Diagnostic Cut-Off Values Based on Lipid Layer Pattern for Dry Eye Disease Subtypes Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Study Design and Diagnostic Criteria
- -
- ADDE subtype: TMH ≤ 0.16 mm and MGLA < 50%.
- -
- EDE subtype: TMH > 0.16 mm and MGLA ≥ 50%.
- -
- MDE subtype: TMH ≤ 0.16 mm and MGLA ≥ 50%.
2.3. Evaluation Procedures
2.3.1. Symptomatology Assessment
2.3.2. Tear Film Osmolarity
2.3.3. Fluorescein Break-Up Time
2.3.4. Corneal Staining
2.3.5. Tear Meniscus Height
2.3.6. Meibomian Gland Loss Area
2.3.7. Lipid Layer Pattern
- -
- First, following the basic Lipid Layer Pattern Guillon’s (LLP-G) scheme in five steps (Open Meshwork, Closed Meshwork, Wave, Amorphous, or Colour) with the intermediate of each as inter-categories [26]. A grade from 1 to 5, with middle steps, was assigned to analyze thickness from thinnest to thickest.
- -
- Secondly, LLP images were classified in four steps following the Lipid Layer Pattern Colour (LLP-C) characteristics scheme (Grey, White, Yellow, Brown or higher) and the intermediate mixtures of colours as inter-categories [27]. A grade from 1 to 4, with middle steps, was assigned to analyze thickness from thinnest to thickest.
2.3.8. Statistical Analysis
3. Results
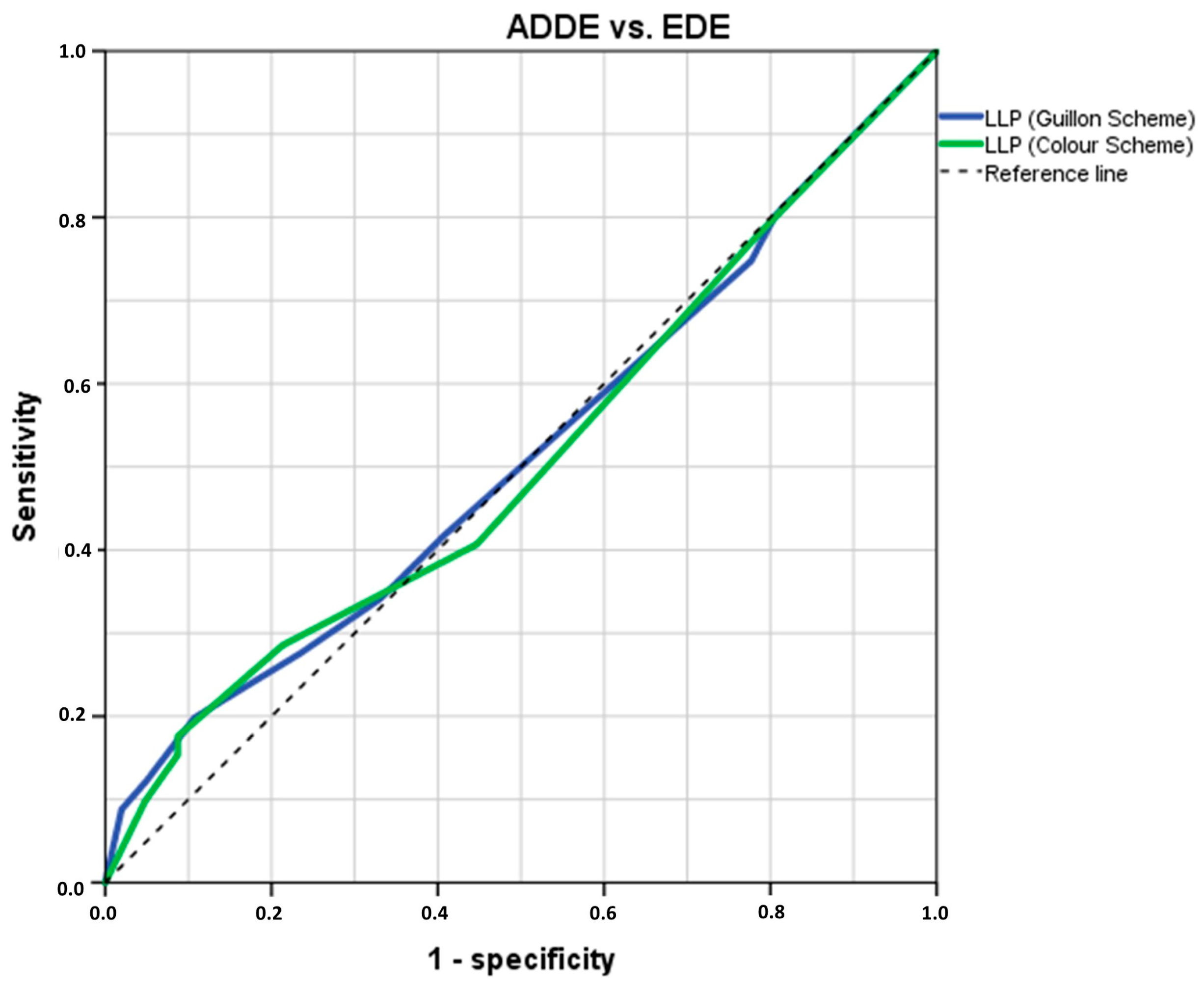
3.1. Analysis of LLPs Cut-Off Threshold Values to Differentiate ADDE from EDE Participants
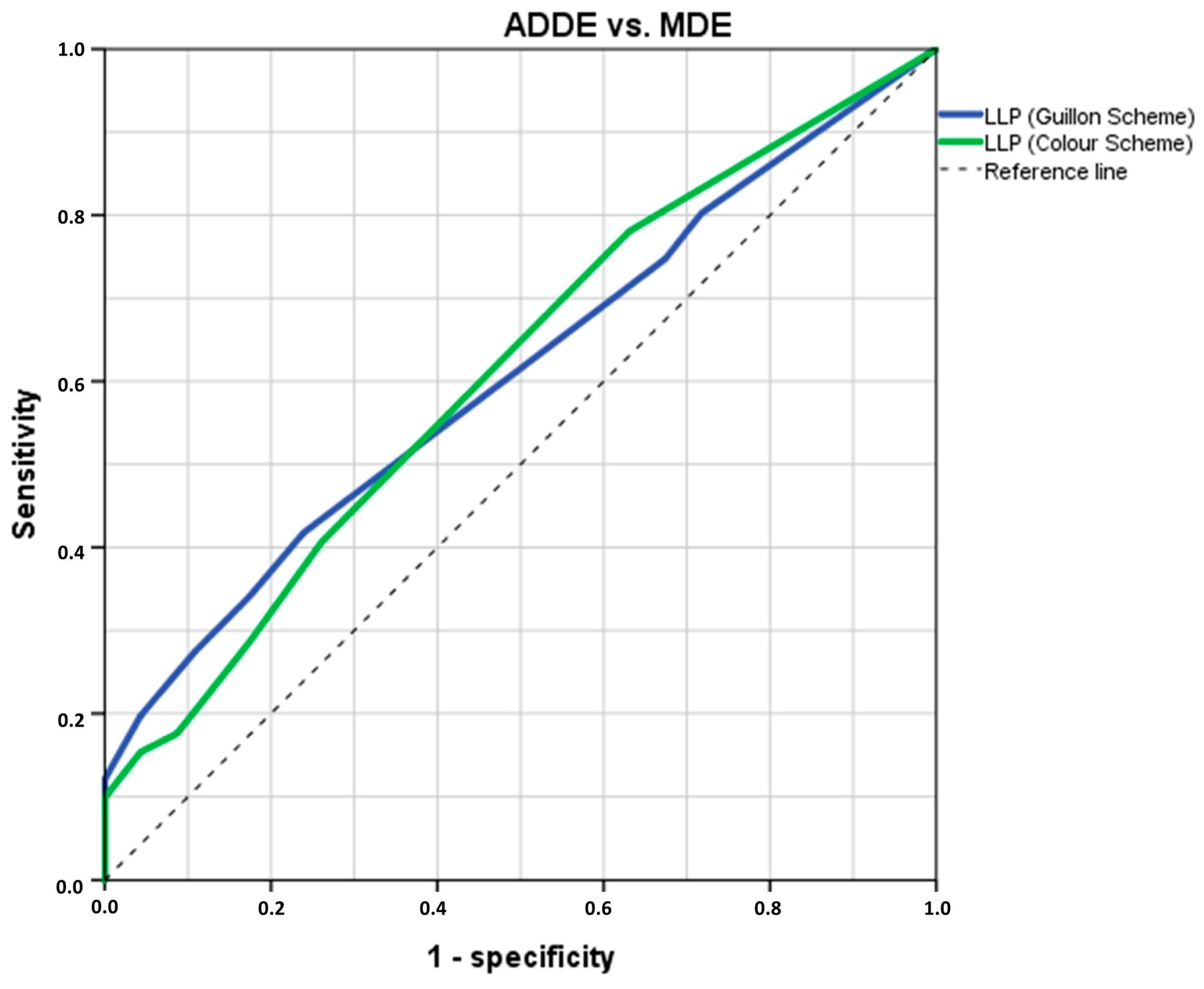
3.2. Analysis of LLPs Cut-Off Threshold Values to Differentiate ADDE from MDE Participants
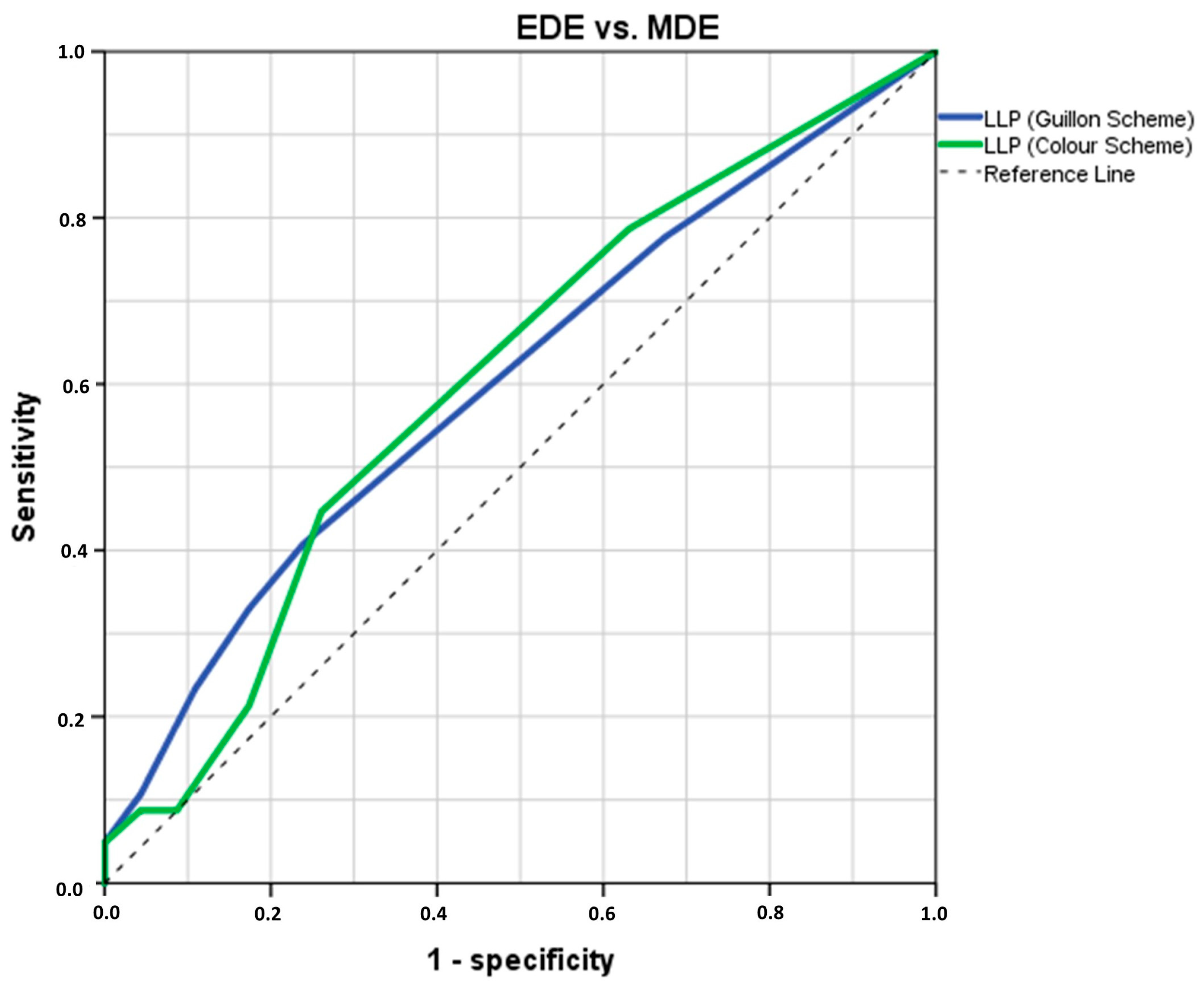
3.3. Analysis of LLPs Cut-Off Threshold Values to Differentiate EDE from MDE Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Cutrupi, F.; De Luca, A.; Di Zazzo, A.; Micera, A.; Coassin, M.; Bonini, S. Real Life Impact of Dry Eye Disease. Semin. Ophthalmol. 2023, 38, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.P. Abnormal lipid layers. Observation, differential diagnosis, and classification. Adv. Exp. Med. Biol. 1998, 438, 309–313. [Google Scholar] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46 (Suppl. S1), S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope. Life 2022, 12, 2007. [Google Scholar] [CrossRef]
- Fineide, F.; Arita, R.; Utheim, T.P. The role of meibography in ocular surface diagnostics: A review. Ocul. Surf. 2021, 19, 133–144. [Google Scholar] [CrossRef]
- Garcia-Resua, C.; Pena-Verdeal, H.; Minones, M.; Giraldez, M.J.; Yebra-Pimentel, E. Interobserver and intraobserver repeatability of lipid layer pattern evaluation by two experienced observers. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2014, 37, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.P. Use of the Tearscope Plus and attachments in the routine examination of the marginal dry eye contact lens patient. Adv. Exp. Med. Biol. 1998, 438, 859–867. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. A cross-sectional study of non-modifiable and modifiable risk factors of dry eye disease states. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2023, 46, 101800. [Google Scholar] [CrossRef]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Longitudinal analysis of variation in status and diagnostic stability of untreated dry eye disease. Ocul. Surf. 2023, 29, 298–300. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal clinically important difference for the ocular surface disease index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Markoulli, M.; Flanagan, J.; Papas, E. The validity of point of care tear film osmometers in the diagnosis of dry eye. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2022, 42, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Xie, W.; Ying, J.; Yao, Y. Evaluation of tear film and meibomian gland function in dry eye patients using Keratograph 5M. Zhejiang Da Xue Xue Bao. Yi Xue Ban J. Zhejiang University. Med. Sci. 2016, 45, 422–428. [Google Scholar] [CrossRef]
- Itokawa, T.; Suzuki, T.; Koh, S.; Hori, Y. Evaluating the Differences Between Fluorescein Tear Break-up Time and Noninvasive Measurement Techniques. Eye Contact Lens 2023, 49, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Le, Q. Analysis of the first tear film break-up point in Sjogren’s syndrome and non-Sjogren’s syndrome dry eye patients. BMC Ophthalmol. 2022, 22, 1. [Google Scholar] [CrossRef]
- Inferrera, L.; Aragona, E.; Wylegala, A.; Valastro, A.; Latino, G.; Postorino, E.I.; Gargano, R.; Orzechowska-Wylegala, B.; Wylegala, E.; Roszkowska, A.M. The Role of Hi-Tech Devices in Assessment of Corneal Healing in Patients with Neurotrophic Keratopathy. J. Clin. Med. 2022, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Lam, A.K.; Tai, S.K.; Chan, J.K.; Ng, R.W. Lower Tear Meniscus Height Measurements Using Keratography and Swept-Source Optical Coherence Tomography and Effect of Fluorescein Instillation Methods. Curr. Eye Res. 2019, 44, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Resua, C.; Santodomingo-Rubido, J.; Lira, M.; Giraldez, M.J.; Vilar, E.Y. Clinical assessment of the lower tear meniscus height. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2009, 29, 487–496. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B. Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2013, 36, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.P. Non-invasive Tearscope Plus routine for contact lens fitting. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 1998, 21 (Suppl. S1), S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Baron, D.F.; Herman, J.P.; Finnemore, V.M.; Exford, J.M.; Hermosa, J.L.; Leahy, C.D.; Glonek, T.; Greiner, J.V. Tear film lipid layer thickness as a function of blinking. Cornea 1994, 13, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Davies, L.N.; Dunne, M.C.; Gilmartin, B. Statistical guidelines for clinical studies of human vision. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2011, 31, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Recommendations for analysis of repeated-measures designs: Testing and correcting for sphericity and use of manova and mixed model analysis. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2017, 37, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- de Monchy, I.; Gendron, G.; Miceli, C.; Pogorzalek, N.; Mariette, X.; Labetoulle, M. Combination of the Schirmer I and phenol red thread tests as a rescue strategy for diagnosis of ocular dryness associated with Sjogren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5167–5173. [Google Scholar] [CrossRef] [PubMed]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Niedernolte, B.; Trunk, L.; Wolffsohn, J.S.; Pult, H.; Bandlitz, S. Evaluation of tear meniscus height using different clinical methods. Clin. Exp. Optom. 2021, 104, 583–588. [Google Scholar] [CrossRef]
- Asiedu, K.; Dzasimatu, S.K.; Kyei, S. Clinical subtypes of dry eye in youthful clinical sample in Ghana. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2019, 42, 206–211. [Google Scholar] [CrossRef]
- Ji, Y.W.; Seong, H.; Seo, J.G.; Park, S.Y.; Alotaibi, M.; Choi, M.; Nam, S.; Kim, T.I.; Lee, H.K.; Seo, K.Y. Evaluation of dry eye subtypes and characteristics using conventional assessments and dynamic tear interferometry. Br. J. Ophthalmol. 2022, 106, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.B.; Fan, N.W.; Lin, P.Y. Value of lipid layer thickness and blinking pattern in approaching patients with dry eye symptoms. Can. J. Ophthalmol. J. Can. D’ophtalmologie 2019, 54, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Lee, J.S.; Kang, S.Y.; Kim, H.M.; Song, J.S. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am. J. Ophthalmol. 2013, 155, 1104–1110.e1102. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Morishige, N.; Fujii, T.; Fukuoka, S.; Chung, J.L.; Seo, K.Y.; Itoh, K. Tear Interferometric Patterns Reflect Clinical Tear Dynamics in Dry Eye Patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3928–3934. [Google Scholar] [CrossRef]
- Remeseiro, B.; Bolon-Canedo, V.; Peteiro-Barral, D.; Alonso-Betanzos, A.; Guijarro-Berdinas, B.; Mosquera, A.; Penedo, M.G.; Sanchez-Marono, N. A methodology for improving tear film lipid layer classification. IEEE J. Biomed. Health Inform. 2014, 18, 1485–1493. [Google Scholar] [CrossRef]
- Rolando, M.; Merayo-Lloves, J. Management Strategies for Evaporative Dry Eye Disease and Future Perspective. Curr. Eye Res. 2022, 47, 813–823. [Google Scholar] [CrossRef]

| Age (Years) * | OSDI (Score) ** | Osmolarity (mOsm/L) * | FBUT (s) ** | Corneal Staining (Oxford Scheme) ** | TMH (mm) * | MGLA ** | LLP-G ** | LLP-C ** | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n = 240) | Mean/ Median | 48.3 | 26.04 | 322.38 | 5.27 | 1.00 | 0.186 | 53.92 | Closed Meshwork | Grey/White |
| SD/IQR | 16.52 | 20.12–37.50 | 18.55 | 3.43–8.15 | 0.00–2.00 | 0.098 | 41.48–59.67 | Open Meshwork/Closed Meshwork—Wave | Grey/White—White | |
| Minimum | 19.0 | 13.36 | 282.00 | 1.29 | 0.00 | 0.060 | 10.24 | Open Meshwork | Grey | |
| Maximum | 81.0 | 83.33 | 400.00 | 65.13 | 4.00 | 0.640 | 82.22 | Colour | Brown or higher |
| Age (Years) * | OSDI (Score) ** | Osmolarity (mOsm/l) * | FBUT (s) ** | Corneal Staining (Oxford Scheme) ** | TMH (mm) * | MGLA ** | LLP-G ** | LLP-C ** | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ADDE (n = 91) | Mean/ Median | 44.54 | 25.00 | 321.47 | 5.29 | 1.00 | 0.128 | 38.47 | Closed Meshwork | Grey/White |
| SD/IRQ | 17.3 | 18.75–34.09 | 17.60 | 3.42–8.85 | 0.00–2.00 | 0.021 | 28.22–43.51 | Open Meshwork/Closed Meshwork—Wave/Amorphous | Grey/White—White/Yellow | |
| Minimum | 19.0 | 13.36 | 292.00 | 1.33 | 0.00 | 0.080 | 10.24 | Open Meshwork | Grey | |
| Maximum | 71.0 | 81.25 | 400.00 | 21.83 | 4.00 | 0.160 | 48.78 | Colour | Brown or higher | |
| EDE (n = 103) | Mean/ Median | 52.7 | 25.0 | 322.56 | 4.79 | 1.00 | 0.260 | 57.02 | Closed Meshwork | Grey/White |
| SD/IRQ | 15.5 | 20.0–36.36 | 19.65 | 3.38–7.38 | 0.00–2.00 | 0.110 | 54.18–62.90 | Closed Meshwork—Wave | Grey/White—White | |
| Minimum | 20.0 | 13.36 | 282.00 | 1.29 | 0.00 | 0.160 | 50.38 | Open Meshwork | Grey | |
| Maximum | 81.0 | 83.33 | 400.00 | 65.13 | 4.00 | 0.640 | 79.56 | Colour | Brown or higher | |
| MDE (n = 46) | Mean/ Median | 46.0 | 35.42 | 323.78 | 5.77 | 1.00 | 0.129 | 59.16 | Closed Meshwork | Grey/White |
| SD/IRQ | 15.1 | 24.43–43.23 | 18.15 | 4.02–9.67 | 0.00–2.00 | 0.022 | 55.70–65.01 | Open Meshwork—Closed Meshwork | Grey-White | |
| Minimum | 20.0 | 13.50 | 284.00 | 1.75 | 0.00 | 0.060 | 50.38 | Open Meshwork | Grey | |
| Maximum | 70.0 | 75.00 | 373.00 | 23.13 | 4.00 | 0.160 | 82.22 | Amorphous | Yellow/Brown or higher | |
| p | 0.001 ‡ | 0.001 † | 0.784 ‡ | 0.190 † | 0.655 † | <0.001 ‡ | <0.001 † | 0.066 † | 0.059 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabucedo-Villamarin, B.; Garcia-Queiruga, J.; Pena-Verdeal, H.; Garcia-Resua, C.; Yebra-Pimentel, E.; Giraldez, M.J. Diagnostic Cut-Off Values Based on Lipid Layer Pattern for Dry Eye Disease Subtypes Assessment. J. Clin. Med. 2025, 14, 623. https://doi.org/10.3390/jcm14020623
Sabucedo-Villamarin B, Garcia-Queiruga J, Pena-Verdeal H, Garcia-Resua C, Yebra-Pimentel E, Giraldez MJ. Diagnostic Cut-Off Values Based on Lipid Layer Pattern for Dry Eye Disease Subtypes Assessment. Journal of Clinical Medicine. 2025; 14(2):623. https://doi.org/10.3390/jcm14020623
Chicago/Turabian StyleSabucedo-Villamarin, Belen, Jacobo Garcia-Queiruga, Hugo Pena-Verdeal, Carlos Garcia-Resua, Eva Yebra-Pimentel, and Maria J. Giraldez. 2025. "Diagnostic Cut-Off Values Based on Lipid Layer Pattern for Dry Eye Disease Subtypes Assessment" Journal of Clinical Medicine 14, no. 2: 623. https://doi.org/10.3390/jcm14020623
APA StyleSabucedo-Villamarin, B., Garcia-Queiruga, J., Pena-Verdeal, H., Garcia-Resua, C., Yebra-Pimentel, E., & Giraldez, M. J. (2025). Diagnostic Cut-Off Values Based on Lipid Layer Pattern for Dry Eye Disease Subtypes Assessment. Journal of Clinical Medicine, 14(2), 623. https://doi.org/10.3390/jcm14020623










