Sleep Bruxism and Hypobaric Hypoxia Exposure: Exploring the Physiological Association
Abstract
1. Introduction
2. Materials and Methods
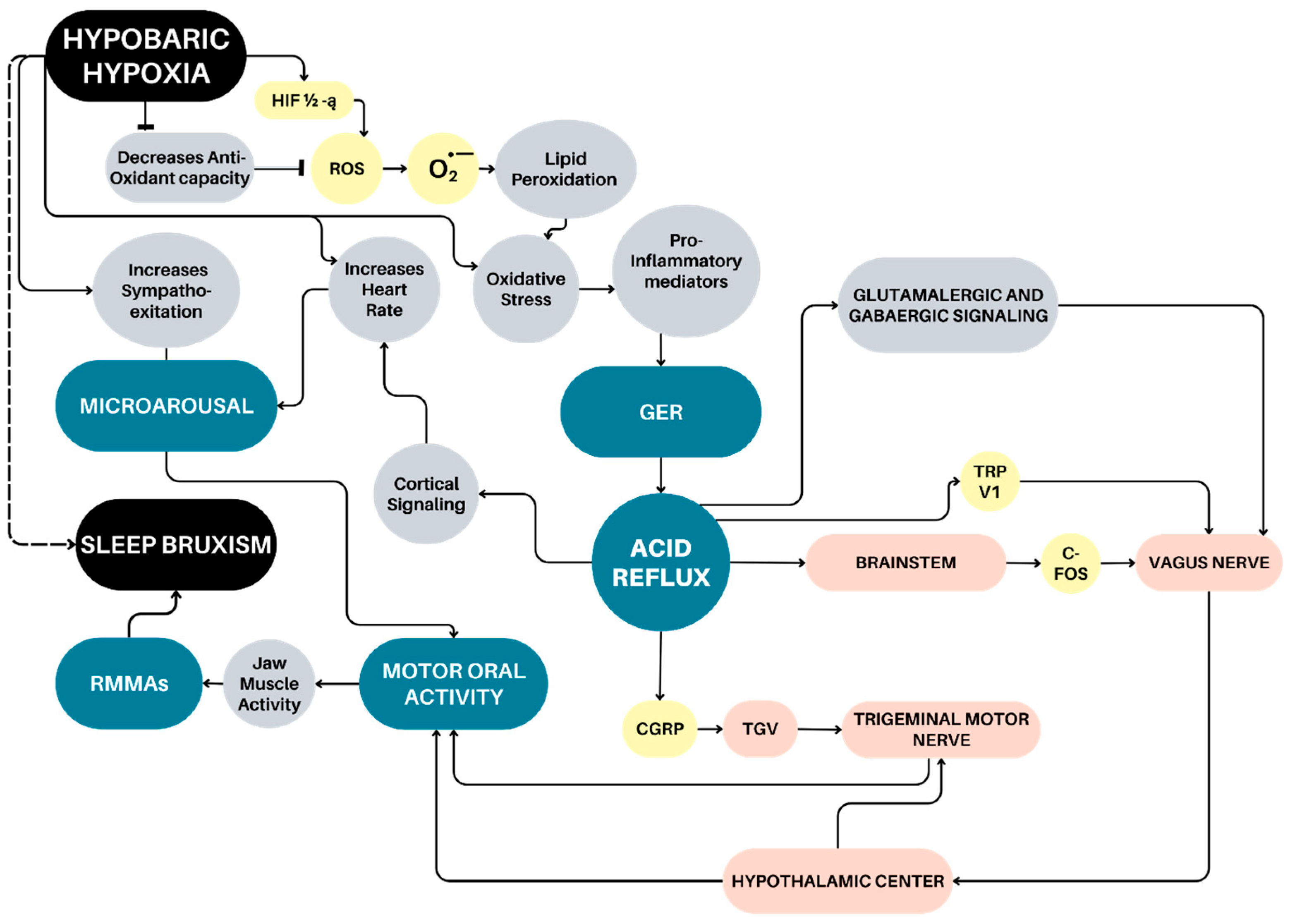
3. Sleep Bruxism Mechanism Associated with Gastroesophageal Reflux
4. Hypobaric Hypoxia and Gastroesophageal Reflux
5. Relationships Among Obstructive Sleep Breathing Disorders, Sleep Arousal and Sleep Bruxism
6. Microarousal-Induced Sleep Bruxism and Hypobaric Hypoxia Exposure
7. Conclusions
Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SB | Sleep bruxism |
| OSA | Obstructive Sleep Apnea |
| CIHH | Chronic Intermittent Hypobaric Hypoxia |
| PaO2 | Partial Pressure of Oxygen |
| TG | Tooth Grinding |
| GER | Gastroesophageal Reflux |
| LES | Lower Esophageal Sphincter |
| RMMA | Rhythmic Masticatory Muscle Activity |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| NTS | Nucleus Tractus Solitarius |
| MotN | The trigeminal motor Nucleus |
| TGV | Trigeminal Nerve |
| c-FOS | Cellular-Fos |
| CGPR | Calcitonin Gene-Related Peptide |
| HIF-1α | Hypoxia-inducible factors |
| NA | Noradrenaline |
| UAO | Upper Airway Obstruction |
| ROS | Reactive Oxygen Species |
| OSBD | Obstructive Sleep Breathing Disorders |
| OSA | Obstructive Sleep Apnea Syndrome |
| EEG | Electroencephalogram |
| REM | Rapid Eye Movement |
| EMG | Electromyography |
| CAP | Cyclic Alternating Pattern |
| AOPP | Advanced Protein Products |
| TBARS | Thiobarbituric Acid-Reacting Substances |
| TAC | Total Antioxidant Capacity |
| cBRS | Reduced Cardiac Baroreflex Sensitivity |
References
- Verhoeff, M.C.; Lobbezoo, F.; Ahlberg, J.; Bender, S.; Bracci, A.; Colonna, A.; Dal Fabbro, C.; Durham, J.; Glaros, A.G.; Häggman-Henrikson, B.; et al. Updating the Bruxism Definitions: Report of an International Consensus Meeting. J. Oral Rehabil. 2025, 52, 1335–1342. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Lavigne, G.J.; Svensson, P.; Lobbezoo, F. Five years after the 2018 consensus definitions of sleep and awake bruxism: An explanatory note. J. Oral Rehabil. 2024, 51, 623–624. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- Svensson, P.; Lavigne, G. Clinical bruxism semantics beyond academic debates: Normo- and patho-bruxism as a new proposal. J. Oral Rehabil. 2020, 47, 547–548. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic basis of sleep bruxism and sleep apnea-response to a medical puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Lobbezoo, F. Bruxism definition: Past, present, and future—What should a prosthodontist know? J. Prosthet. Dent. 2022, 128, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Campello, C.P.; Moraes, S.L.D.; Vasconcelos, B.C.D.E.; Lima, E.L.S.; Pellizzer, E.P.; Lemos, C.A.A.; Muniz, M.T.C. Polymorphisms of the serotonin receptors genes in patients with bruxism: A systematic review. J. Appl. Oral Sci. 2022, 29, e20210262. [Google Scholar] [CrossRef] [PubMed]
- Smardz, J.; Martynowicz, H.; Wojakowska, A.; Wezgowiec, J.; Danel, D.; Mazur, G.; Wieckiewicz, M. Lower serotonin levels in severe sleep bruxism and its association with sleep, heart rate, and body mass index. J. Oral Rehabil. 2022, 49, 422–429. [Google Scholar] [CrossRef]
- Doblado, N.G.; Barrera Mora, J.M.; Dorado, F.P.; Fernández, J.C.R.; Ordeix, G.B.; Escalona, E.E. Relationship Between Bruxism and Obstructive Sleep Apnea: A Systematic Review of the Literature. J. Clin. Med. 2025, 14, 5013. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Siques, P.; López, R.; Romero, R.; León-Velarde, F.; Flores, K.; Lüneburg, N.; Hannemann, J.; Böger, R.H. Long-Term Intermittent Work at High Altitude: Right Heart Functional and Morphological Status and Associated Cardiometabolic Factors. Front. Physiol. 2018, 9, 248. [Google Scholar] [CrossRef]
- Siques, P.; Brito, J.; Flores, K.; Ordenes, S.; Arriaza, K.; Pena, E.; León-Velarde, F.; López de Pablo, Á.L.; Gonzalez, M.C.; Arribas, S. Long-Term Chronic Intermittent Hypobaric Hypoxia Induces Glucose Transporter (GLUT4) Translocation Through AMP-Activated Protein Kinase (AMPK) in the Soleus Muscle in Lean Rats. Front. Physiol. 2018, 9, 799. [Google Scholar] [CrossRef]
- Pena, E.; San Martin-Salamanca, R.; El Alam, S.; Flores, K.; Arriaza, K. Tau Protein Alterations Induced by Hypobaric Hypoxia Exposure. Int. J. Mol. Sci. 2024, 25, 889. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.S.; Sils, I.V.; Staab, J.E.; Fulco, C.S.; Muza, S.R.; Beidleman, B.A. Acute mountain sickness and sleep disturbances differentially influence cognition and mood during rapid ascent to 3000 and 4050 m. Physiol. Rep. 2022, 10, e15175. [Google Scholar] [CrossRef]
- Burtscher, J.; Niedermeier, M.; Hüfner, K.; van den Burg, E.; Kopp, M.; Stoop, R.; Burtscher, M.; Gatterer, H.; Millet, G.P. The interplay of hypoxic and mental stress: Implications for anxiety and depressive disorders. Neurosci. Biobehav. Rev. 2022, 138, 104718. [Google Scholar] [CrossRef]
- Calderon-Jofre, R.; Moraga, D.; Moraga, F.A. The Effect of Chronic Intermittent Hypobaric Hypoxia on Sleep Quality and Melatonin Serum Levels in Chilean Miners. Front. Physiol. 2022, 12, 809360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dunbar, K.B.; Odze, R.D.; Agoston, A.T.; Wang, X.; Su, T.; Nguyen, A.D.; Zhang, X.; Spechler, S.J.; Souza, R.F. Hypoxia-inducible factor-1α mediates reflux-induced epithelial-mesenchymal plasticity in Barrett’s oesophagus patients. Gut 2024, 73, 1269–1279. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Suzuki, Y.; Ozawa, A.; Shibagaki, A.; Okura, K.; Matsuka, Y. Associations between sleep bruxism, oral wetness, and salivary flow: A quantitative analysis. J. Prosthodont. Res. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Azer, S.A.; Goosenberg, E. Gastroesophageal Reflux Disease (GERD), 1st ed.; StatPearls Publishing: Treasure Island, FL, USA, 2025; p. 45. [Google Scholar]
- Ohmure, H.; Kanematsu-Hashimoto, K.; Nagayama, K.; Taguchi, H.; Ido, A.; Tominaga, K.; Arakawa, T.; Miyawaki, S. Evaluation of a Proton Pump Inhibitor for Sleep Bruxism: A Randomized Clinical Trial. J. Dent. Res. 2016, 95, 1479–1486. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Xu, L.; Qi, W.; Dai, J.; Wang, B.; Tian, J.; Fu, X.; Yu, Y. Exacerbation of symptoms, nocturnal acid reflux, and impaired autonomic function are associated with sleep disturbance in gastroesophageal reflux disease patients. Front. Med. 2024, 11, 1438698. [Google Scholar] [CrossRef]
- Thomas, D.C.; Colonna, A.; Manfredini, D. Obstructive sleep apnoea, sleep bruxism and gastroesophageal reflux—Mutually interacting conditions? A literature review. Aust. Dent. J. 2024, 69 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Rompré, P.H.; Montplaisir, J.Y. Sleep bruxism: Validity of clinical research diagnostic criteria in a controlled polysomnographic study. J. Dent. Res. 1996, 75, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.; Chen, J.; Lu, Y.; Guo, J. Obstructive sleep apnea: A follow-up program in its relation to temporomandibular joint disorder, sleep bruxism and orofacial pain. BMC Oral Health 2023, 23, 578. [Google Scholar] [CrossRef]
- Nota, A.; Pittari, L.; Paggi, M.; Abati, S.; Tecco, S. Correlation between Bruxism and Gastroesophageal Reflux Disorder and Their Effects on Tooth Wear. A Systematic Review. J. Clin. Med. 2022, 11, 1107. [Google Scholar] [CrossRef]
- Leech, T.; Peiris, M. Mucosal neuroimmune mechanisms in gastro-oesophageal reflux disease (GORD) pathogenesis. J. Gastroenterol. 2024, 59, 165–178. [Google Scholar] [CrossRef]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Papakosta, V.K. Neurobiology of bruxism: The impact of stress (Review). Biomed. Rep. 2024, 20, 59. [Google Scholar] [CrossRef]
- Tan, X.; Wang, S.; Wu, F.; Zhu, J. Bidirectional correlation between gastroesophageal reflux disease and sleep problems: A systematic review and meta-analysis. PeerJ 2024, 12, e17202. [Google Scholar] [CrossRef]
- Meza, R.C.; Ancatén-González, C.; Chiu, C.Q.; Chávez, A.E. Transient Receptor Potential Vanilloid 1 Function at Central Synapses in Health and Disease. Front. Cell Neurosci. 2022, 16, 864828. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, C.D.; Wang, R.; Xu, Q.J.; Dai Pra, R.; Zhang, L.; Chang, R.B. A multidimensional coding architecture of the vagal interoceptive system. Nature 2022, 603, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Thie, N.M.; Huynh, N.; Miyawaki, S.; Lavigne, G.J. Topical review: Sleep bruxism and the role of peripheral sensory influences. J. Orofac. Pain 2003, 17, 191–213. [Google Scholar] [PubMed]
- Giovanni, A.; Giorgia, A. The neurophysiological basis of bruxism. Heliyon 2021, 7, e07477. [Google Scholar] [CrossRef]
- Frontiers Editorial. The neural substrates of bruxism: Current knowledge and directions. Front. Neurosci. 2024, 18, 1125. [Google Scholar]
- Martynowicz, H.; Lavigne, G.; Kato, T.; Poreba, R.; Michalek-Zrabkowska, M.; Macek, P.; Gac, P.; Wojakowska, A.; Surowiak, P.; Mazur, G. A case-control study on the effect of rhythmic masticatory muscle activity (RMMA) clusters on sleep fragmentation and severity of orofacial muscle pain in sleep bruxism. J. Sleep. Res. 2024, 33, e14072. [Google Scholar] [CrossRef]
- Lara Aparicio, S.Y.; Laureani Fierro, Á.J.; Aranda Abreu, G.E.; Toledo Cárdenas, R.; García Hernández, L.I.; Coria Ávila, G.A.; Rojas Durán, F.; Aguilar, M.E.H.; Manzo Denes, J. Current Opinion on the Use of c-Fos in Neuroscience. NeuroSci 2022, 3, 687–702. [Google Scholar] [CrossRef]
- Singh, B.P.; Singh, N.; Jayaraman, S.; Kirubakaran, R.; Joseph, S.; Muthu, M.S.; Jivnani, H.; Hua, F. Occlusal interventions for managing temporomandibular disorders. Cochrane Database Syst. Rev. 2024, 9, CD012850. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Lopez-Moncayo, L.F.; Muñoz-Alvear, H.D.; Gomez-Sosa, J.F.; Diaz-Barrera, L.E.; Curtidor, H.; Munoz, H.R. Expression of substance P, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Hildreth, M.; Rapaka, S.; Peng, Y.J.; Nanduri, J.; Prabhakar, N.R. Adrenal epinephrine facilitates erythropoietin gene activation by hypoxia through β2 adrenergic receptor interaction with Hif-2α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2025, 328, R75–R80. [Google Scholar] [CrossRef] [PubMed]
- King, R.; Hayes, C.; Donohoe, C.L.; Dunne, M.R.; Davern, M.; Donlon, N.E. Hypoxia and its impact on the tumour microenvironment of gastroesophageal cancers. World J. Gastrointest. Oncol. 2021, 13, 312–331. [Google Scholar] [CrossRef]
- Puzio, M.; Moreton, N.; O’Connor, J.J. Neuroprotective strategies for acute ischemic stroke: Targeting oxidative stress and prolyl hydroxylase domain inhibition in synaptic signalling. Brain Disord. 2022, 5, 100030. [Google Scholar] [CrossRef]
- Li, K.; He, C. Gastric Mucosal Lesions in Tibetans with High-Altitude Polycythemia Show Increased HIF-1A Expression and ROS Production. Biomed. Res. Int. 2019, 2019, 6317015. [Google Scholar] [CrossRef]
- Michaud, A.; Jia, W.L.; Djeddi, D.; Samson, N.; Nadeau, C.; Geha, S.; Praud, J.P. Effects of upper airway obstruction or hypoxia on gastroesophageal reflux in newborn lambs. Pediatr. Res. 2021, 89, 496–501. [Google Scholar] [CrossRef]
- Salvador, R.; Watson, T.J.; Herbella, F.; Dubecz, A.; Polomsky, M.; Jones, C.E.; Raymond, D.R.; Peters, J.H. Association of gastroesophageal reflux and O2 desaturation: A novel study of simultaneous 24-h MII-pH and continuous pulse oximetry. J. Gastrointest. Surg. 2009, 13, 854–861. [Google Scholar] [CrossRef]
- Sharma, P.; Mohanty, S.; Ahmad, Y. Decoding Proteomic cross-talk between hypobaric and normobaric hypoxia: Integrative analysis of oxidative stress, cytoskeleton remodeling, and inflammatory pathways. Life Sci. 2025, 371, 123611. [Google Scholar] [CrossRef]
- Hohenauer, E.; Freitag, L.; Costello, J.T.; Williams, T.B.; Küng, T.; Taube, W.; Herten, M.; Clijsen, R. The effects of normobaric and hypobaric hypoxia on cognitive performance and physiological responses: A crossover study. PLoS ONE 2022, 17, e0277364. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Lv, J.; Bai, L.H.; Yan, X.D.; Zhang, L. Effects of Hypoxemia by Acute High-Altitude Exposure on Human Intestinal Flora and Metabolism. Microorganisms 2023, 11, 2284. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, X.; Zeng, X.; Han, B.; Xie, H.; Wang, W.; Sun, L.; Hu, M.; Gao, Y.; Xiao, W. Gastrointestinal syndrome encountered during a train voyage to high altitudes: A 14-day survey of 69 passengers in China. Travel. Med. Infect. Dis. 2024, 59, 102718. [Google Scholar] [CrossRef]
- Batool, Z.; Amjad Kamal, M.; Shen, B. Advanced treatment strategies for high-altitude pulmonary hypertension employing natural medicines: A review. J. Pharm. Anal. 2025, 15, 101129. [Google Scholar] [CrossRef]
- Hirakawa, K.; Asano, R.; Ueda, J.; Aoki, T.; Tsuji, A.; Ogo, T. Calcium channel blockers in patients with pulmonary arterial hypertension receiving PAH-specific treatment. Int. J. Cardiol. 2024, 406, 132043. [Google Scholar] [CrossRef]
- Souza, R.F. Reflux esophagitis and its role in the pathogenesis of Barrett’s metaplasia. J. Gastroenterol. 2017, 52, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Agoston, A.T.; Dunbar, K.B.; Cipher, D.J.; Zhang, X.; Yu, C.; Cheng, E.; Zhang, Q.; Pham, T.H.; Tambar, U.K.; et al. Hypoxia-inducible factor-2α plays a role in mediating oesophagitis in GORD. Gut 2017, 66, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Zhang, X.; Davis, D.; Nguyen, A.D.; Ustaoglu, A.; Genta, R.M.; Wang, X.; Kale, I.; Ekeanyanwu, R.; Leeds, S. In Obesity, Esophagogastric Junction Fat Impairs Esophageal Barrier Function and Dilates Intercellular Spaces via Hypoxia-Inducible Factor 2α. Gastroenterology 2025, 168, 914–930.e19. [Google Scholar] [CrossRef]
- Lile, I.E.; Hajaj, T.; Veja, I.; Hosszu, T.; Vaida, L.L.; Todor, L.; Stana, O.; Popovici, R.-A.; Marian, D. Comparative Evaluation of Natural Mouthrinses and Chlorhexidine in Dental Plaque Management: A Pilot Randomized Clinical Trial. Healthcare 2025, 13, 1181. [Google Scholar] [CrossRef]
- Marcjasz, P.; Bioły, A.; Buliszak, A.; Babczyńska, M.; Chodkowska, E.; Bielas, K. Sleep Bruxism and Obstructive Sleep Apnea—Shared Pathophysiology or Coincidence? Qual. Sport 2025, 41, 60146. [Google Scholar] [CrossRef]
- Li, D.; Kuang, B.; Lobbezoo, F.; de Vries, N.; Hilgevoord, A.; Aarab, G. Sleep bruxism is highly prevalent in adults with obstructive sleep apnea: A large-scale polysomnographic study. J. Clin. Sleep. Med. 2023, 19, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kuang, B.; Li, D.; Lobbezoo, F.; de Vries, R.; Hilgevoord, A.; De Vries, N.; Huynh, N.; Lavigne, G.; Aarab, G. Associations between sleep bruxism and other sleep-related disorders in adults: A systematic review. Sleep. Med. 2022, 89, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, P.; Polmann, H.; Conti Réus, J.; Massignan, C.; de Souza, B.D.M.; Gozal, D.; Lavigne, G.; Flores-Mir, C.; De Luca Canto, G. Sleep bruxism and obstructive sleep apnea: Association, causality or spurious finding? A scoping review. Sleep 2022, 45, zsac073. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014; pp. 49–52. [Google Scholar]
- Smardz, J.; Wieckiewicz, M.; Wojakowska, A.; Michalek-Zrabkowska, M.; Poreba, R.; Gac, P.; Mazur, G.; Martynowicz, H. Incidence of Sleep Bruxism in Different Phenotypes of Obstructive Sleep Apnea. J. Clin. Med. 2022, 11, 4091. [Google Scholar] [CrossRef]
- Yap, A.U.; Tan, M.W.Y.; Tan, S.H.X.; Chua, A.P. Sleep bruxism events: An epiphenomenon of severe obstructive sleep apnea? Clin. Oral Investig. 2023, 27, 4633–4642. [Google Scholar] [CrossRef]
- Dal Fabbro, C.; Bornhardt-Suazo, T.; Landry Schönbeck, A.; de Meyer, M.; Lavigne, G.J. Understanding the clinical management of co-occurring sleep-related bruxism and obstructive sleep apnea in adults: A narrative and critical review. J. Prosthodont. 2025, 34, 46–61. [Google Scholar] [CrossRef]
- Troester, M.M.; Quan, S.F.; Berry, R.B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; Version 3; American Academy of Sleep Medicine: Darien, IL, USA, 2023; p. 48. [Google Scholar]
- Zhu, Y.; Toyota, R.; Shiraishi, Y.; Katagiri, A.; Yamada, M.; Higashiyama, M.; Toyoda, H.; Lavigne, G.; Kato, T. Sleep architecture as a candidate for phenotyping sleep bruxism: A narrative physiological review. J. Oral Rehabil. 2024, 51, 87–102. [Google Scholar] [CrossRef]
- Miki, H.; Minakuchi, H.; Miyagi, M.; Hara, E.S.; Shigemoto, S.; Suzuki, Y.; Maekawa, K.; Matsuka, Y.; Clark, G.T.; Kuboki, T. Association of masticatory muscle activity with sleep arousal and other concomitant movements during sleep. J. Oral Rehabil. 2020, 47, 281–288. [Google Scholar] [CrossRef]
- Fulek, M.; Wieckiewicz, M.; Szymanska-Chabowska, A.; Michalek-Zrabkowska, M.; Fulek, K.; Lachowicz, G.; Poreba, R.; Mazur, G.; Martynowicz, H. Systematic Review on the Link between Sleep Bruxism and Systemic Chronic Inflammation. Brain Sci. 2023, 13, 1104. [Google Scholar] [CrossRef]
- DiCaro, M.V.; Lei, K.; Yee, B.; Tak, T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. J. Clin. Med. 2024, 13, 3223. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxid. Med. Cell Longev. 2021, 2021, 9681595. [Google Scholar] [CrossRef]
- Fulek, M.; Frosztega, W.; Wieckiewicz, M.; Szymanska-Chabowska, A.; Gac, P.; Poreba, R.; Mazur, G.; Sciskalska, M.; Kepinska, M.; Martuszewski, A.; et al. The link between sleep bruxism and oxidative stress based on a polysomnographic study. Sci. Rep. 2025, 15, 3567. [Google Scholar] [CrossRef]
- Irarrázaval, S.; Allard, C.; Campodónico, J.; Pérez, D.; Strobel, P.; Vásquez, L.; Urquiaga, I.; Echeverría, G.; Leighton, F. Oxidative Stress in Acute Hypobaric Hypoxia. High. Alt. Med. Biol. 2017, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; Pena, E.; Brito, J.; El Alam, S. Oxidative Stress, Kinase Activation, and Inflammatory Pathways Involved in Effects on Smooth Muscle Cells During Pulmonary Artery Hypertension Under Hypobaric Hypoxia Exposure. Front. Physiol. 2021, 12, 690341. [Google Scholar] [CrossRef]
- Pena, E.; Siques, P.; Brito, J.; Arribas, S.M.; Böger, R.; Hannemann, J.; León-Velarde, F.; González, M.C.; López, M.R.; López de Pablo, Á.L. Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia. Int. J. Mol. Sci. 2020, 21, 8576. [Google Scholar] [CrossRef]
- Li, H.S.; Liu, H.J.; Zhang, Y.; Zhang, J.; Yan, H.Y.; Yuan, W.C.; Wang, S.; Yu, S.; Yang, S.Q.; Sun, M.W.; et al. Chronic intermittent hypobaric hypoxia prevents pulmonary arterial hypertension through maintaining eNOS homeostasis. Arch. Biochem. Biophys. 2025, 767, 110340. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Marzorati, M.; Healey, B.; Dellanoce, C.; Vezzoli, A. Effects of Prolonged Exposure to Hypobaric Hypoxia on Oxidative Stress: Overwintering in Antarctic Concordia Station. Oxid. Med. Cell Longev. 2022, 2022, 4430032. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, A.R.; Arce-Álvarez, A.; Ramirez-Campillo, R.; Vásquez-Muñoz, M.; von Igel, M.; Ramírez, M.A.; Del Rio, R.; Andrade, D.C. Baroreflex Modulation During Acute High-Altitude Exposure in Rats. Front. Physiol. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Fisher, J.P.; Roche, J.; Turner, R.; Walzl, A.; Roveri, G.; Gatterer, H.; Siebenmann, C. Hypobaric hypoxia and cardiac baroreflex sensitivity in young women. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1048–H1054. [Google Scholar] [CrossRef]
- Pramsohler, S.; Schilz, R.; Patzak, A.; Rausch, L.; Netzer, N.C. Periodic breathing in healthy young adults in normobaric hypoxia equivalent to 3500 m, 4500 m, and 5500 m altitude. Sleep Breath 2019, 23, 703–709. [Google Scholar] [CrossRef]
- Boos, C.J.; Bye, K.; Sevier, L.; Bakker-Dyos, J.; Woods, D.R.; Sullivan, M.; Quinlan, T.; Mellor, A. High Altitude Affects Nocturnal Non-linear Heart Rate Variability: PATCH-HA Study. Front. Physiol. 2018, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. Int. J. Environ. Res. Public Health 2021, 18, 9248. [Google Scholar] [CrossRef] [PubMed]
- Duman, S. Can melatonin be used for bruxism in children? Eur. Arch. Paediatr. Dent. 2021, 22, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- Strickland, B.; Small, E.; Ryan, M.; Paterson, R. Effectiveness of Continuous Positive Airway Pressure in Alleviating Hypoxemia and Improving Exertional Capacity at Altitude. High Alt. Med. Biol. 2024, 25, 319–325. [Google Scholar] [CrossRef]
- Patrician, A.; Anholm, J.D.; Ainslie, P.N. A narrative review of periodic breathing during sleep at high altitude: From acclimatizing lowlanders to adapted highlanders. J. Physiol. 2024, 602, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena, E.; Yanez, M.P.; Montini, F. Sleep Bruxism and Hypobaric Hypoxia Exposure: Exploring the Physiological Association. J. Clin. Med. 2025, 14, 7176. https://doi.org/10.3390/jcm14207176
Pena E, Yanez MP, Montini F. Sleep Bruxism and Hypobaric Hypoxia Exposure: Exploring the Physiological Association. Journal of Clinical Medicine. 2025; 14(20):7176. https://doi.org/10.3390/jcm14207176
Chicago/Turabian StylePena, Eduardo, Maria Paz Yanez, and Francisca Montini. 2025. "Sleep Bruxism and Hypobaric Hypoxia Exposure: Exploring the Physiological Association" Journal of Clinical Medicine 14, no. 20: 7176. https://doi.org/10.3390/jcm14207176
APA StylePena, E., Yanez, M. P., & Montini, F. (2025). Sleep Bruxism and Hypobaric Hypoxia Exposure: Exploring the Physiological Association. Journal of Clinical Medicine, 14(20), 7176. https://doi.org/10.3390/jcm14207176






