approaches in mobile tongue cancer. Cancer Med. 2012, 1, 128–140. [Google Scholar] [CrossRef]
- Boxberg, M.; Leising, L.; Steiger, K.; Jesinghaus, M.; Alkhamas, A.; Mielke, M.; Pfarr, N.; Götz, C.; Wolff, K.D.; Weichert, W.; et al. Composition and Clinical Impact of the Immunologic Tumor Microenvironment in Oral Squamous Cell Carcinoma. J. Immunol. 2019, 202, 278–291. [Google Scholar] [CrossRef]
- Gaafar, N.M.; Osman, T.A.; Ahmed, I.A.; Elsheikh, M.; Dongre, H.; Jacobsen, M.R.; Mohamed, N.G.; Fromreide, S.; Suleiman, A.M.; Johannessen, A.C.; et al. Characterization of immune cell infiltrate in tumor stroma and epithelial compartments in oral squamous cell carcinomas of Sudanese patients. Clin. Exp. Dent. Res. 2022, 8, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Boldrup, L.; Coates, P.J.; Fahraeus, R.; Wang, L.; Wilms, T.; Norberg-Spaak, L.; Sgaramella, N.; Nylander, K. High immune cytolytic activity in tumor-free tongue tissue confers better prognosis in patients with squamous cell carcinoma of the oral tongue. J. Pathol. Clin. Res. 2019, 5, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.E.; Strauss, L.; Wagner, E.M.; Gooding, W.; Whiteside, T.L. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin. Cancer Res. 2002, 8, 3137–3145. [Google Scholar] [PubMed]
- Gasparoto, T.H.; de Souza Malaspina, T.S.; Benevides, L.; de Melo, E.J., Jr.; Costa, M.R.; Damante, J.H.; Ikoma, M.R.; Garlet, G.P.; Cavassani, K.A.; da Silva, J.S.; et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol. Immunother. 2010, 59, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ranz, M.; Lequerica-Fernández, P.; Rodríguez-Santamarta, T.; Suárez-Sánchez, F.J.; López-Pintor, R.M.; García-Pedrero, J.M.; de Vicente, J.C. Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment. Front. Immunol. 2022, 13, 941351. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulos, K.; Sotiriou, S.; Collins, A.R.; Kartsidis, P.; Schmitt, A.C.; Chen, X.; Khazaie, K.; Hinni, M.L.; Ramsower, C.A.; Zarka, M.A.; et al. Transcriptomic and Immunophenotypic Characterization of Tumor Immune Microenvironment in Squamous Cell Carcinoma of the Oral Tongue. Head. Neck Pathol. 2021, 15, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Salama, A.M.; Valero, C.; Yuan, A.; Khimraj, A.; Saliba, M.; Zanoni, D.K.; Ganly, I.; Ghossein, R.; Patel, S.G.; et al. Histologic evaluation of host immune microenvironment and its prognostic significance in oral tongue squamous cell carcinoma: A comparative study on lymphocytic host response (LHR) and tumor infiltrating lymphocytes (TILs). Pathol. Res. Pract. 2021, 228, 153473. [Google Scholar] [CrossRef]
- Noda, Y.; Ishida, M.; Ueno, Y.; Fujisawa, T.; Iwai, H.; Tsuta, K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: A retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer 2022, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Khan, A.A.; Zaheer, S.; Ranga, S. Tumor-infiltrating lymphocytes in oral cavity squamous cell carcinoma and its association with clinicopathological parameters. Pathol. Res. Pract. 2023, 251, 154882. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Shan, Z.; Liu, Z.; Liu, S.; Yang, L.; Fang, X.; Li, K.; Wang, B.; Deng, Z.; Hu, Y.; et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer Immunol. Immunother. 2020, 69, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. Association of infiltrating cells with microvessel density in oral squamous cell carcinoma. Pol. J. Pathol. 2017, 68, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.-M.; Shoenfeld, Y.; Rojas-Villarraga, A.; Levy, R.A.; Cervera, R. (Eds.) Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Katou, F.; Ohtani, H.; Watanabe, Y.; Nakayama, T.; Yoshie, O.; Hashimoto, K. Differing phenotypes between intraepithelial and stromal lymphocytes in early-stage tongue cancer. Cancer Res. 2007, 67, 11195–11201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Zhai, Y.; Li, F.; Shi, X.; Ying, M. Single-Cell Profiling Reveals Heterogeneity of Primary and Lymph Node Metastatic Tumors and Immune Cell Populations and Discovers Important Prognostic Significance of CCDC43 in Oral Squamous Cell Carcinoma. Front. Immunol. 2022, 13, 843322. [Google Scholar] [CrossRef] [PubMed]
- Zancope, E.; Costa, N.L.; Junqueira-Kipnis, A.P.; Valadares, M.C.; Silva, T.A.; Leles, C.R.; Mendonça, E.F.; Batista, A.C. Differential infiltration of CD8+ and NK cells in lip and oral cavity squamous cell carcinoma. J. Oral. Pathol. Med. 2010, 39, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
- Soopanit, T.; Laokulrath, N.; Chayopasakul, V.; Pongsapich, W. Prognostic value and clinicopathological status of PD-L1 expression and CD8+ TILs in oral squamous cell cancer patients with or without traditional risk factors. Head. Neck 2023, 45, 1017–1025. [Google Scholar] [CrossRef]
- Fang, J.; Li, X.; Ma, D.; Liu, X.; Chen, Y.; Wang, Y.; Lui, V.W.Y.; Xia, J.; Cheng, B.; Wang, Z. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer 2017, 17, 375. [Google Scholar] [CrossRef]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. Immunohistochemical Analysis of Foxp3(+), CD4(+), CD8(+) Cell Infiltrates and PD-L1 in Oral Squamous Cell Carcinoma. Pathol. Oncol. Res. 2018, 24, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, N.M.; Osman, T.A.; Elsheikh, M.; Ahmed, I.A.; Dongre, H.; Fromreide, S.; Suleiman, A.M.; Johannessen, A.C.; Nginamau, E.S.; Costea, D.E. Epithelial PD-L1 expression at tumor front predicts overall survival in a cohort of oral squamous cell carcinomas from Sudan. Clin. Exp. Dent. Res. 2022, 8, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.R.; Chou, C.H.; Liu, C.J.; Lin, Y.C.; Tu, H.F.; Chang, K.W.; Lin, S.C. The Concordant Disruption of B7/CD28 Immune Regulators Predicts the Prognosis of Oral Carcinomas. Int. J. Mol. Sci. 2023, 24, 5931. [Google Scholar] [CrossRef] [PubMed]
- Daroonpan, P.; Ouchi, R.; Zhang, C.; Nagai, S.; Nishii, N.; Kashima, Y.; Tsushima, F.; Harada, H.; Hamagaki, M.; Ikeda, T.; et al. Personal immune profiles: Diversity and prognostic value for oral tongue squamous cell carcinoma evaluated by comprehensive immune parameter analyses with multiplex immunofluorescence. Oral. Oncol. 2023, 143, 106458. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Wermker, K.; Hallermann, C.; Pannier, F.; Hölzle, F.; Modabber, A. Immune checkpoint analysis in lip cancer. J. Craniomaxillofac Surg. 2021, 49, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sukhera, Z.A.; Zafar, N.; Ara, N.; Muneer, S.; Haroon, A.; Saeed, Z. Role Of Cd8+ Tumour-Infiltrating Lymphocytes In Predicting Regional Lymph Node Metastasis In Lip And Oral Cavity Squamous Cell Carcinoma. J. Ayub Med. Coll. Abbottabad 2023, 35, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wirsing, A.M.; Ervik, I.K.; Seppola, M.; Uhlin-Hansen, L.; Steigen, S.E.; Hadler-Olsen, E. Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod. Pathol. 2018, 31, 910–922. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Mao, L.; Yang, Q.C.; Fu, L.Q.; Wu, C.C.; Liu, B.; Sun, Z.J. CD103(+) T and Dendritic Cells Indicate a Favorable Prognosis in Oral Cancer. J. Dent. Res. 2019, 98, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Mattox, A.K.; Lee, J.; Westra, W.H.; Pierce, R.H.; Ghossein, R.; Faquin, W.C.; Diefenbach, T.J.; Morris, L.G.; Lin, D.T.; Wirth, L.J.; et al. PD-1 Expression in Head and Neck Squamous Cell Carcinomas Derives Primarily from Functionally Anergic CD4(+) TILs in the Presence of PD-L1(+) TAMs. Cancer Res. 2017, 77, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Han, N.; Mao, Y.; Li, Y. Increased levels of PD1 and glycolysis in CD4(+) T cells are positively associated with lymph node metastasis in OSCC. BMC Oral. Health 2023, 23, 356. [Google Scholar] [CrossRef]
- Fraga, M.; Yáñez, M.; Sherman, M.; Llerena, F.; Hernandez, M.; Nourdin, G.; Álvarez, F.; Urrizola, J.; Rivera, C.; Lamperti, L.; et al. Immunomodulation of T Helper Cells by Tumor Microenvironment in Oral Cancer Is Associated With CCR8 Expression and Rapid Membrane Vitamin D Signaling Pathway. Front. Immunol. 2021, 12, 643298. [Google Scholar] [CrossRef]
- Xie, P.; Wu, S.; Guo, L.; Ren, J.; Cai, K.; Zhou, M.; Liu, W.; Yang, S. Identification of Candidate Target Genes and Immune Cells in Oral Squamous Cell Carcinoma. Comput. Math. Methods Med. 2021, 2021, 5802110. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, T.M.M.; Monteiro, B.V.B.; Pereira, J.D.S.; Silva, L.A.B.; Nonaka, C.F.W.; Silveira É, J.D.D.; Miguel, M. Assessment of the presence of interleukin 17(+) macrophages and Th17 cells in situ in lip and oral tongue cancer. Hum. Immunol. 2021, 82, 945–949. [Google Scholar] [CrossRef]
- Amaral, M.G.D.; Sena, L.S.B.; Batista, A.C.; MendonÇa, E.F.; GordÓn-NÚÑez, M.A.; Alves, P.M.; Nonaka, C.F.W. FoxP3+ regulatory T cells in oral tongue squamous cell carcinoma in young and older patients. Braz. Oral. Res. 2020, 34, e096. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kubota, A.; Yokose, T.; Furukawa, M.; Matsushita, T.; Katsumata, N.; Oridate, N. Prognostic Role of Tumor-Infiltrating Lymphocytes and Tumor Budding in Early Oral Tongue Carcinoma. Laryngoscope 2021, 131, 2512–2518. [Google Scholar] [CrossRef]
- da Cunha, F.A.F.; Aguiar, M.C.; Souza, L.B.; Pinto, L.P.; Godoy, G.P.; Alves, P.M.; Nonaka, C.F. Immunohistochemical analysis of FoxP3+ regulatory T cells in lower lip squamous cell carcinomas. Braz. Oral. Res. 2016, 30, e130. [Google Scholar] [CrossRef]
- Kouketsu, A.; Sato, I.; Oikawa, M.; Shimizu, Y.; Saito, H.; Tashiro, K.; Yamashita, Y.; Takahashi, T.; Kumamoto, H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral. Maxillofac. Surg. 2019, 48, 1279–1288. [Google Scholar] [CrossRef]
- Surendran, S.; Aboelkheir, U.; Tu, A.A.; Magner, W.J.; Sigurdson, S.L.; Merzianu, M.; Hicks, W.L., Jr.; Suresh, A.; Kirkwood, K.L.; Kuriakose, M.A. T-Cell Infiltration and Immune Checkpoint Expression Increase in Oral Cavity Premalignant and Malignant Disorders. Biomedicines 2022, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, Y.X.; Lao, X.M.; Liang, Y.J.; Liao, G.Q. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral. Oncol. 2016, 53, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Bhan, A.K. A case for regulatory B cells. J. Immunol. 2006, 176, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lu, Y.; Wang, W.; Xie, N.; Yi, C.; Xiong, G.; Xu, X.; Zhang, M.; Wang, C. Prognostic value of tumor-infiltrating immune cells in clinical early-stage oral squamous cell carcinoma. J. Oral. Pathol. Med. 2023, 52, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Huang, Z.; Wei, C.; Wang, Y.; Xiao, H.; Chen, S.; Huang, Z. CD79A work as a potential target for the prognosis of patients with OSCC: Analysis of immune cell infiltration in oral squamous cell carcinoma based on the CIBERSORTx deconvolution algorithm. BMC Oral. Health 2023, 23, 411. [Google Scholar] [CrossRef]
- Bag, S.; Oetjen, J.; Shaikh, S.; Chaudhary, A.; Arun, P.; Mukherjee, G. Impact of spatial metabolomics on immune-microenvironment in oral cancer prognosis: A clinical report. Mol. Cell Biochem. 2024, 479, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Smitha, T.; Rao, K.; Priya, N.S.; Sheethal, H.S.; Chitra, S. Expression of CD 20 B-Lymphocyte in oral epithelial dysplasia and oral squamous cell carcinoma: A comparative immunohistochemistry study. J. Oral. Maxillofac. Pathol. 2023, 27, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Banerjee, A.; Saikia, N.; Phookan, J.; Baruah, M.N.; Baruah, S. Negative regulation of natural killer cell in tumor tissue and peripheral blood of oral squamous cell carcinoma. Cytokine 2015, 76, 123–130. [Google Scholar] [CrossRef]
- Heikkinen, I.; Bello, I.O.; Wahab, A.; Hagström, J.; Haglund, C.; Coletta, R.D.; Nieminen, P.; Mäkitie, A.A.; Salo, T.; Leivo, I.; et al. Assessment of Tumor-infiltrating Lymphocytes Predicts the Behavior of Early-stage Oral Tongue Cancer. Am. J. Surg. Pathol. 2019, 43, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Bag, S.; Chakraborty, P.; Dey, D.; Roy, S.; Jain, P.; Roy, P.; Soong, R.; Majumder, P.P.; Dutt, S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLoS ONE 2020, 15, e0242058. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, Y.G. Regulation of TGF-β receptor activity. Cell Biosci. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- MaruYama, T.; Chen, W.; Shibata, H. TGF-β and Cancer Immunotherapy. Biol. Pharm. Bull. 2022, 45, 155–161. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, P.C.; Vieira É, L.M.; Sousa, A.A.; Teixeira, A.L.; Aguiar, M.C.F. Immunophenotype of neutrophils in oral squamous cell carcinoma patients. J. Oral. Pathol. Med. 2017, 46, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.S.; Corrigan, A.E.; Dadah, H.; George, A.M.; Keshwara, S.M.; Sideris, M.; Szabados, B. Cytokine-based Cancer Immunotherapy: Challenges and Opportunities for IL-10. Anticancer. Res. 2021, 41, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Niklander, S.E. Inflammatory Mediators in Oral Cancer: Pathogenic Mechanisms and Diagnostic Potential. Front. Oral. Health 2021, 2, 642238. [Google Scholar] [CrossRef] [PubMed]
- Caponio, V.C.A.; Zhurakivska, K.; Lo Muzio, L.; Troiano, G.; Cirillo, N. The Immune Cells in the Development of Oral Squamous Cell Carcinoma. Cancers 2023, 15, 3779. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Agag, M.; Smaga, M.; Vaishnaw, Y.; Idrees, M.; Shearston, K.; Farah, C.S. PD-1/PD-L1, Treg-related proteins, and tumour-infiltrating lymphocytes are associated with the development of oral squamous cell carcinoma. Pathology 2022, 54, 409–416. [Google Scholar] [CrossRef]
- Flores-Hidalgo, A.; Phero, J.; Steward-Tharp, S.; Williamson, M.; Paquette, D.; Krishnan, D.; Padilla, R. Immunophenotypic and Gene Expression Analyses of the Inflammatory Microenvironment in High-Grade Oral Epithelial Dysplasia and Oral Lichen Planus. Head. Neck Pathol. 2024, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.P.; Lee, B.K.B.; Lau, S.H.; Kallarakkal, T.G.; Zaini, Z.M.; Lye, B.K.W.; Zain, R.B.; Sathasivam, H.P.; Yeong, J.P.S.; Savelyeva, N.; et al. Transcriptional analysis highlights three distinct immune profiles of high-risk oral epithelial dysplasia. Front. Immunol. 2022, 13, 954567. [Google Scholar] [CrossRef] [PubMed]
- Gasparoto, T.H.; de Souza Malaspina, T.S.; Damante, J.H.; de Mello, E.F., Jr.; Ikoma, M.R.; Garlet, G.P.; Costa, M.R.; Cavassani, K.A.; da Silva, J.S.; Campanelli, A.P. Regulatory T cells in the actinic cheilitis. J. Oral. Pathol. Med. 2014, 43, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Study design | Original research and clinical observational studies. | Case reports, editorials, letters, and reviews |
| Population | Human in vivo research focusing on OSCC or OPMDs. | In vitro research, animal model research, and studies that include pharyngeal diseases where data cannot be separated from the oral cavity. |
| Language | Publications in English. | Publications not in English or not available in full text. |
| Relevance | Studies that directly address the role of TILs in the context of OSCC or OPMDs. | Studies that do not provide specific data on TILs or do not meet the research question’s criteria. |
| Lymphocyte Subtype | Primary Activities | Prognostic Implication |
|---|---|---|
| CD8+ T Cells | Cytotoxic activity, killing tumor cells. Phenotypically activated in the stroma, with an active immune response. | Positive. High concentration linked to better prognosis and outcomes (longer survival time). |
| CD8+ Tissue-Resident Memory T Cells (Trm) | Cytotoxic activity (targeting tumor cells) | Positive. High concentration linked to better prognosis. |
| CD4+ Th1 Cells | Tumor-specific adaptive immunity. Pro-inflammatory cytokine profile. Promotes CD8+ T-cell activity | Positive. High concentration linked to better prognosis. |
| CD4+ Central Memory Cells | Contribute to adaptive immunity | Positive. High concentration linked to better prognosis. |
| CD4+ Th2 Cells | Anti-inflammatory cytokine profile. Supports humoral immunity | Negative. High concentration linked to poorer prognosis. |
| CD4+ Th17 Cells | High plasticity; can enhance immune cell recruitment or tumor growth. Can differentiate into Tregs or Th1 Cells. | Depends on TME composition and tumor site. Effects can be both protumor and antitumor depending on context. |
| CD4+ Regulatory T (Tregs) Cells | Immunosuppressive, inhibits CD8+ T Cells and NK Cells. | Negative. High concentration linked to worse outcomes (decreased overall survival). |
| NKT Cells | Antitumor immune responses. | Positive. High concentration linked to better prognosis. |
| CD20+ B Cells | Antibody production as part of the adaptive humoral immune response. | Positive. High concentration linked to better prognosis and outcomes (longer survival time). |
| Regulatory B (Bregs) Cells | Immunosuppressive, supports Tregs and suppresses T-cells. | Negative. High concentration linked to poorer prognosis (decreased overall survival, advanced stages, higher recurrence). |
| NK Cells | Innate immune system, direct cytotoxicity against tumor cells. | Positive. High concentration linked to better prognosis (longer survival time). |
| Lymphocyte Subtype | Primary Activities | Prognostic Implication |
|---|---|---|
| CD8+ T Cells | Cytotoxic activity, active immune response. Phenotypically activated in mucosa and submucosa. | Positive. High concentration may indicate immune surveillance and delayed transformation. |
| CD4+ Tregs Cells | Immunosuppressive. Down-regulate autoreactive T cells. | Negative. High concentration linked to postoperative recurrence and progression to OSCC. |
| NK Cells | Innate immune system, direct cytotoxicity. Phenotypically activated in mucosa and submucosa. | Positive. High concentration may indicate immune surveillance and delayed transformation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutera, S.; Furchì, O.A.; Pentenero, M. Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review. J. Clin. Med. 2025, 14, 606. https://doi.org/10.3390/jcm14020606
Sutera S, Furchì OA, Pentenero M. Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review. Journal of Clinical Medicine. 2025; 14(2):606. https://doi.org/10.3390/jcm14020606
Chicago/Turabian StyleSutera, Samuele, Olga Anna Furchì, and Monica Pentenero. 2025. "Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review" Journal of Clinical Medicine 14, no. 2: 606. https://doi.org/10.3390/jcm14020606
APA StyleSutera, S., Furchì, O. A., & Pentenero, M. (2025). Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review. Journal of Clinical Medicine, 14(2), 606. https://doi.org/10.3390/jcm14020606







