Quantitative Evaluation of Skeletal, Dental, and Soft Tissue Changes After Orthognathic Surgery: A Cephalometric and Statistical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure Methodology
2.2. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Association of Oral and Maxillofacial Surgeons. Parameters of Care: Indications for Orthognathic Surgery; AAOMS: Rosemont, IL, USA, 2025; Available online: https://aaoms.org/wp-content/uploads/2025/01/ortho_indications.pdf (accessed on 6 October 2025).
- Lin, H.H.; Lo, L.J. Three-Dimensional Computer-Assisted Surgical Simulation and Intraoperative Navigation in Orthognathic Surgery: A Literature Review. J. Formos. Med. Assoc. 2015, 114, 300–307. [Google Scholar] [CrossRef]
- Khechoyan, D.Y. Orthognathic Surgery: General Considerations. Semin. Plast. Surg. 2013, 27, 133–136. [Google Scholar] [CrossRef]
- Seo, H.J.; Choi, Y.K. Current Trends in Orthognathic Surgery. Arch. Craniofac. Surg. 2021, 22, 287. [Google Scholar] [CrossRef] [PubMed]
- Posnick, J.C. Craniofacial and Maxillofacial Surgery in Children and Young Adults; W.B. Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Wirthlin, J.O.; Shetye, P.R. Orthodontist’s Role in Orthognathic Surgery. Semin. Plast. Surg. 2013, 27, 137–144. [Google Scholar] [PubMed]
- Larson, B.E. Orthodontic Preparation for Orthognathic Surgery. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 441–458. [Google Scholar] [CrossRef]
- Klein, K.P.; Kaban, L.B.; Masoud, M.I. Orthognathic Surgery and Orthodontics: Inadequate Planning Leading to Complications or Unfavorable Results. Oral Maxillofac. Surg. Clin. N. Am. 2020, 32, 71–82. [Google Scholar] [CrossRef]
- Edler, R.J. Problems in the Orthodontic Management of Orthognathic Cases. Eur. J. Orthod. 1990, 12, 420–437. [Google Scholar] [CrossRef]
- Quast, A.; Santander, P.; Leding, J.; Klenke, D.; Moser, N.; Schliephake, H.; Meyer-Marcotty, P. Orthodontic Incisor Decompensation in Orthognathic Therapy—Success and Efficiency in Three Dimensions. Clin. Oral Investig. 2021, 25, 4001–4010. [Google Scholar] [CrossRef]
- Cunningham, S.J.; Garratt, A.M.; Hunt, N.P. Development of a Condition-Specific Quality of Life Measure for Patients with Dentofacial Deformity: I. Reliability of the Instrument. Community Dent. Oral Epidemiol. 2000, 28, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Meger, M.N.; Fatturi, A.L.; Gerber, J.T.; Weiss, S.G.; Rocha, J.S.; Scariot, R.; Wambier, L.M. Impact of Orthognathic Surgery on Quality of Life of Patients with Dentofacial Deformity: A Systematic Review and Meta-Analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 265–271. [Google Scholar] [CrossRef]
- Syed, N.; Chiu, G.A.; Korczak, P. Should Patients Take Vitamin D before Mandibular Operations? Br. J. Oral Maxillofac. Surg. 2017, 55, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Bowe, D.C.; Gruber, E.A.; McLeod, N.M.H. Nerve Injury Associated with Orthognathic Surgery. Part 1: UK Practice and Motor Nerve Injuries. Br. J. Oral Maxillofac. Surg. 2016, 54, 362–365. [Google Scholar] [CrossRef]
- Pavlíková, G.; Foltán, R.; Horká, M.; Hanzelka, T.; Borunská, H.; Šedý, J. Piezosurgery in Oral and Maxillofacial Surgery. Int. J. Oral Maxillofac. Surg. 2011, 40, 451–457. [Google Scholar] [CrossRef]
- Li, J.; Ver Berne, J.; Shujaat, S.; Shaheen, E.; Politis, C.; Jacobs, R. Influence of Systemic Comorbidities on the Complications of Orthognathic Surgery: A Scoping Review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e956–e961. [Google Scholar] [CrossRef]
- Staikou, C.; Stavroulakis, E.; Karmaniolou, I. A Narrative Review of Peri-Operative Management of Patients with Thalassaemia. Anaesthesia 2014, 69, 494–510. [Google Scholar] [CrossRef]
- American Association of Oral and Maxillofacial Surgeons (AAOMS). Parameters of Care: Clinical Practice Guidelines for Oral and Maxillofacial Surgery (AAOMS ParCare 2023)—Patient Assessment. J. Oral Maxillofac. Surg. 2023, 81 (Suppl. S11S), e13–e34. Available online: https://aaoms.org/wp-content/uploads/2024/08/parcare_patient_assesment.pdf (accessed on 6 October 2025).
- Schiller, L.A.; Schiller, A.C.; Schiller, A.; Dascalu, A.G.; Brad, S. Bone Augmentation Procedures in a Patient with Acromegaly. Case Rep. Dent. 2020, 2020, 8479502. [Google Scholar] [CrossRef]
- Zieliński, G.; Gawda, P. Defining Effect Size Standards in Temporomandibular Joint and Masticatory Muscle Research. Med. Sci. Monit. 2025, 31, e948365. [Google Scholar] [CrossRef]
- Alam, M.K.; Alfawzan, A.A. Dental Characteristics of Different Types of Cleft and Non-Cleft Individuals. Front. Cell Dev. Biol. 2020, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, J.-B.; Chang, M.-S.; Ryu, J.-J.; Lim, W.H.; Jung, S.-K. Influence of the Depth of the Convolutional Neural Networks on an Artificial Intelligence Model for Diagnosis of Orthognathic Surgery. J. Pers. Med. 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Alfawzan, A.A. Evaluation of Sella Turcica Bridging and Morphology in Different Types of Cleft Patients. Front. Cell Dev. Biol. 2020, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Stăncioiu, A.-A.; Motofelea, A.C.; Hușanu, A.A.; Vasica, L.; Nagib, R.; Popa, A.; Szuhanek, C. Associations of Digital Measurements: Analysis of Orthopantomography versus Lateral Cephalograms for Evaluation of Facial Asymmetry. J. Clin. Med. 2025, 14, 1296. [Google Scholar] [CrossRef] [PubMed]
- Yassir, Y.A.; Salman, A.R.; Nabbat, S.A. The Accuracy and Reliability of WebCeph for Cephalometric Analysis. J. Taibah Univ. Med. Sci. 2022, 17, 57–66. [Google Scholar] [CrossRef]
- Koz, S.; Uslu-Akcam, O. Artificial Intelligence-Supported and App-Aided Cephalometric Analysis: Which One Can We Trust? Diagnostics 2025, 15, 559. [Google Scholar] [CrossRef]
- Szuhanek, C.; Schiller, A.; Grigore, A.; Popa, L. A Guide to Orthodontics; Editura Victor Babeș: Timișoara, Romania, 2019. [Google Scholar]
- Mathapun, J.; Charoemratrote, C. Is Incisor Compensation Related to Skeletal Discrepancies in Skeletal Class III? A Retrospective Cephalometric Study. Diagnostics 2024, 14, 1021. [Google Scholar] [CrossRef]
- Tikku, T.; Khanna, R.; Maurya, R.P.; Verma, S.L.; Srivastava, K.; Kadu, M. Cephalometric Norms for Orthognathic Surgery in North Indian Population Using Nemoceph Software. J. Oral Biol. Craniofac. Res. 2014, 4, 94–103. [Google Scholar] [CrossRef]
- Hong, P.; Kim, Y.H.; Choo, H.; Choi, Y.-J.; Chae, H.S. Selecting Anterior Segmental Osteotomy versus Orthodontic Camouflage Treatment: Importance of the Incisor–Mandibular Plane Angle. Appl. Sci. 2025, 15, 4849. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Yi, Q.L. How Do I Interpret a Confidence Interval? Transfusion 2016, 56, 1680–1683. [Google Scholar] [CrossRef]
- Lai, H.-C.; Denadai, R.; Ho, C.-T.; Lin, H.-H.; Lo, L.-J. Effect of Le Fort I Maxillary Advancement and Clockwise Rotation on the Anteromedial Cheek Soft Tissue Change in Patients with Skeletal Class III Pattern and Midface Deficiency: A 3D Imaging-Based Prediction Study. J. Clin. Med. 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Z.; Yan, J.; Chen, L.; Piao, Z.G. Evaluation of Facial Soft Tissue Thickness in Asymmetric Mandibular Deformities after Orthognathic Surgery. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 37. [Google Scholar] [CrossRef] [PubMed]
- Psomiadis, S.; Sifakakis, I.; Iatrou, I.; Gkantidis, N. Aesthetic Impact of Orthognathic Surgery vs. Orthodontic Camouflage in Class II Division 1 Patients with Convex Facial Profile: A Follow-Up Using Combined Frontal and Profile Views. J. Clin. Med. 2025, 14, 4277. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, V.A.; Pistilli, R.; Govoni, F.A.; Di Nezza, S.; Tarascio, L.; Pica, F.; De Paolis, L.; Celebrini, A.; Magliacani, V.; Bellocchi, G.; et al. Maxillomandibular Advancement (MMA) Surgery Improves Obstructive Sleep Apnea: CAD/CAM vs. Traditional Surgery. Appl. Sci. 2025, 15, 9149. [Google Scholar] [CrossRef]
- Nike, E.; Radzins, O.; Vuollo, V.; Slaidina, A.; Abeltins, A. Changes in Facial Soft Tissue Asymmetry in Class II Patients One Year after Orthognathic Surgery. J. Clin. Med. 2025, 14, 2912. [Google Scholar] [CrossRef]
- Perrotti, G.; Baccaglione, G.; Clauser, T.; Scaini, R.; Grassi, R.; Testarelli, L.; Reda, R.; Testori, T.; Del Fabbro, M. Total Face Approach (TFA) 3D Cephalometry and Superimposition in Orthognathic Surgery: Evaluation of the Vertical Dimensions in a Consecutive Series. Methods Protoc. 2021, 4, 36. [Google Scholar] [CrossRef]
- Alam, M.K.; Nowrin, S.A.; Shahid, F.; AlHarby, H.; Abutayyem, H.; Alswairki, H.J.; El-Din Mohamed, S.K. Orthognathic versus Camouflage Treatment of Class III Malocclusion: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 3314. [Google Scholar] [CrossRef]
- Psomiadis, S.; Gkantidis, N.; Sifakakis, I.; Iatrou, I. Perceived Effects of Orthognathic Surgery versus Orthodontic Camouflage Treatment of Convex Facial Profile Patients. J. Clin. Med. 2023, 13, 91. [Google Scholar] [CrossRef]
- Van Vlijmen, O.J.C.; Maal, T.; Bergé, S.J.; Bronkhorst, E.M.; Katsaros, C.; Kuijpers-Jagtman, A.M. A Comparison between 2D and 3D Cephalometry on CBCT Scans of Human Skulls. Int. J. Oral Maxillofac. Surg. 2010, 39, 156–160. [Google Scholar] [CrossRef]
- Swennen, G.R.; Schutyser, F.A.; Hausamen, J.E. Three-Dimensional Cephalometry: A Color Atlas and Manual; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Xia, J.J.; Gateno, J.; Teichgraeber, J.F.; Christensen, A.M.; Lasky, R.E.; Lemoine, J.J.; Liebschner, M.A. Accuracy of the Computer-Aided Surgical Simulation (CASS) System in the Treatment of Patients with Complex Craniomaxillofacial Deformity: A Pilot Study. J. Oral Maxillofac. Surg. 2007, 65, 248–254. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, H.W.; Moon, J.H.; Yu, Y.; Kim, H.; Her, S.B.; Lee, S.J. Automated Identification of Cephalometric Landmarks: Part 1—Comparisons between the Latest Deep-Learning Methods YOLOv3 and SSD. Angle Orthod. 2019, 89, 903–909. [Google Scholar] [CrossRef] [PubMed]
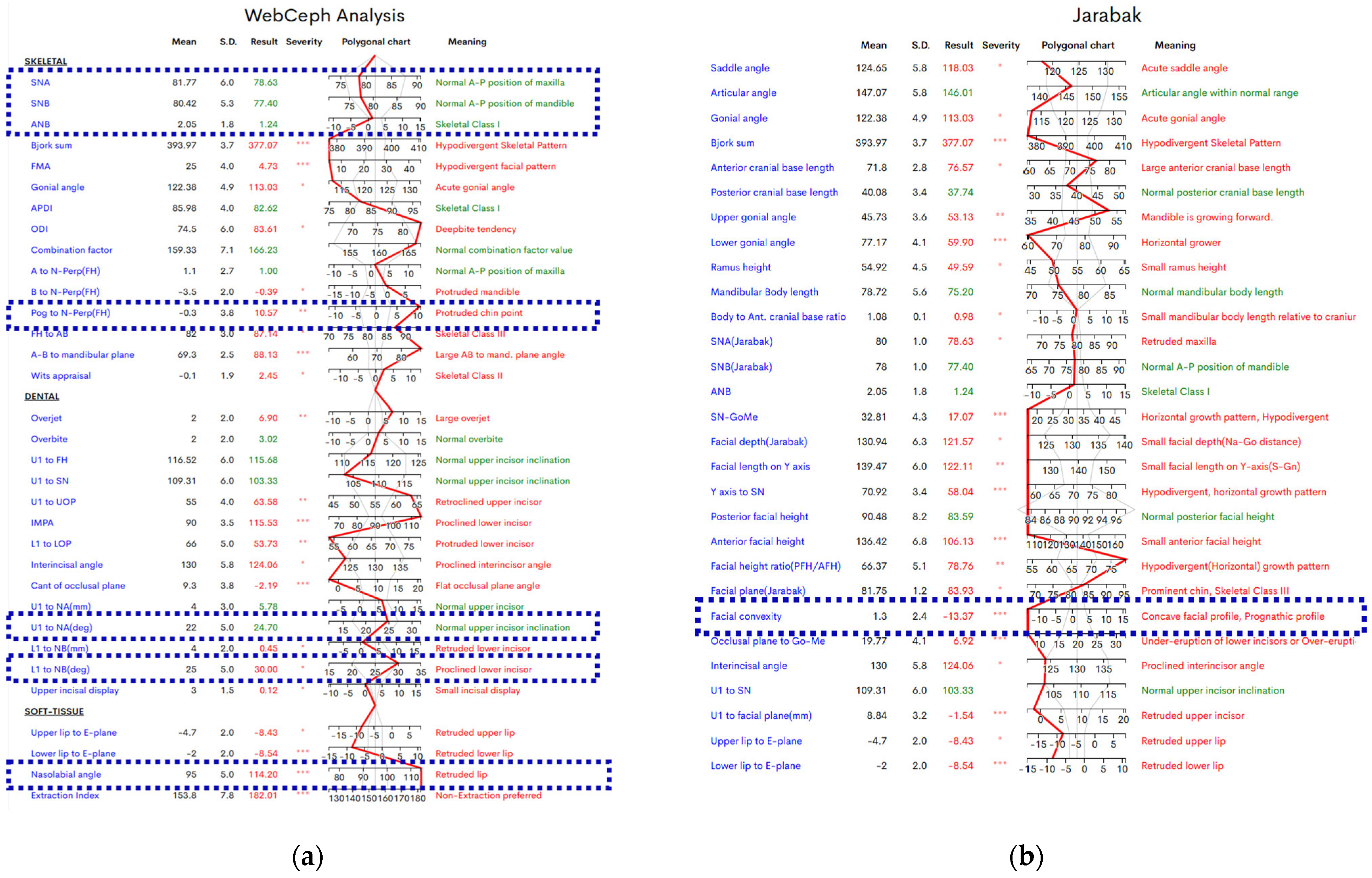

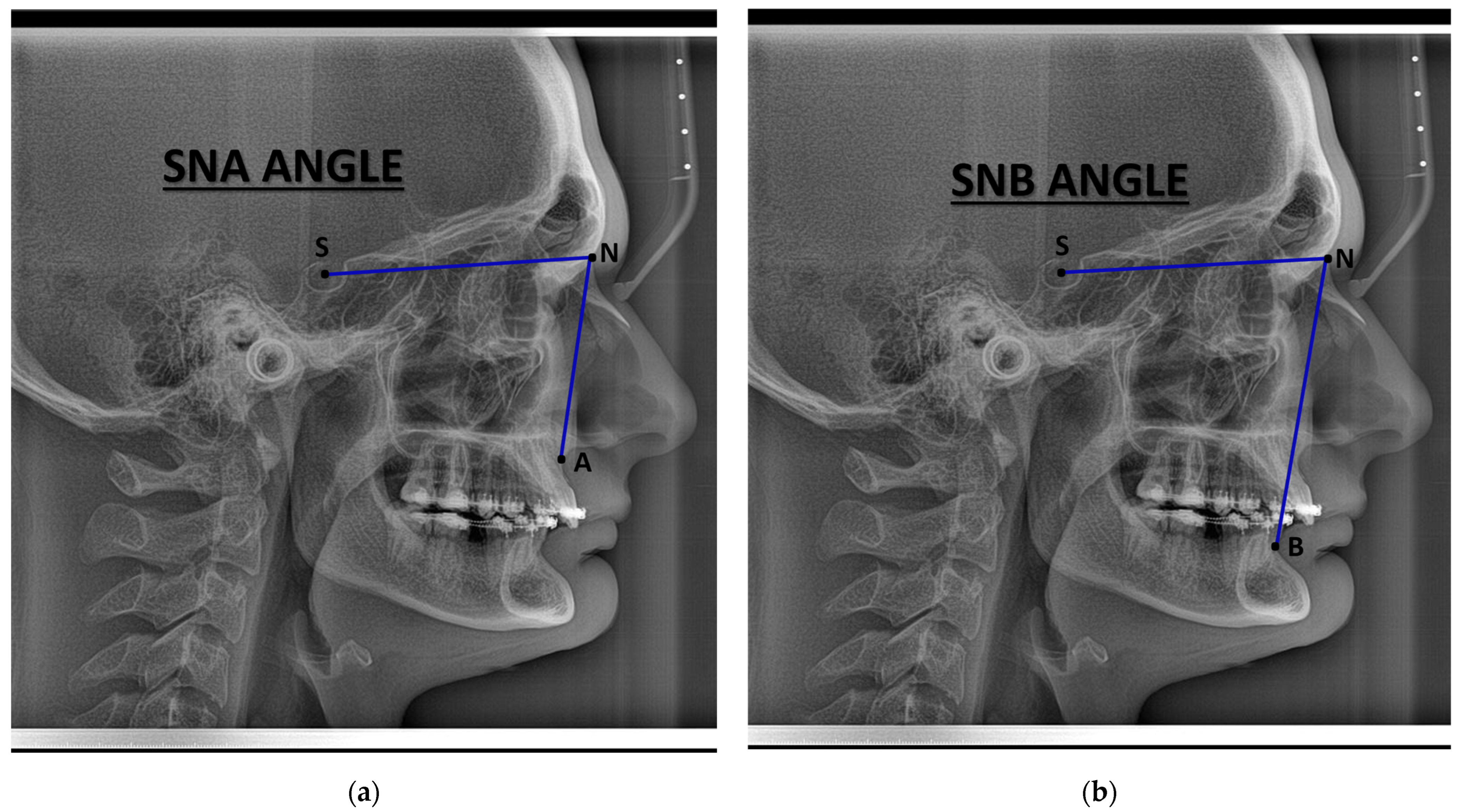
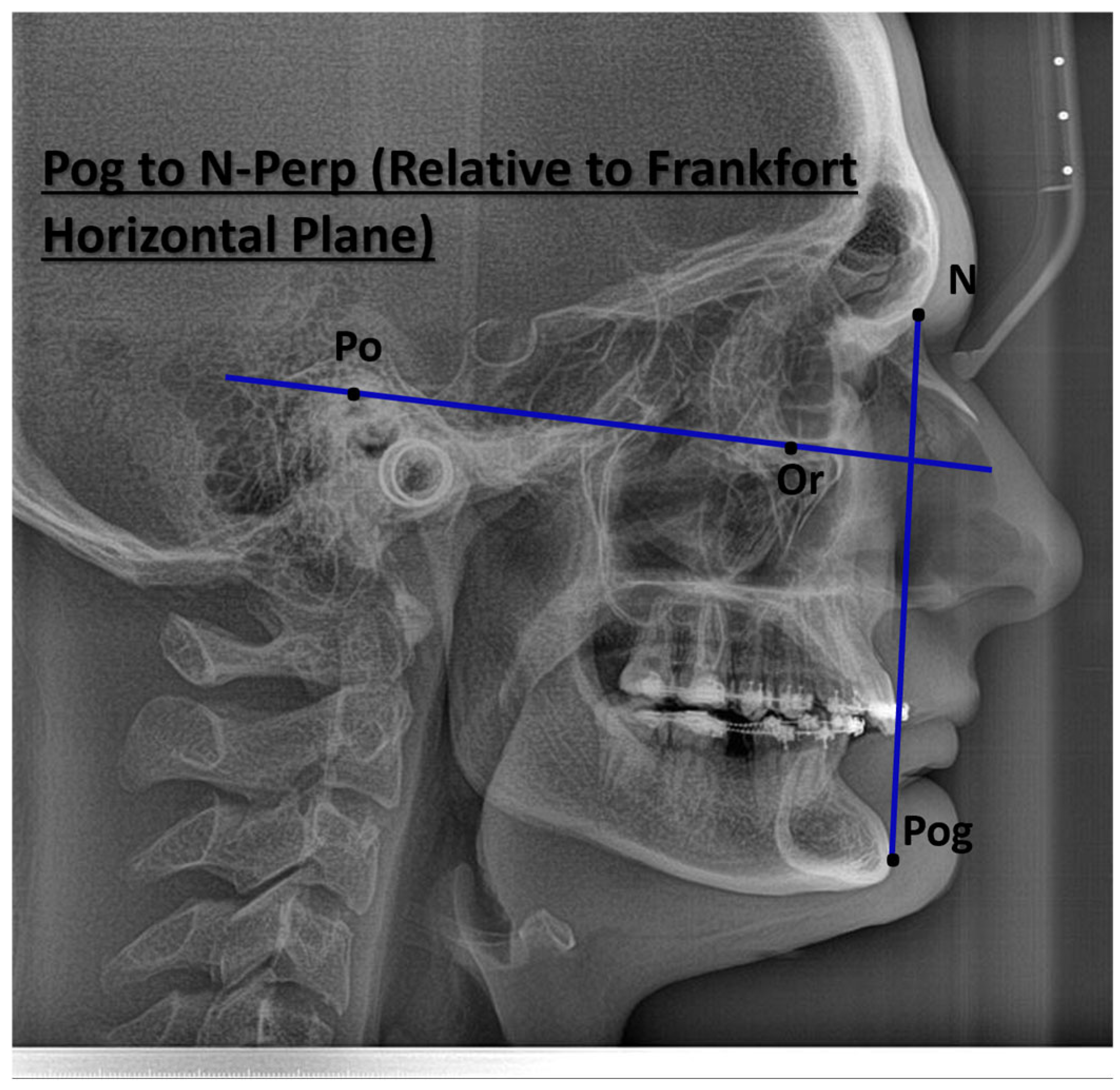
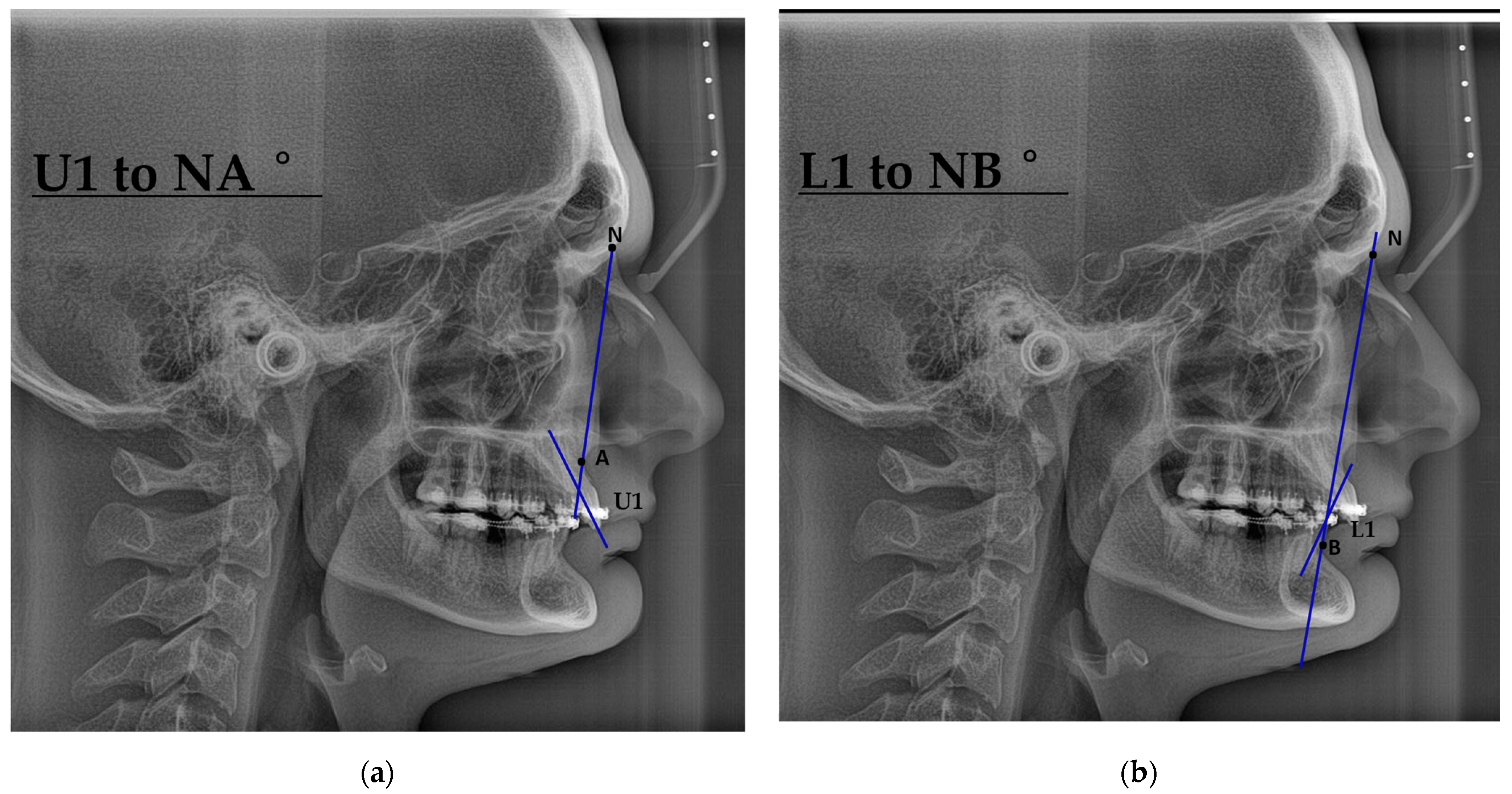

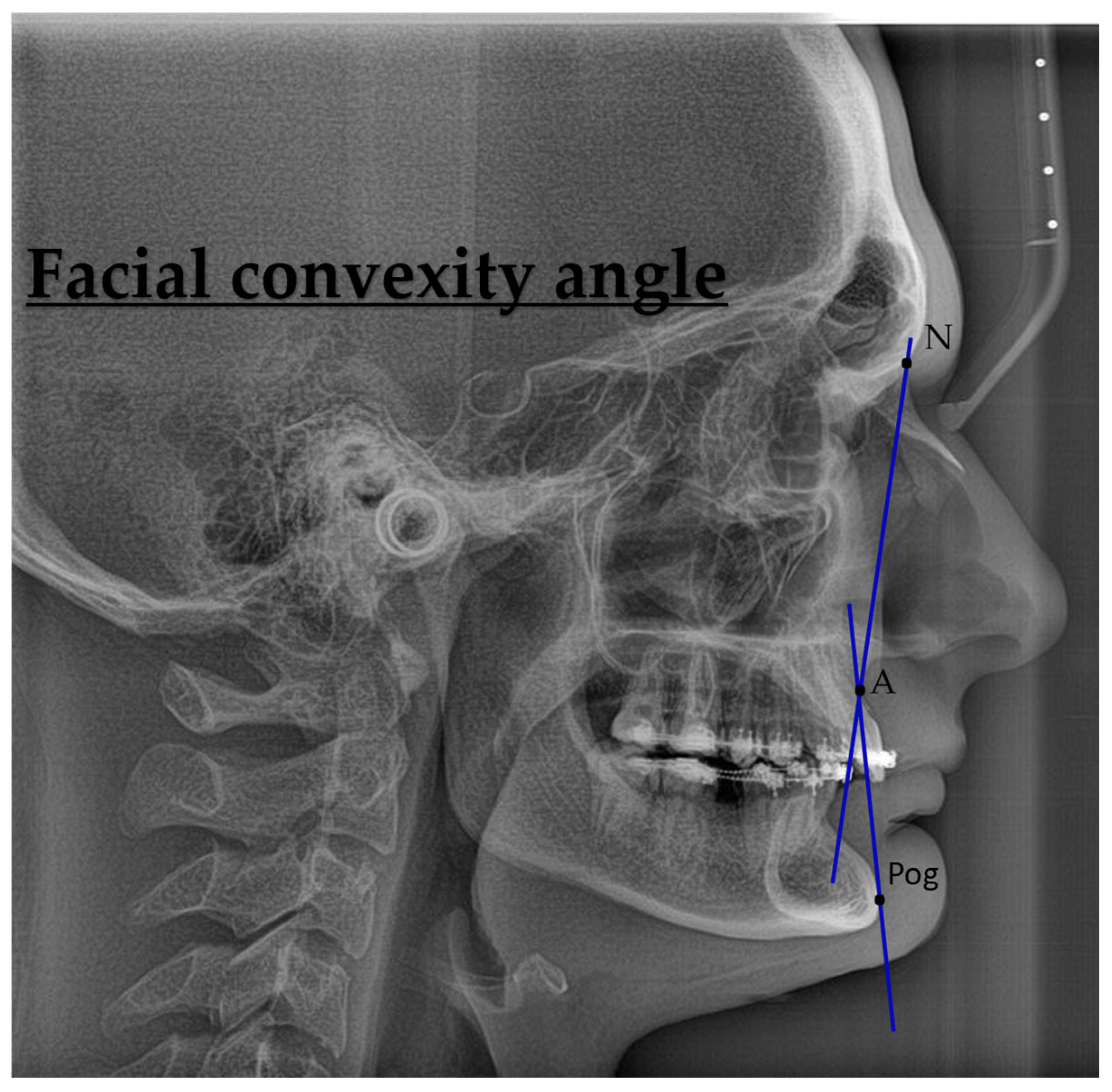
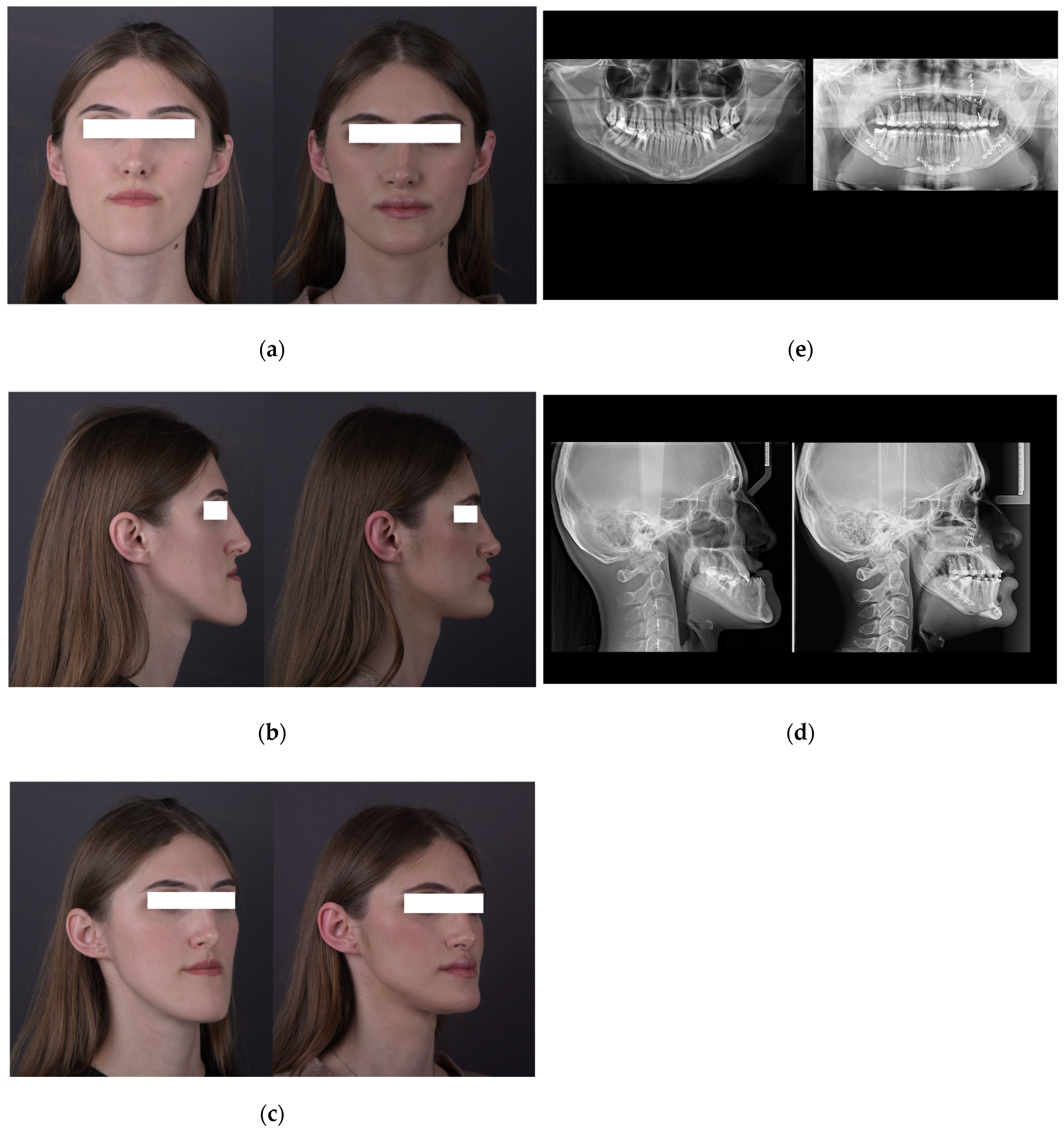
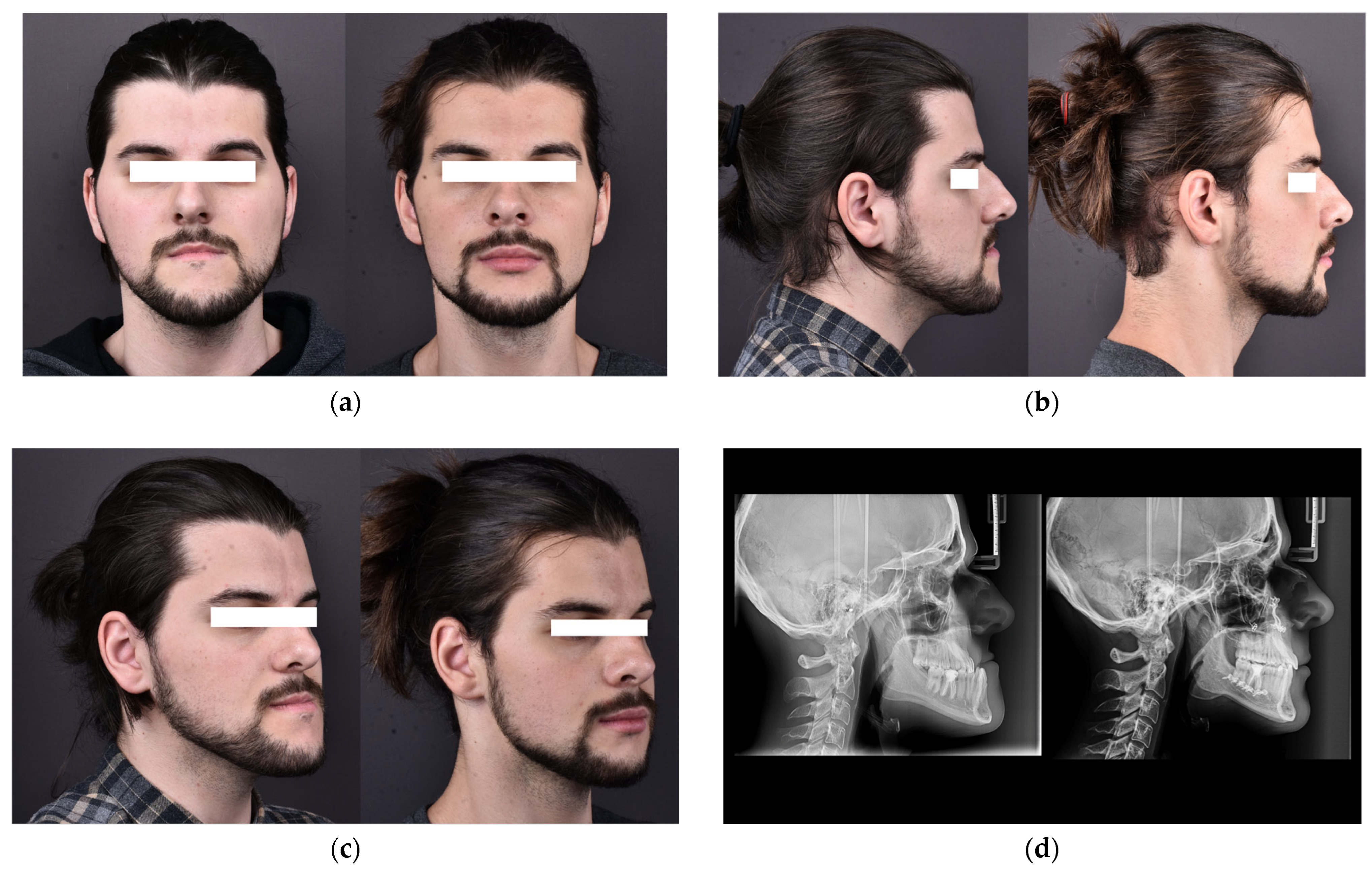

| Characteristic | Preoperative Median (IQR) | Postoperative Median (IQR) |
|---|---|---|
| Demographics | ||
| Age (years) | 28.0 (24.0, 33.0) | – |
| Sex, n (%) | Female: 11 (44%) Male: 14 (56%) | – |
| Skeletal parameters | ||
| SNA (°) | 83.6 (79.9, 84.9) | 86.3 (80.4, 89.0) |
| SNB (°) | 83.5 (76.8, 87.2) | 84.0 (81.0, 86.0) |
| ANB (°) | 0.9 (−4.9, 5.3) | 3.0 (−0.2, 4.5) |
| Pogonion to N-Perpendicular FH (mm) | 1.87 (−0.94, 2.31) | 1.4 (0.4, 2.6) |
| Dental parameters | ||
| U1–NA (°) | 26 (21, 28) | 22 (19, 29) |
| L1–NB (°) | 24 (17, 27) | 25 (19, 30) |
| Soft tissue parameters | ||
| Nasolabial angle (°) | 102 (92, 114) | 105 (91, 117) |
| Facial convexity (°) | 0 (−12, 7) | 2 (−6, 5) |
| Variable | Mean Difference | SE Difference | t | df | p-Value |
|---|---|---|---|---|---|
| SNA (°) | −3.123 | 1.24 | −2.511 | 24 | 0.019 |
| SNB (°) | −1.147 | 1.41 | −0.811 | 24 | 0.426 |
| ANB (°) | −1.980 | 1.00 | −1.977 | 24 | 0.060 |
| Pogonion to N-Perpendicular FH (mm) | −1.571 | 1.21 | −1.296 | 24 | 0.207 |
| U1–NA (°) | 0.465 | 1.87 | 0.249 | 24 | 0.806 |
| L1–NB (°) | −2.968 | 1.55 | −1.915 | 24 | 0.067 |
| Nasolabial angle (°) | −6.950 | 8.83 | −0.787 | 24 | 0.439 |
| Facial convexity (°) | −0.502 | 4.12 | −0.122 | 24 | 0.904 |
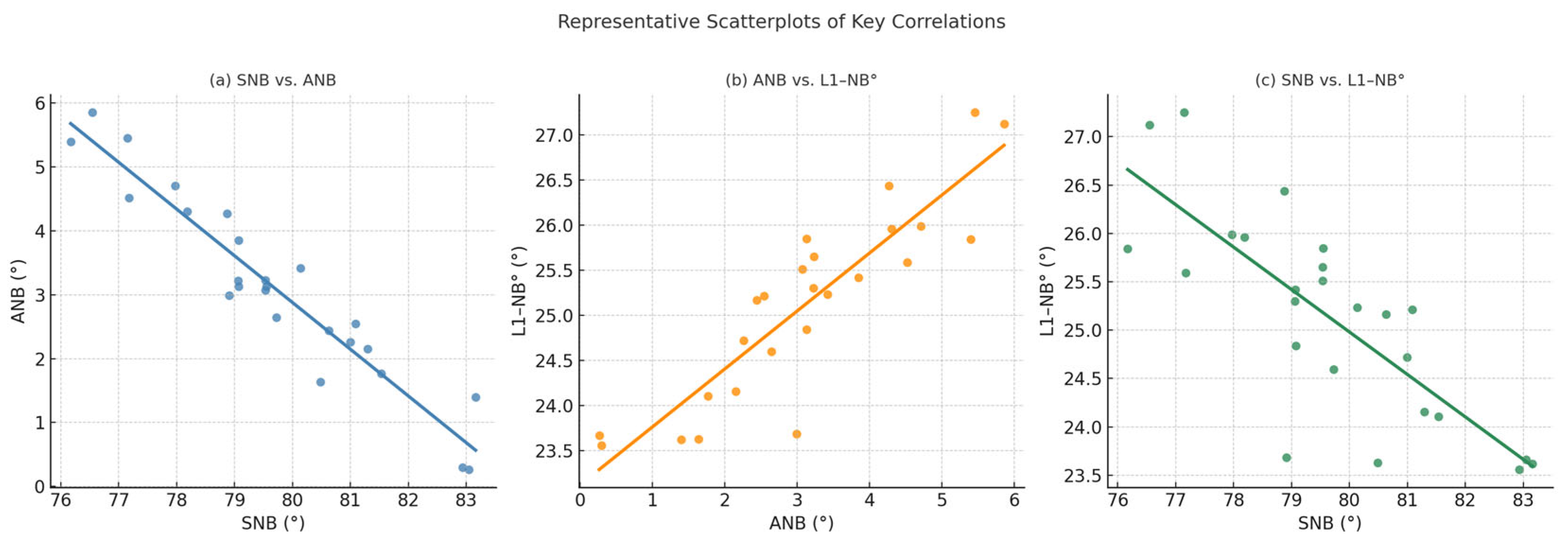
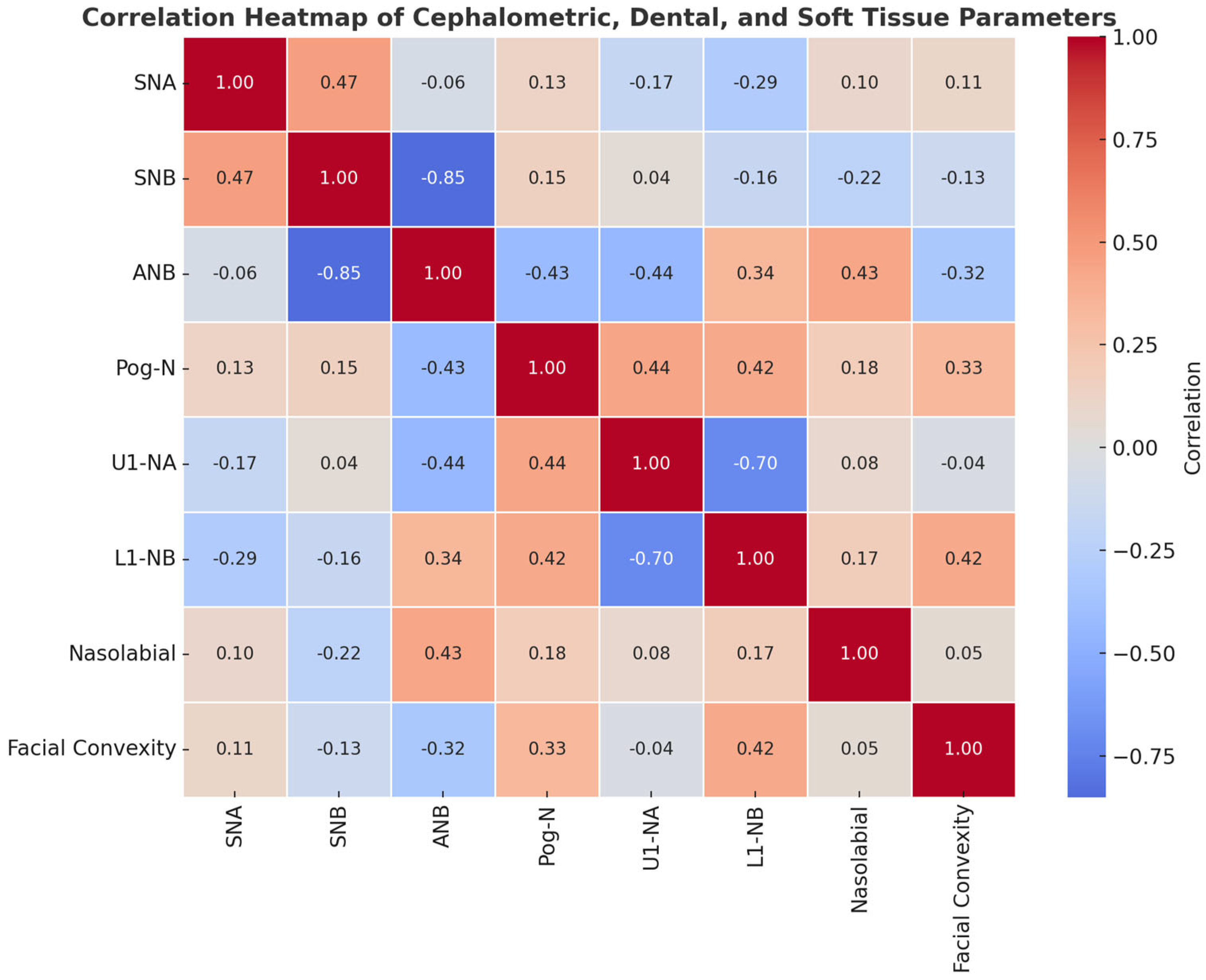
| Variable | Age | SNA-PRE-OP | SNB | ANB | Pog to N-Perp FH | U1-NA° | L1-NB° | Nasolabial Angle | Facial Convexity |
|---|---|---|---|---|---|---|---|---|---|
| Age | — | ||||||||
| SNA-PRE-OP | 0.016 (−0.382, 0.408), p = 0.941 | ||||||||
| SNB | −0.080 (−0.460, 0.326), p = 0.705 | 0.473 (0.096, 0.732), p = 0.017 | |||||||
| ANB | 0.099 (−0.308, 0.476), p = 0.637 | 0.058 (−0.345, 0.443), p = 0.784 | −0.852 (−0.933, −0.689), p < 0.001 | ||||||
| Pog to N-Perp FH | −0.305 (−0.625, 0.102), p = 0.138 | −0.066 (−0.450, 0.338), p = 0.753 | 0.463 (0.082, 0.725), p = 0.020 | −0.563 (−0.784, −0.216), p = 0.003 | |||||
| U1-NA° | −0.051 (−0.437, 0.351), p = 0.808 | −0.064 (−0.448, 0.340), p = 0.762 | 0.382 (−0.015, 0.675), p = 0.059 | −0.471 (−0.730, −0.093), p = 0.017 | 0.333 (−0.072, 0.643), p = 0.104 | ||||
| L1-NB° | 0.013 (−0.384, 0.406), p = 0.952 | 0.031 (−0.369, 0.421), p = 0.884 | −0.559 (−0.781, −0.210), p = 0.004 | 0.652 (0.346, 0.833), p < 0.001 | −0.244 (−0.583, 0.167), p = 0.240 | −0.022 (−0.414, 0.376), p = 0.917 | |||
| Nasolabial angle | −0.084 (−0.464, 0.322), p = 0.691 | 0.325 (−0.081, 0.638), p = 0.113 | −0.159 (−0.522, 0.252), p = 0.447 | 0.373 (−0.026, 0.669), p = 0.066 | −0.056 (−0.441, 0.347), p = 0.791 | −0.124 (−0.495, 0.285), p = 0.553 | 0.348 (−0.054, 0.653), p = 0.088 | ||
| Facial convexity | 0.348 (−0.055, 0.653), p = 0.089 | −0.048 (−0.435, 0.354), p = 0.819 | −0.406 (−0.691, −0.013), p = 0.044 | 0.432 (0.045, 0.707), p = 0.031 | −0.309 (−0.628, 0.098), p = 0.132 | −0.230 (−0.573, 0.182), p = 0.268 | 0.419 (0.029, 0.699), p = 0.037 | 0.106 (−0.302, 0.481), p = 0.614 |
| Variable | SNA-POST-OP | SNB After | ANB After | Pog to N-Perp FH After | U1-NA° After | L1-NB° After | Nasolabial Angle After | Facial Convexity After |
|---|---|---|---|---|---|---|---|---|
| SNA-POST-OP | — | |||||||
| SNB After | 0.410 (0.018, 0.693), p = 0.042 | |||||||
| ANB After | 0.097 (−0.310, 0.474), p = 0.644 | −0.459 (−0.723, −0.078), p = 0.021 | ||||||
| Pog to N-Perp FH After | 0.133 (−0.276, 0.502), p = 0.525 | 0.153 (−0.258, 0.517), p = 0.465 | −0.425 (−0.702, −0.036), p = 0.034 | |||||
| U1-NA° After | −0.168 (−0.528, 0.243), p = 0.421 | 0.036 (−0.364, 0.425), p = 0.864 | −0.435 (−0.709, −0.049), p = 0.030 | 0.440 (0.054, 0.711), p = 0.028 | ||||
| L1-NB° After | −0.288 (−0.613, 0.121), p = 0.163 | −0.157 (−0.520, 0.254), p = 0.454 | 0.340 (−0.064, 0.648), p = 0.097 | 0.421 (0.032, 0.700), p = 0.036 | −0.696 (−0.856, −0.415), p < 0.001 | |||
| Nasolabial angle After | 0.102 (−0.306, 0.478), p = 0.628 | −0.225 (−0.569, 0.187), p = 0.280 | 0.435 (0.049, 0.709), p = 0.030 | 0.186 (−0.226, 0.541), p = 0.373 | 0.082 (−0.323, 0.463), p = 0.695 | 0.174 (−0.238, 0.532), p = 0.406 | ||
| Facial convexity After | 0.108 (−0.300, 0.482), p = 0.609 | −0.135 (−0.503, 0.275), p = 0.520 | −0.327 (−0.639, 0.078), p = 0.111 | 0.329 (−0.076, 0.641), p = 0.109 | −0.045 (−0.432, 0.357), p = 0.831 | 0.420 (0.029, 0.699), p = 0.037 | 0.053 (−0.350, 0.439), p = 0.802 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avrămuț, R.-P.; Stăncioiu, A.-A.; Talpos, S.; Motofelea, A.C.; Popa, M.; Szuhanek, C. Quantitative Evaluation of Skeletal, Dental, and Soft Tissue Changes After Orthognathic Surgery: A Cephalometric and Statistical Analysis. J. Clin. Med. 2025, 14, 7336. https://doi.org/10.3390/jcm14207336
Avrămuț R-P, Stăncioiu A-A, Talpos S, Motofelea AC, Popa M, Szuhanek C. Quantitative Evaluation of Skeletal, Dental, and Soft Tissue Changes After Orthognathic Surgery: A Cephalometric and Statistical Analysis. Journal of Clinical Medicine. 2025; 14(20):7336. https://doi.org/10.3390/jcm14207336
Chicago/Turabian StyleAvrămuț, Robert-Paul, Andra-Alexandra Stăncioiu, Serban Talpos, Alexandru Cătălin Motofelea, Malina Popa, and Camelia Szuhanek. 2025. "Quantitative Evaluation of Skeletal, Dental, and Soft Tissue Changes After Orthognathic Surgery: A Cephalometric and Statistical Analysis" Journal of Clinical Medicine 14, no. 20: 7336. https://doi.org/10.3390/jcm14207336
APA StyleAvrămuț, R.-P., Stăncioiu, A.-A., Talpos, S., Motofelea, A. C., Popa, M., & Szuhanek, C. (2025). Quantitative Evaluation of Skeletal, Dental, and Soft Tissue Changes After Orthognathic Surgery: A Cephalometric and Statistical Analysis. Journal of Clinical Medicine, 14(20), 7336. https://doi.org/10.3390/jcm14207336







